 |
 |
| Korean J Intern Med > Volume 39(5); 2024 > Article |
|
Abstract
Background/Aims
Methods
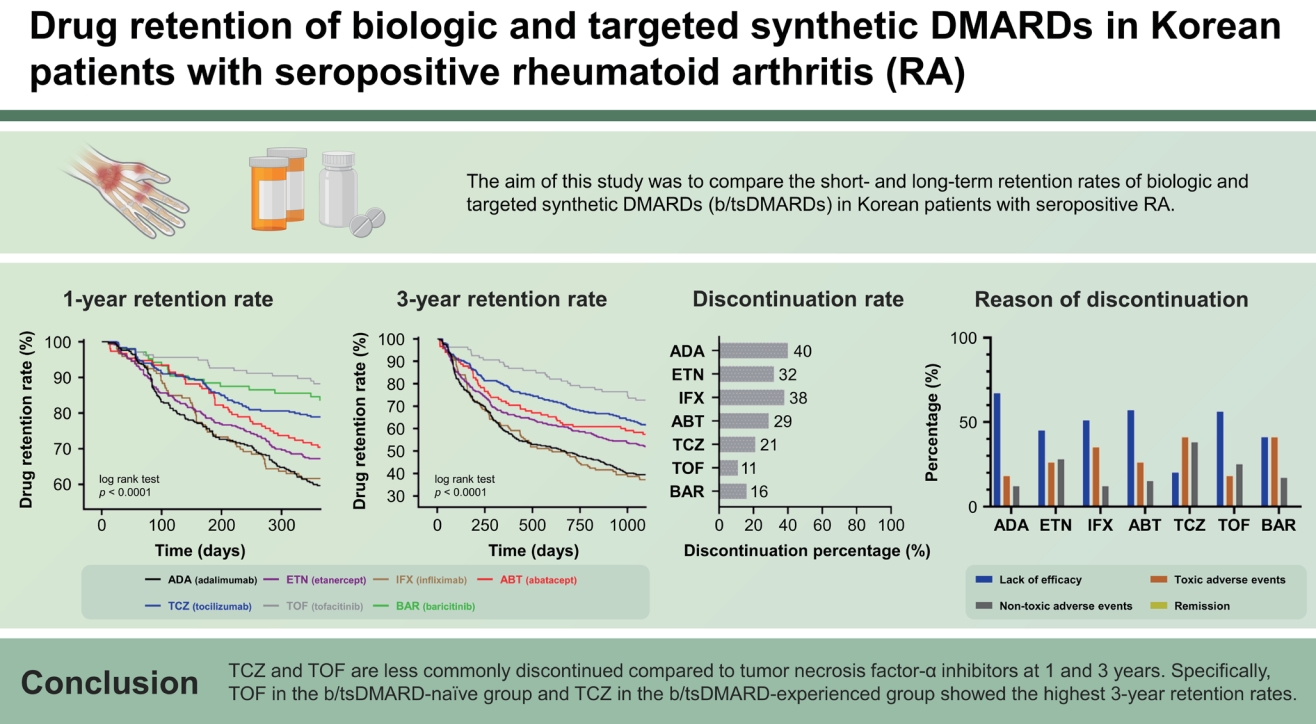
Results
Notes
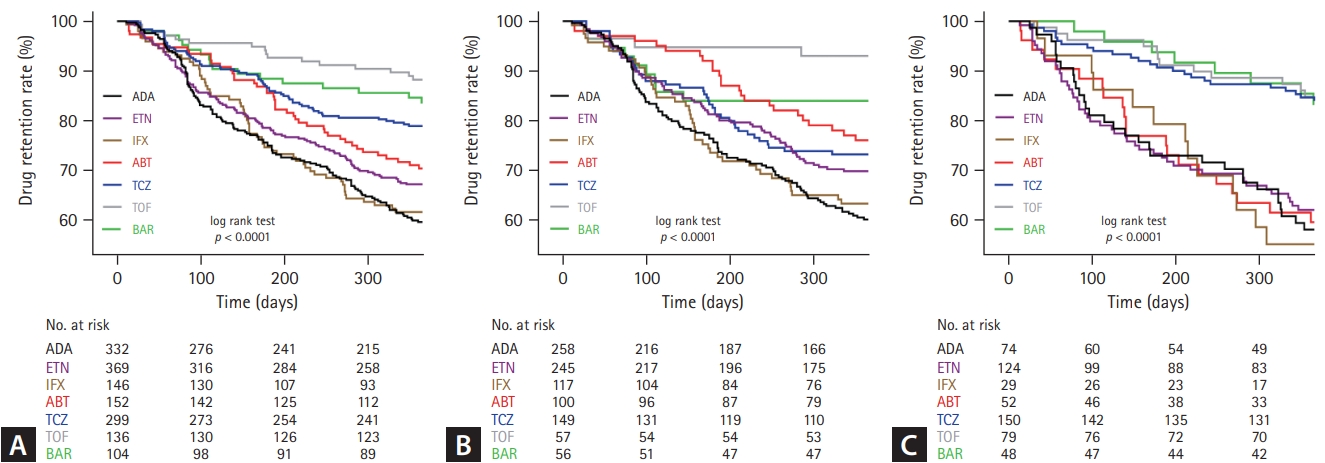
Figure┬Ā1.
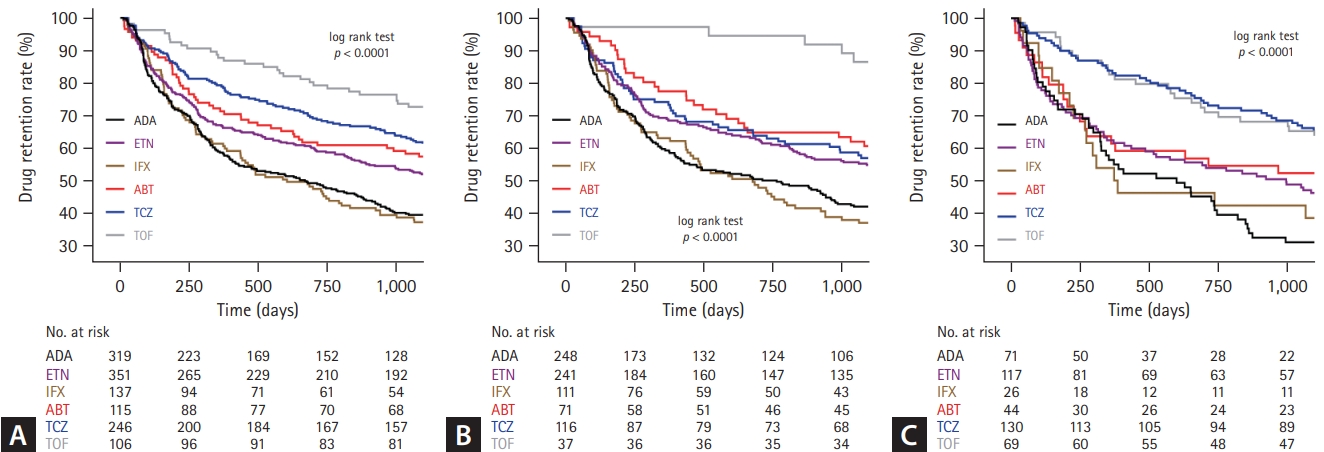
Figure┬Ā2.
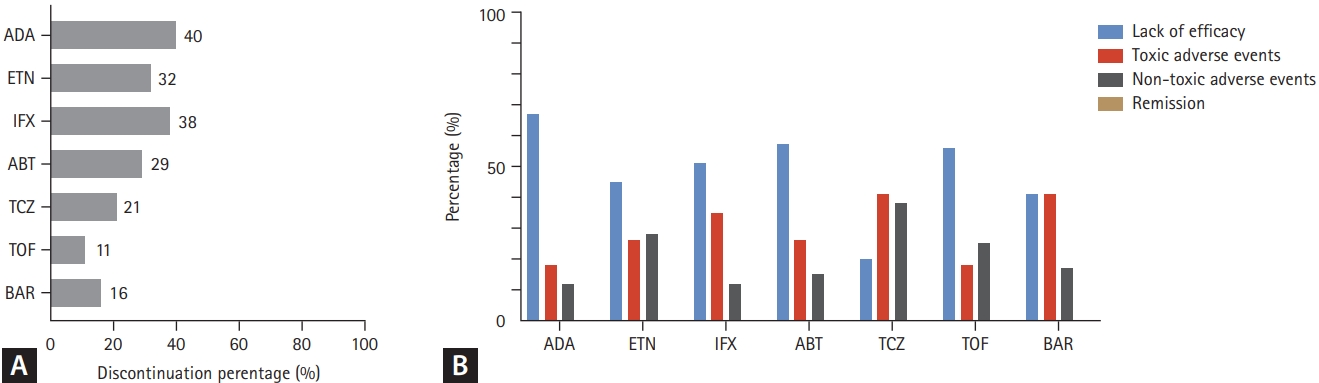
Figure┬Ā3.
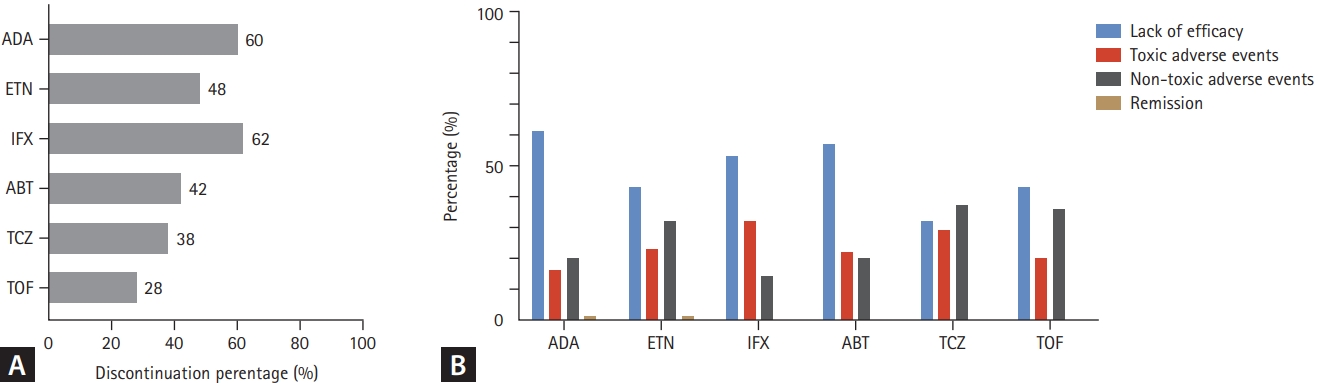
Figure┬Ā4.
Table┬Ā1.
Values are presented as median (interquartile range) or number (%).
b/tsDMARD, biological or targeted synthetic disease-modifying anti-rheumatic drug; RA, rheumatoid arthritis; RF, rheumatoid factor; CCP, cyclic citrullinated peptide; CCI, Charlson comorbidity index; csDMARDs, conventional synthetic disease-modifying anti-rheumatic drugs; MTX, methotrexate; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; TNFi, tumor necrosis factor inhibitors.
Table┬Ā2.
| Variable |
1-year follow-up group |
|||
|---|---|---|---|---|
|
Naïve |
Experienced |
|||
| Univariable HR (95% CI) | Multivariable HR (95% CI) | Univariable HR (95% CI) | Multivariable HR (95% CI) | |
| Age, per year (yr) | 1.00 (0.99ŌĆō1.01) | 1.02 (1.00ŌĆō1.03)* | 1.02 (0.99ŌĆō1.04) | |
| Sex, female | 0.78 (0.58ŌĆō1.04) | 0.81 (0.60ŌĆō1.10) | 1.01 (0.62ŌĆō1.66) | |
| RA disease duration, per year | 0.99 (0.98ŌĆō1.01) | 1.01 (0.99ŌĆō1.03) | ||
| RF positivity | 1.42 (0.83ŌĆō2.43) | 1.34 (0.78ŌĆō2.30) | 0.61 (0.31ŌĆō1.20) | 0.57 (0.29ŌĆō1.14) |
| Anti-CCP ab positivity | 1.15 (0.70ŌĆō1.91) | 1.06 (0.59ŌĆō1.91) | ||
| High CCI (> 4) | 1.21 (0.96ŌĆō1.53) | 1.19 (0.93ŌĆō1.51) | 1.54 (1.12ŌĆō2.11)** | 1.09 (0.68ŌĆō1.76) |
| MTX combination | 1.09 (0.76ŌĆō1.56) | 1.11 (0.72ŌĆō1.71) | ||
| Corticosteroids combination | 1.39 (0.94ŌĆō2.04) | 1.40 (0.94ŌĆō2.07) | 1.34 (0.83ŌĆō2.17) | |
| Elevated ESR (> 40 mm/h) | 1.64 (1.29ŌĆō2.09)*** | 1.24 (0.96ŌĆō1.62) | 1.51 (1.09ŌĆō2.09)* | 1.13 (0.78ŌĆō1.63) |
| Elevated CRP (> 1 mg/dL) | 1.12 (0.89ŌĆō1.42) | 1.11 (0.81ŌĆō1.52) | ||
| Type of b/tsDMARDs | ||||
| ŌĆāAdalimumab | Ref | Ref | Ref | Ref |
| ŌĆāEtanercept | 0.72 (0.53ŌĆō0.97)* | 0.66 (0.49ŌĆō0.89)** | 0.92 (0.58ŌĆō1.44) | 0.85 (0.54ŌĆō1.35) |
| ŌĆāInfliximab | 0.92 (0.64ŌĆō1.31) | 0.95 (0.67ŌĆō1.36) | 1.06 (0.52ŌĆō2.02) | 0.94 (0.49ŌĆō1.82) |
| ŌĆāAbatacept | 0.53 (0.34ŌĆō0.82)** | 0.75 (0.45ŌĆō1.23) | 0.96 (0.55ŌĆō1.68) | 1.04 (0.59ŌĆō1.85) |
| ŌĆāTocilizumab | 0.63 (0.44ŌĆō0.91)* | 0.82 (0.54ŌĆō1.24) | 0.32 (0.19ŌĆō0.55)*** | 0.34 (0.20ŌĆō0.59)*** |
| ŌĆāTofacitinib | 0.15 (0.05ŌĆō0.40)*** | 0.24 (0.09ŌĆō0.69)** | 0.30 (0.16ŌĆō0.59)*** | 0.42 (0.20ŌĆō0.90)* |
| ŌĆāBaricitinib | 0.36 (0.18ŌĆō0.72)** | 0.63 (0.30ŌĆō1.32) | 0.33 (0.15ŌĆō0.72)** | 0.43 (0.18ŌĆō1.01) |
| Starting year of b/tsDMARDs | ||||
| ŌĆā2008ŌĆō2014 | Ref | Ref | Ref | Ref |
| ŌĆāŌēź 2015 | 0.50 (0.39ŌĆō0.64)*** | 0.63 (0.50ŌĆō0.88)** | 0.47 (0.34ŌĆō0.65)*** | 0.66 (0.42ŌĆō1.03) |
b/tsDMARD, biological or targeted synthetic disease modifying anti-rheumatic drug; HR, hazard ratio; CI, confidence interval; RA, rheumatoid arthritis; RF, rheumatoid factor; CCP, cyclic citrullinated peptide; CCI, Charlson comorbidity index; MTX, methotrexate; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
Table┬Ā3.
| Variable |
3-year follow-up group |
|||
|---|---|---|---|---|
|
Naïve |
Experienced |
|||
| Univariable HR (95% CI) | Multivariable HR (95% CI) | Univariable HR (95% CI) | Multivariable HR (95% CI) | |
| Age, per year (yr) | 0.99 (0.98ŌĆō1.00) | 0.99 (0.98ŌĆō1.00) | 1.01 (1.00ŌĆō1.02) | 1.01 (1.00ŌĆō1.02) |
| Sex, female | 0.94 (0.72ŌĆō1.23) | 0.82 (0.56ŌĆō1.20) | ||
| RA disease duration, per year | 0.99 (0.98ŌĆō1.00) | 0.99 (0.98ŌĆō1.00) | 1.01 (0.99ŌĆō1.02) | |
| RF positivity | 1.21 (0.79ŌĆō1.84) | 0.84 (0.44ŌĆō1.58) | ||
| Anti-CCP ab positivity | 1.13 (0.75ŌĆō1.71) | 1.16 (0.71ŌĆō1.90) | ||
| High CCI (> 4) | 0.97 (0.79ŌĆō1.19) | 1.34 (1.02ŌĆō1.75)* | 1.15 (0.77ŌĆō1.72) | |
| MTX combination | 1.16 (0.84ŌĆō1.59) | 1.10 (0.74ŌĆō1.64) | ||
| Corticosteroid combination | 1.20 (0.87ŌĆō1.64) | 1.30 (0.87ŌĆō1.93) | 1.34 (0.90ŌĆō2.00) | |
| Elevated ESR (> 40 mm/h) | 1.60 (1.26ŌĆō1.93)*** | 1.33 (1.06ŌĆō1.67)* | 1.42 (1.07ŌĆō1.88) | 1.28 (0.94ŌĆō1.74) |
| Elevated CRP (> 1 mg/dL) | 1.09 (0.89ŌĆō1.33) | 1.10 (0.84ŌĆō1.45) | ||
| Type of b/tsDMARDs | ||||
| ŌĆāAdalimumab | Ref | Ref | Ref | Ref |
| ŌĆāEtanercept | 0.70 (0.54ŌĆō0.89)* | 0.70 (0.54ŌĆō0.90)** | 0.72 (0.49ŌĆō1.04)* | 0.66 (0.45ŌĆō0.96)* |
| ŌĆāInfliximab | 1.09 (0.82ŌĆō1.45) | 1.17 (0.87ŌĆō1.56) | 0.87 (0.49ŌĆō1.53) | 0.79 (0.45ŌĆō1.41) |
| ŌĆāAbatacept | 0.56 (0.37ŌĆō0.84)** | 0.78 (0.50ŌĆō1.22) | 0.62 (0.37ŌĆō1.04) | 0.66 (0.39ŌĆō1.12) |
| ŌĆāTocilizumab | 0.65 (0.47ŌĆō0.90)** | 0.81 (0.57ŌĆō1.17) | 0.37 (0.25ŌĆō0.55)*** | 0.36 (0.24ŌĆō0.55)*** |
| ŌĆāTofacitinib | 0.16 (0.07ŌĆō0.39)*** | 0.25 (0.10ŌĆō0.62)** | 0.39 (0.24ŌĆō0.63)*** | 0.52 (0.29ŌĆō0.92)* |
| Starting year of b/tsDMARDs | ||||
| ŌĆā2008ŌĆō2014 | Ref | Ref | Ref | Ref |
| ŌĆāŌēź 2015 | 0.58 (0.46ŌĆō0.73)*** | 0.74 (0.55ŌĆō0.98)* | 0.58 (0.43ŌĆō0.77)*** | 0.73 (0.50ŌĆō1.07) |
b/tsDMARD, biological or targeted synthetic disease modifying anti-rheumatic drug; HR, hazard ratio; CI, confidence interval; RA, rheumatoid arthritis; RF, rheumatoid factor; CCP, cyclic citrullinated peptide; CCI, Charlson comorbidity index; MTX, methotrexate; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
REFERENCES
- TOOLS
-
METRICS

- Related articles
-
Perioperative and anesthetic management of patients with rheumatoid arthritis2022 July;37(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print


