 |
 |
|
|
|
Abstract
Background/Aims
Methods
Results
Acknowledgments
Figure┬Ā3.

Figure┬Ā4.

Figure┬Ā5.

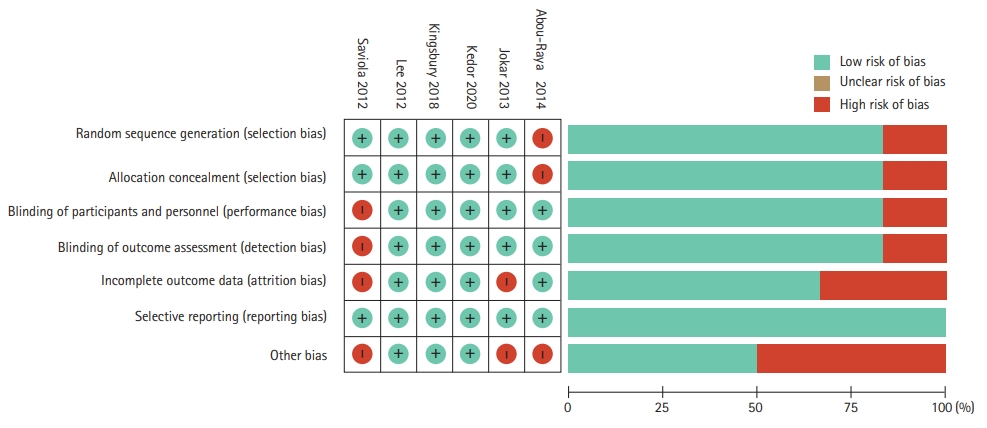
Table┬Ā1.
| Study, country, trial name, trial registration number | Publication type | Group | Sample size, n | Women | Age, yr | BMI, kg/m2 | OA type | KL gradea/ACR | Follow-up | Key outcome measures | Details of interventions and dose | Funding | RoB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saviola et al. (2012) [25], Italy, NA, NA | Full-text | HCQ | 14 | 13 (95) | 63.5 ┬▒ 7.41 | NR | Hand OA | Radiographic criteriab/NR | 12 mo | VAS | 400 mg tablet daily for 30 days, 200 mg maintenance daily for 11 months | NR | High |
| DreiserŌĆÖs score | |||||||||||||
| Synovitis assed using UI | |||||||||||||
| CLO | 21 | 23 (95) | 60.0 ┬▒ 7.13 | Morning stiffness | 300 mg i.v. in 250 cc of physiological saline solution for 7 days, followed by 100 mg i.m. maintenance dose for 14 days/3 months | ||||||||
| Physician and Patient Global Assessment | |||||||||||||
| ESR, CRP, ALP┬Ė RF | |||||||||||||
| Jokar et al. (2013) [32], Iran, NA, IRCT138709121479N1 | Full-text | HCQ | 21 | 21 (100) | 48.3 ┬▒ 11.14 | 25.6 ┬▒ 3.05 | Knee OA | 2, 3/ACR | 24 wk | WOMAC | 200 mg tablets twice daily for 6 months | University | Low |
| PLB | 23 | 22 (95) | 47.6 ┬▒ 8.54 | 25.4 ┬▒ 2.79 | 2, 3/ACR | Placebo tablets of 200 mg twice daily for 6 months | |||||||
| Abou-Raya et al. (2014) [24], Egypt, NA, NA | Abstract | HCQ | 83 | NR | NR | NR | Knee OA | 3, 4/ACR | 36 wk | VAS | 400 mg daily | NR | High |
| PLB | 83 | 3, 4/ACR | WOMAC function | Placebo | |||||||||
| ADL | |||||||||||||
| Kingsbury et al. (2018) [23], UK, HERO, ISRCTN91859104/EudraCT 2011-004300-38 | Full-text | HCQ | 124 | 97 (78) | 62.8 ┬▒ 9.1 | 28.4 ┬▒ 5.4 | Hand OA | NR/ACR | 12 mo | NRS | 200, 300, or 400 mg daily with dosage calculated according to ideal body weight for a maximum of 6.5 mg/kg | Non-profit organisation | Low |
| AUSCAN pain and function | |||||||||||||
| OAQoL | |||||||||||||
| SF-12 | |||||||||||||
| Kallman score | |||||||||||||
| PLB | 124 | 106 (85) | 62.5 ┬▒ 9.2 | 29.3 ┬▒ 6.2 | Placebo | ||||||||
| Lee et al. (2018) [22], Netherlands, FABIO, NCT01148043 | Full-text | HCQ | 98 | 86 (88) | 57.7 ┬▒ 8.2 | NR | Hand OA | 1, 2, or 3/ACR | 24 wk | VAS | 400 mg daily | University | Low |
| PLB | 98 | 82 (84) | 58.3 ┬▒ 7.0 | 1, 2, or 3/ACR | AUSCAN | Placebo | |||||||
| AIMS2-SF | |||||||||||||
| Kedor et al. (2020) [33], Germany, OA-TREAT, ISRCTN46445413/EUDRA CT 2011-001689-16 | Abstract | HCQ | 75 | 68 (90.6) | 52.4 ┬▒ 8.1 | NR | Hand OA | NR/ACR | 52 wk | HAQ | 200ŌĆō400 mg daily | University | Low |
| PLB | 78 | 60 (76.9) | 50.2 ┬▒ 6.6 | AUSCAN pain and function | Matching placebo | ||||||||
| Morning stiffness | |||||||||||||
| SF-36 | |||||||||||||
| ESR | |||||||||||||
| Modified Kallmann score |
Values are presented as number (%) or mean ┬▒ SD.
BMI, body mass index; OA, osteoarthritis; KL grade, Kellgren-Lawrence classification grades of radiographic OA; ACR; American College of Rheumatology; RoB, risk of bias; NA, not applicable; HCQ, hydroxychloroquine; CLO, clodronate; NR, not reported; VAS, visual analog scale; UI, ultrasound imaging; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; ALP, alkaline phosphatase; RF, rheumatoid factor; i.v., intravenous; i.m., intramuscular; PLB, placebo; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index Score; ADL, activities of daily living; NRS, numeric rating scale; AUSCAN, Australian/Canadian Osteoarthritis Index; OAQoL, osteoarthritis quality of life scale; SF-12, 12-item short form survey; AIMS2-SF, Arthritis Impact Measurement Scale 2 short form; HAQ, health assessment questionnaire; SF-36, 36-item short form survey.
Table┬Ā2.
| Outcome | No. of RCTs | No. of patients | Effect estimate (95% CI) |
|---|---|---|---|
| Pain | |||
| ŌĆāHand OA [22,23,25,33] | 4 | 632 | SMD 0.14 (ŌĆō0.20 to 0.48) |
| ŌĆāKnee OA [24,32]a | 2 | 210 | SMD ŌĆō0.72 (ŌĆō1.57 to 0.14) |
| Dysfunction | |||
| ŌĆāHand OA [23,33] | 2 | 401 | SMD 0.08 (ŌĆō0.23 to 0.40) |
| ŌĆāKnee OA [24,32]a | 2 | 210 | SMD ŌĆō0.48 (ŌĆō0.82 to ŌĆō0.14) |
| Adverse event [22,24,25,32] | 4 | 441 | RD 0.04 (ŌĆō0.02 to 0.10) |
| Serious adverse event [23,33] | 2 | 401 | RD ŌĆō0.04 (ŌĆō0.10 to 0.01) |
RCT, randomized controlled trial, CI, confidence interval; OA, osteoarthritis; SMD, standardized mean difference; RD, risk difference.
a Abou-Raya et al. [24] was published as an abstract in 2013 and is still not published as full-text; the author did not make the data available upon email request.
REFERENCES
-
METRICS

- Related articles
-
Efficacy of hydroxychloroquine for knee osteoarthritis2022 January;37(1)





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


