 |
 |
| Korean J Intern Med > Volume 26(3); 2011 > Article |
|
Abstract
Background/Aims
The aim of this study was to assess the efficacy and safety of monthly oral 150 mg ibandronate in women with postmenopausal osteoporosis (PMO).
Methods
A systematic review and meta-analysis were performed to determine treatment efficacy and safety outcomes between monthly oral 150 mg ibandronate and weekly 70 mg alendronate, daily 2.5 mg ibandronate, and a placebo.
Results
Eight randomized controlled trials were included in this systematic review and meta-analysis. Once-monthly 150 mg ibandronate therapy was clinically comparable to weekly 70 mg alendronate, showing increased bone mineral density (BMD) in both the lumbar spine and total hip. Pooled data from two cross-over trials showed that significantly more women with PMO preferred once-monthly ibandronate therapy to once-weekly alendronate therapy (relative risk [RR], 2.422; 95% confidence interval [CI], 2.111 to 2.825; p < 1 ├Ś 10-8) and found the monthly ibandronate regimen more convenient than the weekly alendronate regimen (RR, 3.096; 95% CI, 2.622 to 3.622; p < 1 ├Ś 10-8). Monthly 150 mg ibandronate therapy resulted in a significantly higher change in BMD of the lumbar spine than with the placebo. A once monthly 150 mg regimen produced greater increases in lumbar spine, total hip, femoral neck, and trochanter BMD than daily treatment, with a similar incidence of adverse events between the groups.
Osteoporosis is a skeletal disorder characterized by low bone mass and microarchitectural deterioration of bone tissue, predisposing patients to increased fracture risk [1]. Osteoporosis is the leading underlying cause of fractures, particularly in postmenopausal women, due to the loss of estrogen-mediated suppression of bone resorption [2]. More than 50% of adults 50 years of age or older are estimated to have osteoporosis. Of these, almost 70% are women with postmenopausal osteoporosis (PMO) [3].
Bisphosphonates are the standard first-line treatment and the most widely used therapy for PMO. However, long-term compliance with therapy is necessary for optimal outcomes, because poor adherence is associated with smaller decreases in bone turnover, lower increases in bone mineral density (BMD), and a significantly greater risk for fracture [4]. Although suboptimal adherence to oral bisphosphonates compromises therapeutic outcomes in patients with PMO, long-term compliance to current oral bisphosphonates is poor [5]. This is a major limitation for long-term effective therapy of bisphosphonates for osteoporosis.
Oral bisphosphonates, given once daily or once weekly, are currently the mainstay treatment in patients with PMO [6]. However, oral once daily or once weekly bisphosphonates are inconvenient and challenging for some patients, leading to a decrease in adherence to treatment and a reduction in antifracture efficacy [7]. Although weekly bisphosphonate dosing is associated with better adherence than daily dosing, adherence rates are suboptimal [8]. No more than 31-44% of women with PMO are compliant with their weekly bisphosphonate therapy after 1 year [3,7]. The availability of a less frequent bisphosphonate regimen would offer patients greater convenience and improve therapeutic adherence [3].
Ibandronate is a potent oral bisphosphonate approved by the US Food and Drug Administration for use as a once monthly oral or quarterly intravenous (IV) treatment for PMO [9]. Once monthly oral and quarterly IV ibandronate have been approved and marketed worldwide including Europe, the US, and Asia [10]. Ibandronate has greater antiresorptive efficacy than either alendronate or risedronate in animal models [10]. The high potency, safety, and tolerability profile of ibandronate enable its increased dosing interval, while maintaining an advantageous therapeutic profile. A once monthly oral 150 mg dose is the recommended available dosage regimen [11]. The aim of this study was to assess the efficacy and safety of a monthly oral 150 mg ibandronate dose, compared with a daily and monthly bisphosphonate regimen or a placebo, in women with PMO.
We performed a literature search for randomized controlled trials (RCTs) that examined monthly oral 150 mg ibandronate treatment for osteoporosis. Literature searches were performed using MEDLINE (to June 2010) and the Cochrane Controlled Trials Register (to June 2010) to identify relevant studies. The following key words and subject terms were used in the searches: "ibandronate," "bone mineral density," and "osteoporosis." All references in the articles were reviewed to identify additional studies not included in the electronic databases. RCTs were included if they met the following criteria: the study compared monthly oral 150 mg ibandronate with a placebo and daily or weekly bisphosphonate for efficacy or safety.
The following information was extracted from each study: first author, year of publication, country in which the study was conducted, menopausal state, length of follow-up, preference and convenience numbers, skeletal sites evaluated for BMD, mean and standard deviation of BMD (change-from-baseline), and safety outcomes. Change-from-baseline was reported as a percent change. The safety outcome was the number of patients who had experienced side effects, gastrointestinal (GI) adverse effects, withdrawal, and withdrawal due to adverse effects. We quantified the methodological qualities of studies using Jadad scores [12]. These assessments were based on: 1) whether the randomization method was appropriate, 2) whether double blindness was mentioned in the trial and whether the trial was appropriately performed; and 3) whether the number of patients who withdrew, and their reasons, were clearly stated. Jadad scores ranged from 0 to 5, and higher scores denoted better trial quality.
The trial outcome effect sizes were expressed as the relative risk (RR) for binary data, such as number of adverse effects or weighted mean difference (WMD) for changes in BMD and corresponding 95% confidence intervals (CIs). We assessed within- and between-study variation and heterogeneity using Cochran's Q-statistics [13]. The heterogeneity test was used to assess the null hypothesis that all studies were evaluating the same effect. When a significant Q-statistic (p < 0.10) indicated heterogeneity across studies, the random effect model was used for the meta-analysis and, if not, the fixed effect model was used. The fixed effect model assumes that all studies estimate the same underlying effect and considers only within-study variations. We quantified the effect of heterogeneity using I2 = 100% ├Ś (Q - df) / Q [14], where I2 measures the degree of inconsistency between studies and determines whether the percent total variation across studies is due to heterogeneity rather than to chance. I2 ranges between 0% and 100%; I2 values of 25%, 50%, and 75% are referred to as low, moderate, and high estimates. Statistical analyses were conducted using the comprehensive meta-analysis computer program (Biosta, Englewood, NJ, USA).
Twenty-one studies were identified by electronic and manual searches, and 11 were selected for a full-text review based on titles and abstracts [15-25]. However, three of the 11 were excluded; one study was a RCT that did not compare monthly ibandronate [23], and two contained duplicate data [24,25]. Thus, eight studies met the inclusion criteria [15-22]. Five studies addressed monthly 150 mg ibandronate vs. weekly 70 mg alendronate [15,18-21], but two were cross-over studies [18,21]. Two studies assessed monthly 150 mg ibandronate vs. placebo [16,17,26], and one study considered monthly 150 mg ibandronate vs. daily 2.5 mg ibandronate [22]. Relevant features of the studies included in the systematic review and meta-analysis are provided in Table 1. Follow-up periods ranged from 6 to 24 months. Jadad scores ranged from 1 to 3, and the median score was 3 (Table 1). We performed a meta-analysis if there were at least two comparisons.
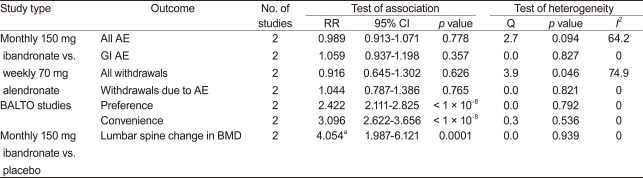
Three RCTs compared monthly 150 mg ibandronate with weekly 70 mg alendronate in patients with PMO [15,19,20] (Table 1). However, two studies were about the same subjects [15,19]. The study by Emkey et al. [15] reported additional data from the monthly oral therapy with ibandronate for osteoporosis intervention (MOTION) study from Miller et al. [19] The MOTION study revealed that once-monthly ibandronate was clinically comparable to weekly alendronate and increased BMD after 12 months in both the lumbar spine and total hip [19]. Mean relative 12-month changes were 5.1% and 5.5% in lumbar spine and 2.9% and 3.0% in total hip BMD with once-monthly ibandronate and weekly alendronate, respectively. Emkey et al. [15] revealed comparable efficacy of once monthly 150 mg ibandronate therapy in terms of BMD response. The percentage of patients with mean lumbar spine and total hip BMD gains above baseline (responders) were 90% and 87%, respectively, for ibandronate and 92% and 90%, respectively, for alendronate.
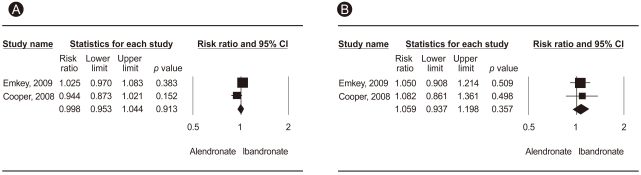
No significant difference was observed between the two treatment regimens in terms of side effects such as all adverse effects, GI adverse effects, number of withdrawals, and withdrawals due to adverse effects (Table 2, Fig. 1). Cooper et al. [20] compared treatment compliance in patients receiving either once monthly ibandronate plus a patient support program (PSP), or once weekly alendronate. Compliance was significantly higher in the ibandronate/PSP group than that in the alendronate group (56.6% [306/541] vs. 38.6% [198/513], p < 0.0001), with 47% relative improvement in the ibandronate/PSP group. Significantly more patients discontinued the study from the alendronate group (25.3%, 134/529), as compared to the ibandronate/PSP group (19.6%, 107/547, p = 0.023).
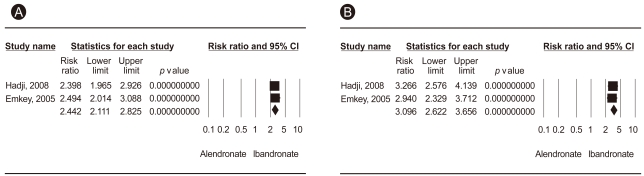
Two randomized open-label, cross-over trials were conducted to assess preference and convenience for once-monthly ibandronate vs. once-weekly alendronate [18,21]. The primary endpoint was the percentage of patients who preferred the ibandronate monthly regimen to the alendronate weekly regimen. The secondary endpoint was the percentage of patients perceiving that the monthly ibandronate regimen was more convenient than weekly dosing of alendronate. Pooled data from two RCTs showed differences between the two groups in terms of preference and convenience (RR, 2.422, 95% CI, 2.111 to 2.825, p < 1 ├Ś 10-8; RR, 3.096, 95% CI, 2.622 to 3.622, p < 1 ├Ś 10-8, respectively) (Table 2, Fig. 2). Significantly more women with PMO preferred once monthly ibandronate therapy to once weekly alendronate therapy and found the monthly ibandronate regimen more convenient than the weekly alendronate regimen.
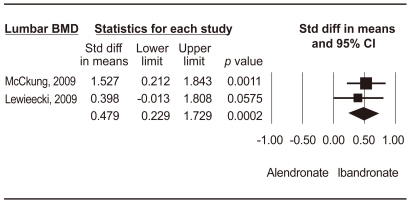
Two RCTs were conducted on monthly 150 mg ibandronate vs. a placebo [16,17,27]. McClung et al. [16] showed that subjects treated with ibandronate achieved larger increases in lumbar spine BMD after 1 year, as compared to a placebo group (3.7% vs. -0.4%, p < 0.0001). Lewiecki et al. [17] revealed that ibandronate increased total hip and lumbar spine BMD more than a placebo at 12 months (differences: 2.2%, p = 0.005, 4.3%, p < 0.001). Monthly 150 mg ibandronate therapy showed significantly higher changes in BMD of the lumbar spine than a placebo (WMD, 4.054; 95% CI, 1.987 to 6.121; p = 0.0001) (Fig. 3).
A 2-year, randomized, double blind, parallel group, phase III, non-inferiority study (monthly oral ibandronate in ladies, MOBILE) was conducted comparing monthly 150 mg ibandronate vs. daily 2.5 mg ibandronate [22]. Substantial increases in lumbar spine BMD were seen in both groups (5.0 and 6.6% in the daily and once monthly groups, p < 0.001). The once monthly 150 mg regimen produced greater increases in total hip, femoral neck, and trochanter BMD (p < 0.05). A similar proportion of patients in the once monthly and daily treatments withdrew from treatment. The incidences of adverse events, drug related adverse events, and drug-related adverse events leading to withdrawal were balanced between the treatments. The once monthly 150 mg regimen was superior to the daily regimen.
Between-study heterogeneity was found during analyses of all adverse events and all withdrawals of monthly 150 mg ibandronate vs. weekly 70 mg alendronate. It was difficult to correlate the funnel plot, because of the small number of studies included. Egger's test could not be conducted due to the small number of studies.
We systemically combined and reviewed the clinical data of eight RCTs that examined monthly oral 150 mg ibandronate treatment for osteoporosis to assess the efficacy and safety of monthly oral 150 mg ibandronate in women with PMO. Once-monthly 150 mg ibandronate therapy was clinically comparable to weekly 70 mg alendronate without a significant difference in side effects. Use of the higher dose resulted in concerns about increased adverse effects. The most common adverse effects reported with bisphosphonates affect the upper GI system. Monthly oral ibandronate shows adverse effect frequencies, including GI adverse effects, that are similar to weekly alendronate.
Adherence to weekly dosing is improved, as compared to daily regimens, but remains suboptimal [7]. One of the proposed benefits of once monthly ibandronate therapy is improved compliance [28]. Adherence was calculated as the ratio of the total days of therapy to the number of days of follow-up, and we tested compliance with the medication possession ratio, which was defined as the proportion of days in which patients had a supply of medication. Significantly more women with PMO preferred once monthly ibandronate therapy to once weekly alendronate therapy, and the monthly ibandronate regimen was more convenient than the weekly alendronate regimen. Noninferiority studies are widely accepted for demonstrating therapeutic equivalence between alternative regimens and are particularly useful for comparing the efficacy of a novel agent or regimen to an established therapy [22]. Once-monthly ibandronate was noninferior to, and clinically comparable to, weekly alendronate with an increased BMD in both the lumbar spine and total hip after 12 months of treatment [22].
The currently available monthly oral ibandronate regimen was approved in 2005, based on the 2-year MOBILE bridging trial [22]. At 2 years, substantial increases in lumbar spine BMD were observed in the once monthly (6.6%) and daily (5.0%) groups. Significantly greater percentages of patients achieved measurable 2-year BMD gains in the lumbar spine and total hip with the 150 mg/mon regimen vs. 2.5 mg/day. The once monthly 150 mg regimen produced greater increases in BMD than the daily treatment, with a similar incidence of adverse events between the groups. The monthly 150 mg ibandronate dose was superior to the daily 2.5 mg dose.
Previous meta-analyses have shown dose-dependent efficacy of ibandronate [27,29]. The efficacy of ibandronate in reducing nonvertebral fracture risk was evaluated in two meta-analyses [27,29]. Patients were assigned to dose-level groups, based on annual cumulative exposure (ACE) to ibandronate. The once-daily 2.5 mg and once monthly oral 150 mg regimens are equivalent to an ACE of 5.5 and 10.8 mg, respectively. A high dose level, including once-monthly 150 mg (ACE Ōēź 10.8 mg), showed a significant reduction in the rate of nonvertebral fractures than did a lower dose (2.5 mg daily regimen; ACE, 5.5 mg) (adjusted hazard ratio, 0.620; 95% CI, 0.396 to 0.974; p = 0.038). Ibandronate regimens with an ACE Ōēź 10.8 mg showed a 38% reduction for all nonvertebral fractures versus daily oral ibandronate (ACE, 5.5 mg). The effect of ibandronate on nonvertebral fractures was dose-dependent.
The present systematic review and meta-analysis has several shortcomings that must be considered. First, the possibility of publication bias is always a concern, and it should be recognized that publication bias is difficult to exclude, particularly when the number of incorporated studies is small, as in the present study. Second, heterogeneity of clinical features, such as, race, age, and study quality, which is of fundamental importance to a meta-analysis, may confound meta-analysis findings. Third, although our systematic review included eight RCTs, the subgroup meta-analysis was based on a smaller number of studies. Only two RCTs were included in our meta-analysis for each subject; thus, findings should be regarded with caution. Third, all of the studies included in this systematic review were funded by drug companies. Therefore, the results should be interpreted with caution.
In conclusion, once monthly 150 mg ibandronate therapy was noninferior and clinically comparable to weekly 70 mg alendronate. Strong patient preference and convenience for monthly ibandronate over weekly alendronate was found in women with PMO. Monthly 150 mg ibandronate was superior to, and as well tolerated as, daily treatment. The availability of a less frequent bisphosphonate regimen, such as once monthly ibandronate, enables patients to choose a dosing regimen that best fits their lifestyle and improves patient satisfaction and adherence to osteoporosis treatment, which increases the effectiveness of osteoporosis therapy. Once monthly 150 mg ibandronate provided an effective therapeutic option for PMO.
References
1. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 1993;94:646ŌĆō650PMID : 8506892.


2. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17:1726ŌĆō1733PMID : 16983459.


3. Simonelli C, Burke MS. Less frequent dosing of bisphosphonates in osteoporosis: focus on ibandronate. Curr Med Res Opin 2006;22:1101ŌĆō1108PMID : 16846543.


4. Yood RA, Emani S, Reed JI, Lewis BE, Charpentier M, Lydick E. Compliance with pharmacologic therapy for osteoporosis. Osteoporos Int 2003;14:965ŌĆō968PMID : 14504697.


5. McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J. Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas 2004;48:271ŌĆō287PMID : 15207894.


6. Reginster JY, Felsenberg D, Cooper C, et al. A new concept for bisphosphonate therapy: a rationale for the development of monthly oral dosing of ibandronate. Osteoporos Int 2006;17:159ŌĆō166PMID : 15959614.


7. Cramer JA, Amonkar MM, Hebborn A, Altman R. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin 2005;21:1453ŌĆō1460PMID : 16197664.


8. Kendler D, Kung AW, Fuleihan Gel-H, et al. Patients with osteoporosis prefer once weekly to once daily dosing with alendronate. Maturitas 2004;48:243ŌĆō251PMID : 15207890.


9. Barrett J, Worth E, Bauss F, Epstein S. Ibandronate: a clinical pharmacological and pharmacokinetic update. J Clin Pharmacol 2004;44:951ŌĆō965PMID : 15317823.


10. Frampton JE, Perry CM. Ibandronate: a review of its use in the management of postmenopausal osteoporosis. Drugs 2008;68:2683ŌĆō2707PMID : 19093707.


11. Rossini M, Viapiana O, Gatti D, Adami S. Once-monthly oral ibandronate in postmenopausal osteoporosis: translation and updated review. Clin Ther 2009;31:1497ŌĆō1510PMID : 19695399.


12. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1ŌĆō12PMID : 8721797.


13. Davey Smith G, Egger M. Meta-analyses of randomised controlled trials. Lancet 1997;350:1182. PMID : 9343537.


14. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539ŌĆō1558PMID : 12111919.


15. Emkey R, Delmas PD, Bolognese M, et al. Efficacy and tolerability of once-monthly oral ibandronate (150 mg) and once-weekly oral alendronate (70 mg): additional results from the Monthly Oral Therapy With Ibandronate For Osteoporosis Intervention (MOTION) study. Clin Ther 2009;31:751ŌĆō761PMID : 19446148.


16. McClung MR, Bolognese MA, Sedarati F, Recker RR, Miller PD. Efficacy and safety of monthly oral ibandronate in the prevention of postmenopausal bone loss. Bone 2009;44:418ŌĆō422PMID : 18950736.


17. Lewiecki EM, Keaveny TM, Kopperdahl DL, et al. Once-monthly oral ibandronate improves biomechanical determinants of bone strength in women with postmenopausal osteoporosis. J Clin Endocrinol Metab 2009;94:171ŌĆō180PMID : 18840641.


18. Hadji P, Minne H, Pfeifer M, et al. Treatment preference for monthly oral ibandronate and weekly oral alendronate in women with postmenopausal osteoporosis: A randomized, crossover study (BALTO II). Joint Bone Spine 2008;75:303ŌĆō310PMID : 18069036.


19. Miller PD, Epstein S, Sedarati F, Reginster JY. Once-monthly oral ibandronate compared with weekly oral alendronate in postmenopausal osteoporosis: results from the head-to-head MOTION study. Curr Med Res Opin 2008;24:207ŌĆō213PMID : 18042311.


20. Cooper A, Drake J, Brankin E. PERSIST Investigators. Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results from the PERSIST study. Int J Clin Pract 2006;60:896ŌĆō905PMID : 16800837.



21. Emkey R, Koltun W, Beusterien K, et al. Patient preference for once-monthly ibandronate versus once-weekly alendronate in a randomized, open-label, cross-over trial: the Boniva Alendronate Trial in Osteoporosis (BALTO). Curr Med Res Opin 2005;21:1895ŌĆō1903PMID : 16368038.


22. Reginster JY, Adami S, Lakatos P, et al. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis 2006;65:654ŌĆō661PMID : 16339289.



23. Stakkestad JA, Lakatos P, Lorenc R, Sedarati F, Neate C, Reginster JY. Monthly oral ibandronate is effective and well tolerated after 3 years: the MOBILE long-term extension. Clin Rheumatol 2008;27:955ŌĆō960PMID : 18180976.


24. Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res 2005;20:1315ŌĆō1322PMID : 16007327.


25. Reginster JY, Wilson KM, Dumont E, Bonvoisin B, Barrett J. Monthly oral ibandronate is well tolerated and efficacious in postmenopausal women: results from the monthly oral pilot study. J Clin Endocrinol Metab 2005;90:5018ŌĆō5024PMID : 15972582.


26. Epstein S, Jeglitsch M, McCloskey E. Update on monthly oral bisphosphonate therapy for the treatment of osteoporosis: focus on ibandronate 150 mg and risedronate 150 mg. Curr Med Res Opin 2009;25:2951ŌĆō2960PMID : 19835464.


27. Harris ST, Blumentals WA, Miller PD. Ibandronate and the risk of non-vertebral and clinical fractures in women with postmenopausal osteoporosis: results of a meta-analysis of phase III studies. Curr Med Res Opin 2008;24:237ŌĆō245PMID : 18047776.


Figure┬Ā1
Meta-analysis of all studies (A) and gastrointestinal adverse effects (B) for monthly 150 mg ibandronate vs. weekly 70 mg alendronate. CI, confidence interval.
Figure┬Ā2
Meta-analysis of preference (A) and convenience (B) for monthly 150 mg ibandronate vs. weekly 70 mg alendronate. CI, confidence interval.
Figure┬Ā3
Meta-analysis of change in lumbar spine bone mineral density for monthly 150 mg ibandronate vs. placebo. CI, confidence interval.
Table┬Ā1
Characteristics of individual studies included in the systematic review and meta-analysis
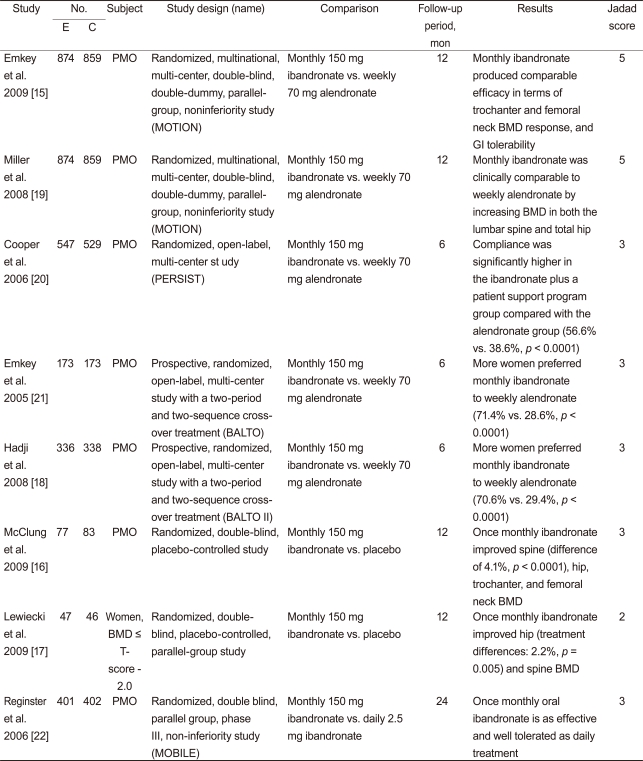
E, experimental group; C, control group; PMO, postmenopausal osteoporosis; MOTION, the monthly oral therapy with ibandronate for osteoporosis intervention study; PERSIST, the persistence study of ibandronate versus alendronate; BALTO, the Bonviva alendronate trial in osteoporosis; MOBILE, the monthly oral ibandronate in ladies; BMD, bone mineral density; GI, gastrointestinal.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



