 |
 |
|
|
|
Abstract
Background/Aims
Data on the immunoprotective status against measles, mumps, rubella, varicella zoster virus (VZV), hepatitis A virus (HAV), and Epstein-Barr virus (EBV) infection in patients with inflammatory bowel disease (IBD) are still lacking. Therefore, we investigated the seropositivity rates for viral infectious diseases and the associated factors in Korean patients with IBD.
Methods
In this retrospective cohort study, serum immunoglobulin G antibody positivity rates against measles virus, mumps virus, rubella virus, VZV, HAV, and EBV viral capsid antigen (VCA) were measured in patients with CrohnŌĆÖs disease or ulcerative colitis (UC) who first visited the IBD clinic. Seropositivity rates and their associated factors were analyzed.
Results
Between January 2016 and December 2018, 263 patients were enrolled (male, 167 [67.3%]; UC, 134 [50.9%]). The median age at serological test was 30 years (interquartile range, 22 to 46). The seropositivity rates were 84.0%, 85.2%, 66.5%, 87.4%, 50.0%, and 93.7% for measles, mumps, rubella, VZV, HAV, and EBV, respectively. Younger age at serological test was associated with seronegative status for measles (adjusted odds ratio [aOR], 0.92; 95% confidence interval [CI], 0.88 to 0.96), VZV (aOR, 0.83; 95% CI, 0.74 to 0.93), and HAV (aOR, 0.93; 95% CI, 0.91 to 0.95). Furthermore, IBD type-UC was associated with seronegative status against VZV (aOR, 0.33; 95% CI, 0.11 to 0.99).
As inflammatory bowel disease (IBD) is considered to develop as a result of dysregulated immune response to the gut microbes in individuals with genetic susceptibility [1], immunosuppressive medications are the mainstay of medical therapy to achieve and maintain clinical and endoscopic remission of IBD [2].
However, the use of immunosuppressive agents is associated with an increased risk of infection [3,4]. In addition to the use of immunosuppressants, patients with IBD are susceptible to various infections because of other disease-associated risk factors such as disease severity, malnutrition, requirement for hospitalization, and need for interventions such as parenteral nutrition or surgery, exposing patients to the risk of infection [5].
Therefore, patients with IBD are at an increased risk of developing vaccine-preventable diseases (VPD) such as measles, mumps, rubella, hepatitis A, hepatitis B, chickenpox, and herpes zoster, and this risk is thought to be higher in patients with IBD receiving immunosuppressive medications [6]. However, it is unclear whether patients with IBD will respond properly to vaccinations against common VPDs. Although several studies have demonstrated that patients with IBD treated with immunosuppressive therapy have an impaired immune response to vaccinations [7ŌĆō12], the current guidelines and consensus recommend routine age-appropriate vaccinations for patients with IBD [3,6,13ŌĆō15].
The Epstein-Barr virus (EBV) can cause opportunistic infections in immunocompromised hosts [16]. Moreover, EBV infection is related to the development of lymphoproliferative disorders, and patients with IBD receiving thiopurine therapy have an increased risk of lymphoma [17ŌĆō19]. EBV serological screening before the initiation of thiopurine therapy is thought to be useful for physicians to assess the risk of lymphoproliferative disorders in patients with IBD [3,20].
Several studies have investigated the immune status of patients with IBD against various viral infectious diseases. DeBruyn et al. [12] reported relatively lower seropositivity rates for routine childhood VPDs (including measles/mumps/rubella [MMR], diphtheria/tetanus, varicella, hepatitis B virus, and hepatitis A virus [HAV]), cytomegalovirus, and EBV in spite of high coverage rates for routine childhood vaccines in Canadian pediatric patients with IBD. Cleveland et al. [21] reported that only 81% of adult patients with IBD in the United States were immune to measles, and most non-immune patients were receiving immunosuppressive therapy. Naganuma et al. [22] reported seropositivity rates for measles virus, mumps virus, rubella virus, and varicella zoster virus (VZV) in Japanese patients with IBD, and the seropositivity rates were low even in patients with an infection history.
So far, studies have shown that the seroprotection rates against VPDs tend to be low in patients with IBD. However, data on the seroprevalence of various viruses such as measles virus, mumps virus, rubella virus, HAV, VZV, and EBV among patients with IBD in Asia, including Korea, are still lacking. Therefore, we investigated the seropositivity rates for these viruses and the associated factors in Korean patients with IBD.
We enrolled consecutive patients with either ulcerative colitis (UC) or CrohnŌĆÖs disease (CD) who first visited the IBD clinic of Asan Medical Center, a tertiary referral center in Seoul, Korea, between January 2016 and December 2018, and who were checked for the immunoglobulin G (IgG) antibodies against measles virus, mumps virus, rubella virus, VZV, HAV, and EBV viral capsid antigen (VCA). Patients were excluded if they were aged < 18 or > 80 years at serological test, of non-Korean ethnicity by familial history, or diagnosed as having unclassified IBD.
Serum samples were collected from patients and tested immediately within 2 hours or stored at 0┬░C to 10┬░C until testing. Quantitative chemiluminescence immunoassay by Liaison XL (DiaSorin, Saluggia, Italy) was performed for IgGs against measles virus, mumps virus, rubella virus, VZV, and EBV VCA. A measles virus IgG titer of < 13.5 AU (arbitrary units)/mL was reported as negative; Ōēź 16.5 AU/mL, as positive; and between 13.5 and 16.5 AU/mL, as equivocal level [23]. Mumps virus IgG and rubella virus IgG titers of < 9.0 AU/mL were reported as negative; Ōēź 11.0 AU/mL, as positive; and between 9.0 and 11.0 AU/mL, as an equivocal level [24,25]. A VZV IgG titer of < 150 mIU/mL was reported as negative; and Ōēź 150 mIU/mL, as positive [26]. An EBV VCA IgG titer of < 20 U/mL was reported as negative; and Ōēź 20 U/mL, as positive [27]. Quantitative electrochemiluminescence immunoassay by Cobas e602 (Roche, Basel, Switzerland) was performed for anti-HAV IgG, with a titer of < 20 U/mL was reported as negative; and Ōēź 20 U/mL, as positive [28].
Serological and demographic data (sex, age, and family history of IBD in first-degree relatives), and medical history were retrospectively collected. Medical history included IBD type (UC or CD), date of IBD diagnosis, disease duration at serological test, disease characteristics (extent of UC, and location and behavior of CD), history of bowel resection in CD, and current IBD medications at serological test. Medications were categorized as systemic corticosteroids (prednisone, methylprednisone, and beclomethasone dipropionate), immunomodulators (azathioprine, 6-mercaptopurine, and methotrexate), anti-tumor necrosis factor-╬▒ agents (infliximab, infliximab biosimilar, adalimumab, and golimumab), and other biologics (ustekinumab and vedolizumab). Current medication was defined as use of drugs within 90 days of the serological test.
Immune status against measles virus, mumps virus, rubella virus, VZV, HAV, and EBV were categorized as seropositive or seronegative status. A positive serological result for each virus was defined as seropositive status, and an equivocal or negative result was defined as a seronegative status.
Descriptive statistics were used to summarize the patientsŌĆÖ demographic characteristics and expressed as median values with their interquartile ranges (IQRs) or numbers with their percentages (%), where appropriate. The proportions of subjects with seropositivity were presented as percentages with their 95% confidence intervals (CIs). Multivariable logistic regression analysis with backward selection was performed to identify potential clinical factors associated with seronegativity using the following variables: sex; age at serological test; disease duration at serological test; family history of IBD; IBD type; IBD subphenotype such as extent of UC, location of CD, behavior of CD, and bowel resection history in CD; and IBD medications. Selected variables were included in a logistic regression model, and final adjusted odds ratios (aORs) of each selected variable were calculated. Statistical significance was defined as a p value of < 0.05. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA). A subgroup analysis was also performed for the subjects who were not receiving any immunosuppressive therapy, categorized as ŌĆ£none or 5-aminosalicylic acidsŌĆØ treatment.
Between January 2016 and December 2018, a total of 263 patients underwent serological tests for measles, mumps, and rubella. Of this total, 151, 262, and 111 had undergone serological tests for VZV, HAV, and EBV, respectively. The baseline characteristics of the 263 study subjects are summarized in Table 1. Of the total, 134 patients (50.9%) had UC and 129 (49.1%) had CD, with 177 (67.3%) being males. The median ages at diagnosis of IBD and serological test were 28 years (IQR, 20 to 41) and 30 years (IQR, 22 to 47), respectively. The median ages at diagnosis and serological test of UC patients were 37 years (IQR, 26 to 51) and 40 years (IQR, 28 to 55), respectively, while CD patients tended to be younger than UC patients: the median ages at diagnosis and serological test were 21 years (IQR, 18 to 28) and 23 years (IQR, 21 to 32), respectively. One hundred and eighty-two patients (69.2%) were not receiving any immunosuppressive therapy, and the rest were receiving any kind of immunosuppressant at serological test.
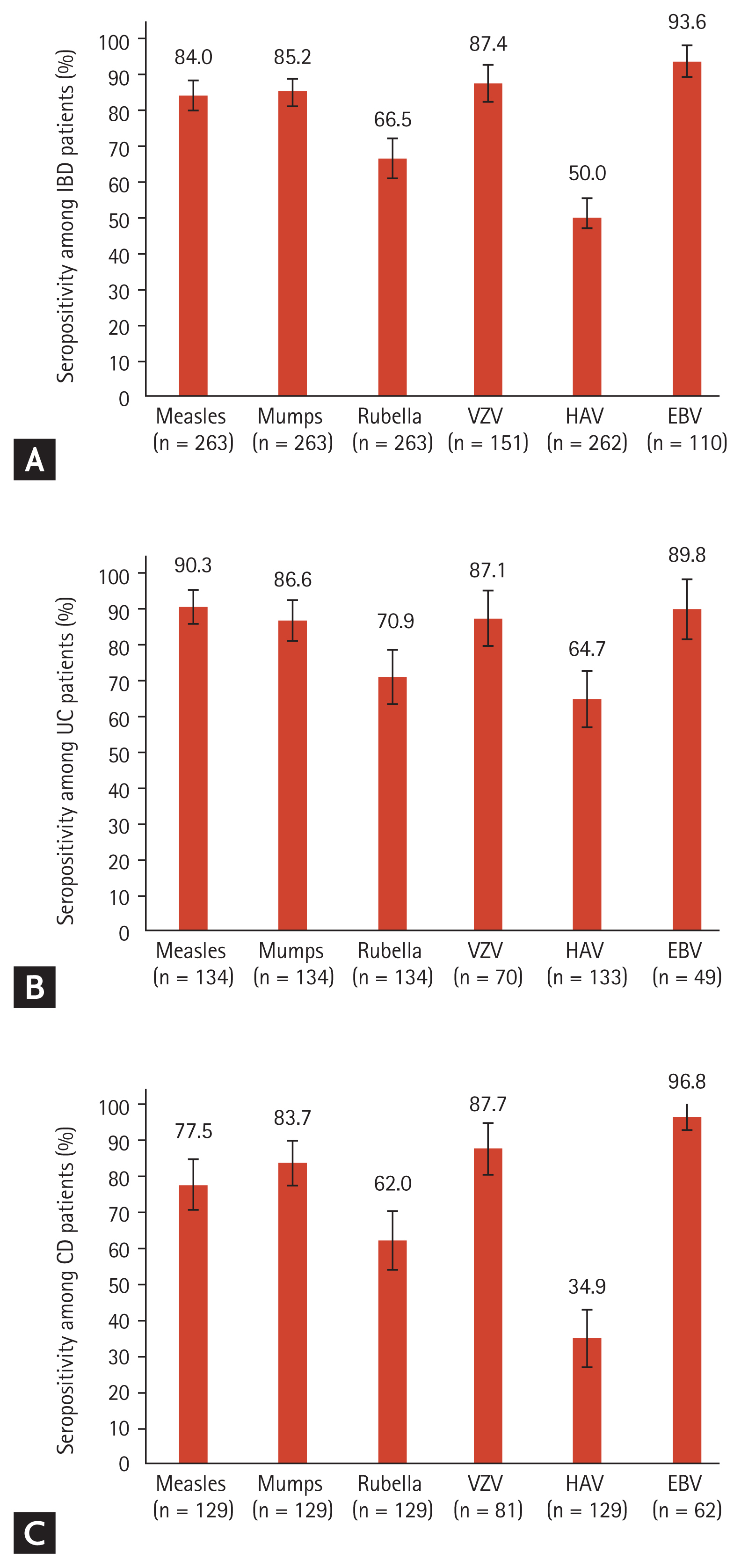
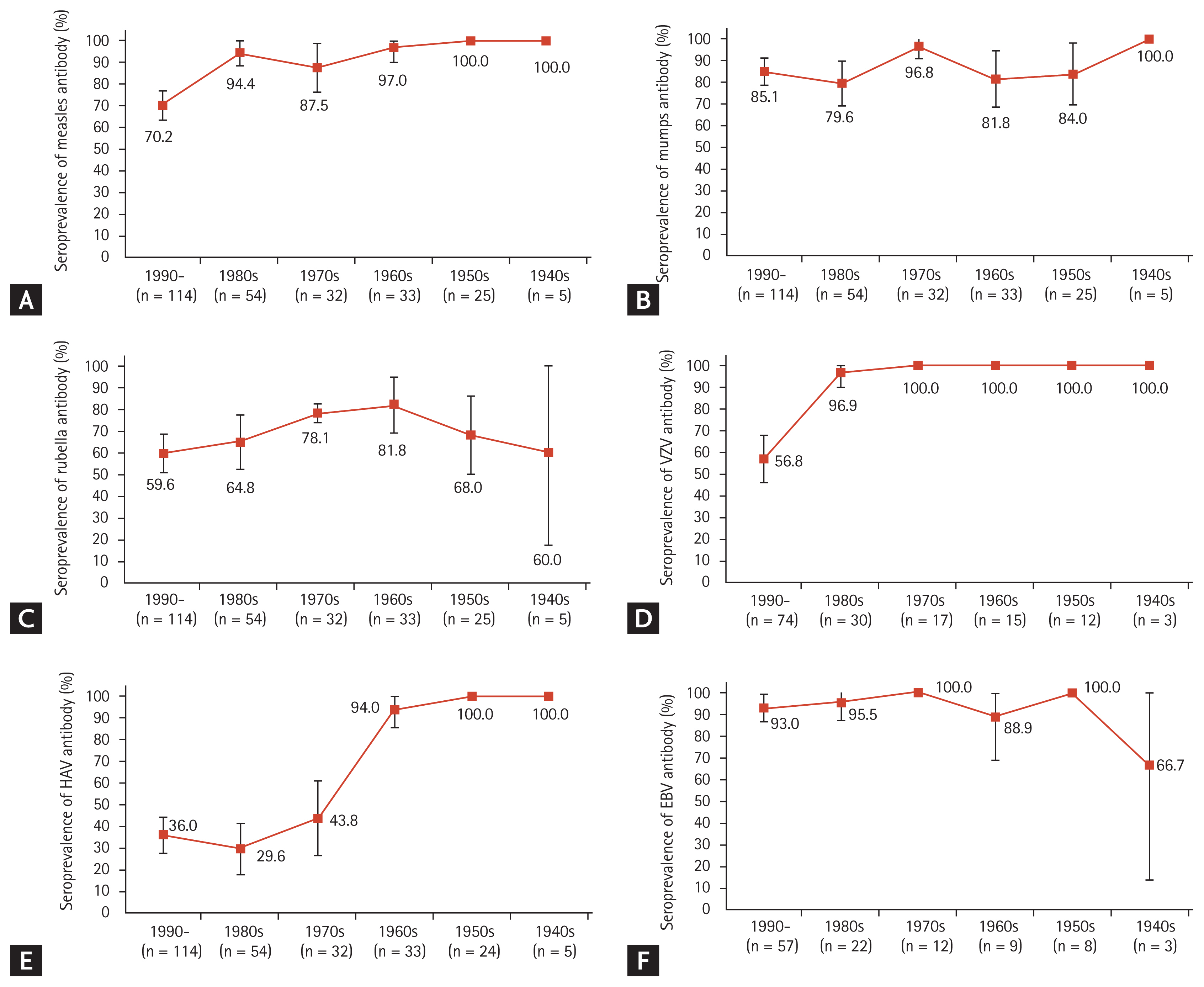
The results of the serological tests are summarized in Fig. 1. Of the 263 patients, 84.0% (95% CI, 79.6% to 88.4%), 85.2% (95% CI, 80.9% to 89.5%), and 66.5% (95% CI, 60.8% to 72.2%) were seropositive for measles, mumps, and rubella virus, respectively. The seropositivity rates for VZV and HAV were 87.4% (95% CI, 82.1% to 92.7%) of 151 patients and 50.0% (95% CI, 44.0% to 56.0%) of 262 patients, respectively. The seropositivity rate for EBV VCA was 93.7% (95% CI, 89.2% to 98.2%) in 111 patients. The seropositivity rates categorized by UC and CD are also shown in Fig. 1. Patients with CD showed numerically lower seropositivity rates for measles, mumps and rubella virus, and it showed almost half seropositivity rate for HAV compared with that observed in patients with UC. The seroprevalence data of IgG antibodies against each virus stratified by birth year are shown in Fig. 2. The seropositivity rate for measles tended to decrease in the younger age group, especially in the patients born in the 1990s (Fig. 2A). The seropositivity rate for VZV was lowest in the 1990s birth cohorts (Fig. 2D). The seropositivity rate for HAV sharply declined from > 90% to < 50% in the 1970s birth cohorts (Fig. 2E). On the contrary, there was no significant difference in seropositivity rates for mumps, rubella, and EBV according to birth cohorts (Fig. 2B, 2C, and 2F).
Multivariable logistic regression model with backward selection was performed to identify factors potentially associated with seronegativity. Selected variables were included in a logistic regression model and final aORs were calculated (Table 2). Younger age at serological test was associated with seronegative status for measles (aOR, 0.92; 95% CI, 0.88 to 0.96), VZV (aOR, 0.83; 95% CI, 0.74 to 0.93), and HAV (aOR, 0.93; 95% CI, 0.91 to 0.95). Furthermore, IBD type-UC was associated with seronegativity for VZV (aOR, 0.33; 95% CI, 0.11 to 0.99). Among patients with UC, younger age at serological test was associated with seronegativity for measles (aOR, 0.94; 95% CI, 0.84 to 0.97), VZV (aOR, 0.85; 95% CI, 0.74 to 0.96), and HAV (aOR, 0.92; 95% CI, 0.90 to 0.95). Moreover, family history of IBD was associated with seronegativity for measles (aOR, 5.04; 95% CI, 1.02 to 24.94) among UC patients. Among patients with CD, younger age at serological test was associated with seronegative status for measles (aOR, 0.90; 95% CI, 0.84 to 0.97), VZV (aOR, 0.79; 95% CI, 0.63 to 0.99), and HAV (aOR, 0.95; 95% CI, 0.91 to 0.98).
We conducted a subgroup analysis for 182 subjects who were not receiving any immunosuppressive therapy. Of the 182 patients, 119 (65.4%) were male. The median ages at IBD diagnosis and at serological test were 30 years (IQR, 22 to 42) and 32 years (IQR, 23 to 48), respectively. The median disease duration at serological test was 2 months (IQR, 0 to 31). Of the 182 patients, 107 (58.8%) had UC and 75 (41.2%) had CD. Their baseline characteristics are shown in Supplementary Table 1, and the results of their serological tests are summarized in Supplementary Fig. 1. Of the 182 patients, 83.0% (95% CI, 77.5% to 88.5%), 85.2% (95% CI, 80.0% to 90.4%), and 67.6% (95% CI, 60.8% to 74.4%) were seropositive against measles, mumps, and rubella, respectively. The seropositivity rate for VZV was 88.9% (96/108; 95% CI, 83.0% to 94.8%), and 52.5% (95/181) of the patients (95% CI, 45.2% to 59.8%) were positive for anti-HAV IgG. Additionally, EBV VCA IgG positivity was observed in 92.0% (69/75) of the patients (95% CI, 85.9% to 98.1%). Through multivariable logistic regression analysis with backward selection and a logistic regression model, final aORs were calculated for selected variables. The results are shown in Supplementary Table 2. Younger age at serological test was associated with seronegative status for measles (OR, 0.91; 95% CI, 0.87 to 0.96) and HAV (OR, 0.93; 95% CI, 0.91 to 0.95).
In this study, we evaluated the seroprevalence of vaccine-preventable viral infectious diseases and EBV among Korean patients with IBD, and the clinical factors associated with seronegativity.
In a previous Canadian study, among pediatric patients with IBD, only 65.8% were seropositive for measles virus IgG [12]. Similarly, a Japanese study on IBD patients aged between 16 and 71 years, reported an IgG seropositivity rate for measles virus of 66.2% [22]. Our study showed a higher seropositivity rate for measles than those reported for Canadian and Japanese patients with IBD [12,22]. In South Korea, after the introduction of the measles-containing vaccine in 1965 and the national immunization program against measles started in 1985, the number of measles cases largely decreased [29ŌĆō31]. In 2001, after the measles outbreak from 2000 to 2001, a 5-year elimination program, including catch-up vaccination and keep-up programs targeting the 8- to 16-year-old population to ensure the two-dose administration of MMR vaccine, was started [31]. After the 5-year elimination program, almost all Koreans born after 1985 are thought to have been immunized. The World Health Organization also declared measles eliminated in South Korea in 2006 [31]. However, occasional measles outbreaks still occur every 3 to 4 years, and several epidemiological studies on measles in Korea have been conducted since 2010 [30,32,33]. Choe et al. [32] published a seroepidemiological study in 2012, reporting that the measles virus IgG titer showed a half U-shaped curve pattern, and that young adolescents had the lowest seropositivity rate. Kang et al. [33] also investigated the seroprevalence of measles among Korean population in 2017, reporting that the overall seropositivity rate was 71.5%, and the age-specific seropositivity rate was lowest between the 16- and 22-year-old age groups. In a recent Korean study of > 7,000 healthcare workers, Jung et al. [34] reported that the seropositivity rate for measles among healthcare workers declined sharply in the 1990s birth cohorts, which is consistent with our study results. Secondary vaccine failure or waning immunity, in which antibody levels decrease over time after vaccination is presumed to be the main reason for the low seropositivity rate in the immunized population [32ŌĆō35]. In addition, primary vaccine failure could be considered to be another reason [32]. A similar seroprevalence pattern was also reported in Europe [36ŌĆō38] and the United States [39], and this is consistent with our results, showing that younger age was associated with seronegativity for measles. In contrast, in another study conducted in the Chicago, among IBD patients aged 18 or older, age at serological test and disease duration were associated with measles antibody titers, and the antibody titers were significantly lower in subjects aged 50 years or more [21]. These results may be due to differences in the characteristics of the study populations, vaccination programs, and vaccination rates between the studies.
The seropositivity rate for mumps virus in our study was 85.2%. The seropositivity rate was 60.5% in Canadian pediatric patients with IBD [12] and 63.3% in Japanese patients with IBD [22]. Kim et al. [29] reported that the seropositivity rate for mumps virus in the general Korean population was approximately 80%. Our study subjects showed a higher seropositivity rate than those in previous studies and a similar rate with the general Korean population [12,22,29]. Similar to that for measles, the intensive national immunization strategy using MMR vaccine in Korea might have contributed to the high seropositivity rates.
Our study showed that the seropositivity rate for rubella virus was 66.5%. In previous studies, the rates were 79.1% in Canadian pediatric patients with IBD [12], 70.5% in Japanese patients with IBD [22], and 95.3% in the United States general population [39]. In previous Korean studies, the seropositivity rate against rubella virus was 77.1% in children [40] and 64.5% in the general population [41]. The seropositivity rate for rubella virus in our study appears to be generally lower than those in studies from other countries, and similar to the data of the general Korean population. The reason for the lower seropositivity rate for rubella virus than those for measles virus and mumps virus in Korea is difficult to explain because the national immunization program was conducted using MMR vaccine. At least, the rubella virus IgG titer after administration of MMR vaccine is known to decrease over time [42], probably explaining the low seropositivity rate (65.5%) and relatively high equivocal level (8.0%) in our study.
The seropositivity rate for VZV was 95.0% in Japanese patients with IBD [22], 70.5% in Canadian pediatric patients with IBD [12], and 85.8% in the general Korean population [43]. Our result of 87.4% seropositivity rate for VZV is similar to that among the general Korean population [43]. Our results showed that younger age at serological test was associated with seronegativity for VZV. This association could be attributed to a presumably higher proportion of subjects with past chickenpox infection in the older population. In Korea, since varicella vaccination was introduced only in 2005 and all of our study subjects were born before 2005, most of the immunized subjects might have acquired immunity through past chickenpox infection. In addition, our study showed that IBD type-UC was associated with seronegativity for VZV compared with CD. A Canadian study with pediatric IBD patients reported that IBD type-UC tended to be associated with seronegativity for VZV, but there was no statistical significance [12]. There have been limited data regarding the association between IBD type and immunity against VZV. There might be effects of other unadjusted factors, such as vaccination history, which was not considered in this study. For patients who lack immunity to VZV, vaccination against varicella should be considered before starting immunosuppressive therapy because the most commonly used VZV vaccines for chickenpox or herpes zoster are live attenuated vaccines, which should be avoided during high-level immunosuppression [14].
In our study, the seropositivity rate for HAV was only 50.0%, and younger age at serological test was associated with the seronegative status for HAV. In the general Korean population, Lee et al. [44] reported that the seropositivity rate for HAV was 53.8% between 2008 and 2010, and Kim et al. [45] demonstrated a seropositivity rate of 62.2% in 2014. Those studies also showed that age-specific seropositivity rates were lowest in the twenties age groups born in the 1980s and 1990s [44,45]. The seropositivity rate for HAV in our study is similar to those reported in the general Korean population, and the low rate can be attributed to relatively young age of the subjects. The median birth year of our subjects who were tested for anti-HAV IgG was 1987, and 64.1% (168/262) of the patients were born between the 1980 and 2000. The seropositivity rate for HAV in young adults born in the 1980s and 1990s appears to be low because urbanization and the improvement of sanitary conditions resulted in the reduction of childhood exposure to HAV and subsequent acquiring of immunity [45ŌĆō47]. Moreover, in Korea, HAV vaccine was introduced in 1997 and included in the national mandatory immunization program for children in 2015 [48], which suggests that vaccination rate against HAV among our study patients would have been low because only 41 patients (15.6%) were born after 1997.
The seropositivity rate for EBV was 93.7% in our study. Worldwide, more than 90% of adults have been reported to acquire immunity to EBV after experiencing primary infection during childhood and adolescence [49]. De Francisco et al. [20] reported an overall seroprevalence of EBV of 97.4% among Spanish patients with IBD over the age of 17, which is similar to our results. Our results also suggest that the seroprevalence is similar between patients with IBD and the general population in Korea.
In our study, CD patients showed generally lower seropositivity rates for measles, mumps, and rubella virus and almost half seropositivity rate for HAV, compared with patients with UC. Considering that younger age at serological test was associated with seronegativity for measles and HAV, this observation could be the results of the difference in age distribution between CD and UC patients.
Among patients with UC, family history of IBD was shown to be associated with seronegativity for measles. However, there has been limited data regarding the association between seroprevalence of measles and family history of IBD, among UC patients. Moreover, since the number of study subjects with a positive family history of IBD was limited in our study (n = 12), further larger-scaled studies are required.
In terms of IBD medication, our results demonstrated no significant association between IBD medication and seropositivity for measles, mumps, rubella, VZV, HAV, and EBV. These results correspond to those of previous studies conducted in Chicago, Wisconsin, Alberta, and Japan [12,21,22,50].
In the subgroup analysis for subjects who were not receiving any immunosuppressive therapy, the seropositivity rate for each viral disease was generally similar to those of the entire study subjects. On the contrary, IBD type was not associated with seronegativity for VZV.
This study has some limitations. First, it was a retrospective study performed in a tertiary referral center, which led to referral bias. Second, the vaccination and previous infectious disease histories of each subject were unavailable, and their associations with seropositivity could not be analyzed. However, even if the information has been collected on the basis of the patientsŌĆÖ recall, the memory of patients could have been inaccurate and unreliable, as documented in a previous study [22]. Although the vaccination record of each individual is available in the online database of the Korea Centers for Disease Control and Prevention, most data for adults are unavailable. Due to a lack of serological tests for IgM antibodies, as well as a lack of vaccination history, it was not possible to distinguish past infection or immunization from active infection. Finally, because our study did not evaluate the serological status of the non-IBD population, we could not compare the results between IBD and non-IBD populations.
In conclusion, the seropositivity rates for viral infectious diseases in Korean patients with IBD, treated with or without immunosuppressive agents, were similar to those in the general Korean population. Younger age at serological test was associated with seronegative status for measles virus, VZV, and HAV. Moreover, IBD type-UC was associated with seronegativity for VZV. Therefore, evaluation of immunity to measles virus, VZV, and HAV, especially in the younger age group is required, and proper vaccination should be implemented.
1. The seropositivity rates against vaccine-preventable diseases in the Korean patients with inflammatory bowel disease (IBD), treated with or without immunosuppressive agents, were similar to those in the general Korean population.
2. Younger age at serological test was associated with seronegative status for measles virus, varicella zoster virus (VZV), and hepatitis A virus (HAV). IBD type-ulcerative colitis was associated with seronegative status against VZV.
3. Evaluation of immunity to measles virus, VZV, and HAV, especially in the younger age group, is required, and proper vaccination should be implemented.
Conflict of Interest
Conflict of interest
Byong Duk Ye received a research grant from Celltrion and Pfizer Korea; consulting fees from Abbvie Korea, Celltrion, Chong Kun Dang Pharm., Daewoong Pharma., Ferring Korea, Janssen Korea, Kangstem Biotech, Kuhnil Pharm., LG Chem., Medtronic Korea, Shire Korea, Takeda Korea, IQVIA, Cornerstones Health, Robarts Clinical Trials Inc., and Takeda; speaking fees from Abbvie Korea, Celltrion, Ferring Korea, Janssen Korea, Pfizer Korea, Shire Korea, Takeda Korea, and IQVIA. Suk-Kyun Yang received a research grant from Janssen Korea. However, none of these funding sources has any commercial interest in relation to this study.
Acknowledgments
This study was supported by a grant (number: 2020IT0012) from the Asan Institute for Life Sciences, Seoul, Korea.
Figure┬Ā1
Seropositivity of viruses. (A) Inflammatory bowel disease (IBD) patients, (B) ulcerative colitis (UC) patients, (C) CrohnŌĆÖs disease (CD) patients. The error bars denote 95% confidence intervals. VZV, varicella zoster virus; HAV, hepatitis A virus; EBV, Epstein-Barr virus.
Figure┬Ā2
Seroprevalence of (A) measles, (B) mumps, (C) rubella, (D) varicella zoster virus (VZV), (E) hepatitis A virus (HAV), and (F) Epstein-Barr virus (EBV) antibodies among the groups according to the decade of birth. The error bars denote 95% confidence intervals.
Table┬Ā1
Baseline characteristics of the patients
| Characteristic | UC (n = 134) | CD (n = 129) | All patients (n = 263) |
|---|---|---|---|
| Male sex | 84 (62.7) | 93 (72.1) | 177 (67.3) |
| Age at diagnosis, yr | 37 (26ŌĆō51) | 21 (18ŌĆō28) | 28 (20ŌĆō41) |
| Age at serological test, yr | 40 (28ŌĆō55) | 23 (21ŌĆō32) | 30 (22ŌĆō47) |
| Disease duration at serological test, mon | 11 (0ŌĆō51) | 4 (0ŌĆō60) | 6 (0ŌĆō59) |
| Family history of IBD | 12 (9.0) | 6 (4.7) | 18 (6.8) |
| Disease extent of UC at serological test | |||
| ŌĆāProctitis | 54 (40.3) | ||
| ŌĆāLeft-sided colitis | 44 (32.8) | ||
| ŌĆāExtensive colitis | 36 (26.9) | ||
| Disease location of CD at serological test | |||
| ŌĆāIleum | 27 (21.0) | ||
| ŌĆāColon | 7 (5.4) | ||
| ŌĆāIleocolon | 95 (73.6) | ||
| ŌĆāUpper gastrointestinal involvement | 28 (21.7) | ||
| Disease behavior of CD at serological test | |||
| ŌĆāNon-stricturing, non-penetrating | 82 (63.6) | ||
| ŌĆāStricturing | 24 (18.6) | ||
| ŌĆāPenetrating | 23 (17.8) | ||
| ŌĆāPerianal disease | 63 (48.8) | ||
| Bowel resection history at serological test among CD patients | 18 (14.0) | ||
| Current medication | |||
| ŌĆāNone or 5-ASA | 107 (79.9) | 75 (58.0) | 182 (69.2) |
| ŌĆāCorticosteroidsa | 16 (12.0) | 9 (7.0) | 25 (9.5) |
| ŌĆāImmunomodulatorsb | 5 (3.7) | 20 (15.5) | 25 (9.5) |
| ŌĆāAnti-TNF-╬▒ agentsc | 2 (1.5) | 12 (9.3) | 14 (5.3) |
| ŌĆāCorticosteroidsa + Immunomodulatorsb | 2 (1.5) | 5 (3.9) | 7 (2.6) |
| ŌĆāCorticosteroidsa + Anti-TNF-╬▒ agentsc | 1 (0.7) | 2 (1.6) | 3 (1.2) |
| ŌĆāImmunomodulatorsb + Anti-TNF-╬▒ agentsc | 1 (0.7) | 5 (3.9) | 6 (2.3) |
| ŌĆāCorticosteroidsa + Immunomodulatorsb + Anti-TNF-╬▒ agentsc | 0 | 1 (0.8) | 1 (0.4) |
Table┬Ā2
Clinical factors associated with seronegativity
REFERENCES
1. Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res 2018;16:26ŌĆō42.



2. Zenlea T, Peppercorn MA. Immunosuppressive therapies for inflammatory bowel disease. World J Gastroenterol 2014;20:3146ŌĆō3152.



3. Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2014;8:443ŌĆō468.


4. Bonovas S, Fiorino G, Allocca M, et al. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol 2016;14:1385ŌĆō1397.


5. Ananthakrishnan AN, McGinley EL. Infection-related hospitalizations are associated with increased mortality in patients with inflammatory bowel diseases. J Crohns Colitis 2013;7:107ŌĆō112.


6. Reich J, Wasan S, Farraye FA. Vaccinating patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2016;12:540ŌĆō546.


7. Cullen G, Bader C, Korzenik JR, Sands BE. Serological response to the 2009 H1N1 influenza vaccination in patients with inflammatory bowel disease. Gut 2012;61:385ŌĆō391.


8. Lee CK, Kim HS, Ye BD, et al. Patients with CrohnŌĆÖs disease on anti-tumor necrosis factor therapy are at significant risk of inadequate response to the 23-valent pneumococcal polysaccharide vaccine. J Crohns Colitis 2014;8:384ŌĆō391.


9. Park SH, Yang SK, Park SK, et al. Efficacy of hepatitis A vaccination and factors impacting on seroconversion in patients with inflammatory bowel disease. Inflamm Bowel Dis 2014;20:69ŌĆō74.


10. DeBruyn J, Fonseca K, Ghosh S, et al. Immunogenicity of influenza vaccine for patients with inflammatory bowel disease on maintenance infliximab therapy: a randomized trial. Inflamm Bowel Dis 2016;22:638ŌĆō647.


11. Wasan SK, Zullow S, Berg A, Cheifetz AS, Ganley-Leal L, Farraye FA. Herpes zoster vaccine response in inflammatory bowel disease patients on low-dose immunosuppression. Inflamm Bowel Dis 2016;22:1391ŌĆō1396.


12. DeBruyn JC, Soon IS, Fonseca K, et al. Serologic status of routine childhood vaccines, cytomegalovirus, and Epstein-Barr virus in children with inflammatory bowel disease. Inflamm Bowel Dis 2019;25:1218ŌĆō1226.


13. Sands BE, Cuffari C, Katz J, et al. Guidelines for immunizations in patients with inflammatory bowel disease. Inflamm Bowel Dis 2004;10:677ŌĆō692.


14. Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol 2017;112:241ŌĆō258.


15. Inflammatory Bowel Disease Group, Chinese Society of Gastroenterology, Chinese Medical Association. Evidence-based consensus on opportunistic infections in inflammatory bowel disease (republication). Intest Res 2018;16:178ŌĆō193.




16. Sitki-Green DL, Edwards RH, Covington MM, Raab-Traub N. Biology of Epstein-Barr virus during infectious mononucleosis. J Infect Dis 2004;189:483ŌĆō492.


17. Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009;374:1617ŌĆō1625.


18. Beaugerie L. Lymphoma: the b├¬te noire of the long-term use of thiopurines in adult and elderly patients with inflammatory bowel disease. Gastroenterology 2013;145:927ŌĆō930.


19. Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA 2017;318:1679ŌĆō1686.



20. De Francisco R, Castano-Garcia A, Martinez-Gonzalez S, et al. Impact of Epstein-Barr virus serological status on clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther 2018;48:723ŌĆō730.


21. Cleveland NK, Rodriquez D, Wichman A, Pan I, Melmed GY, Rubin DT. Many inflammatory bowel disease patients are not immune to measles or pertussis. Dig Dis Sci 2016;61:2972ŌĆō2976.


22. Naganuma M, Nagahori M, Fujii T, Morio J, Saito E, Watanabe M. Poor recall of prior exposure to varicella zoster, rubella, measles, or mumps in patients with IBD. Inflamm Bowel Dis 2013;19:418ŌĆō422.


23. DiaSorin. LIAISON® Measles IgG [Internet] Saluggia (IT): DiaSorin, 2021. [cited 2021 Jun 30]. Available from: https://www.diasorin.com/sites/default/files/allegati_prodotti/scheda_measles_igg.pdf
.
24. DiaSorin. LIAISON® Mumps IgG [Internet] Saluggia (IT): DiaSorin, 2021. [cited 2021 Jun 30]. Available from: https://www.diasorin.com/sites/default/files/allegati_prodotti/scheda_mumps_igg.pdf
.
25. DiaSorin. LIAISON® Rubella IgG II [Internet] Saluggia (IT): DiaSorin, 2021. [cited 2021 Jun 30]. Available from: https://www.diasorin.com/sites/default/files/allegati_prodotti/ese_scheda_rubella_rev.02_low.pdf
.
26. DiaSorin. LIAISON® VZV IgG [Internet] Saluggia (IT): DiaSorin, 2021. [cited 2021 Jun 30]. Available from: https://www.diasorin.com/sites/default/files/allegati_prodotti/ese_scheda_vzv_rev_02_low.pdf
.
27. DiaSorin. Epstein-barr virus, VCA IgG, EBNA IgG, EBV IgM, EA IgG [Internet] Saluggia (IT): DiaSorin, 2021. [cited 2021 Jun 30]. Available from: https://www.diasorin.com/sites/default/files/allegati_prodotti/ese_scheda_ebv_low_1.pdf
.
28. Roche. Elecsys Anti-HAV [Internet] Indianapolis (IN): Roche Diagnostics, 2020. [cited 2021 Jun 30]. Available from: https://pim-eservices.roche.com/LifeScience/Document/7edde42f-b3f6-e911-fa90-005056a71a5d
.
29. Kim SS, Han HW, Go U, Chung HW. Sero-epidemiology of measles and mumps in Korea: impact of the catch-up campaign on measles immunity. Vaccine 2004;23:290ŌĆō297.


30. Yang TU, Kim JW, Eom HE, et al. Resurgence of measles in a country of elimination: interim assessment and current control measures in the Republic of Korea in early 2014. Int J Infect Dis 2015;33:12ŌĆō14.


31. Choe YJ, Jee Y, Oh MD, Lee JK. Measles elimination activities in the Western Pacific region: experience from the Republic of Korea. J Korean Med Sci 2015;30(Suppl 2):S115ŌĆōS121.



32. Choe YJ, Bae GR. Current status of measles in the Republic of Korea: an overview of case-based and seroepidemiological surveillance scheme. Korean J Pediatr 2012;55:455ŌĆō461.



33. Kang HJ, Han YW, Kim SJ, et al. An increasing, potentially measles-susceptible population over time after vaccination in Korea. Vaccine 2017;35:4126ŌĆō4132.


34. Jung J, Kim SK, Kwak SH, Hong MJ, Kim SH. Seroprevalence of measles in healthcare workers in South Korea. Infect Chemother 2019;51:58ŌĆō61.



35. Paunio M, Hedman K, Davidkin I, et al. Secondary measles vaccine failures identified by measurement of IgG avidity: high occurrence among teenagers vaccinated at a young age. Epidemiol Infect 2000;124:263ŌĆō271.



36. Pena-Rey I, Martinez de Aragon V, Mosquera M, de Ory F, Echevarria JE. Measles Elimination Plan Working Group in Spain. Measles risk groups in Spain: implications for the European measles-elimination target. Vaccine 2009;27:3927ŌĆō3934.


37. Emek M, Islek D, Atasoylu G, et al. Association between seroprevalence of measles and various social determinants in the year following a measles outbreak in Turkey. Public Health 2017;147:51ŌĆō58.


38. Smetana J, Chlibek R, Hanovcova I, et al. Decreasing seroprevalence of measles antibodies after vaccination: possible gap in measles protection in adults in the Czech Republic. PLoS One 2017;12:e0170257.



39. Lebo EJ, Kruszon-Moran DM, Marin M, et al. Seroprevalence of measles, mumps, rubella and varicella antibodies in the United States population, 2009ŌĆō2010. Open Forum Infect Dis 2015;2:ofv006.

40. Ki MR, Choi BY, Kim MH, Shin YJ, Park TS. Rubella seroprevalence in Korean children. J Korean Med Sci 2003;18:331ŌĆō336.



41. Jeong KP, Kim MR, Woo HO, Youn HS. Prevalence of rubella antibodies in the Southern Central Korea. J Korean Pediatr Soc 1995;38:786ŌĆō793.
42. LeBaron CW, Forghani B, Matter L, et al. Persistence of rubella antibodies after 2 doses of measles-mumps-rubella vaccine. J Infect Dis 2009;200:888ŌĆō899.


43. Lee H, Cho HK, Kim KH. Seroepidemiology of varicella-zoster virus in Korea. J Korean Med Sci 2013;28:195ŌĆō199.



44. Lee H, Cho HK, Kim JH, Kim KH. Seroepidemiology of hepatitis A in Korea: changes over the past 30 years. J Korean Med Sci 2011;26:791ŌĆō796.



45. Kim KA, Lee A, Ki M, Jeong SH. Nationwide seropositivity of hepatitis A in Republic of Korea from 2005 to 2014, before and after the outbreak peak in 2009. PLoS One 2017;12:e0170432.



46. Lednar WM, Lemon SM, Kirkpatrick JW, Redfield RR, Fields ML, Kelley PW. Frequency of illness associated with epidemic hepatitis A virus infections in adults. Am J Epidemiol 1985;122:226ŌĆō233.


47. Yoon JG, Choi MJ, Yoon JW, et al. Seroprevalence and disease burden of acute hepatitis A in adult population in South Korea. PLoS One 2017;12:e0186257.



48. Heo JY, Song JY, Noh JY, et al. Low level of immunity against hepatitis A among Korean adolescents: vaccination rate and related factors. Am J Infect Control 2013;41:e97ŌĆōe100.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



