 |
 |
| Korean J Intern Med > Volume 39(5); 2024 > Article |
|
Abstract
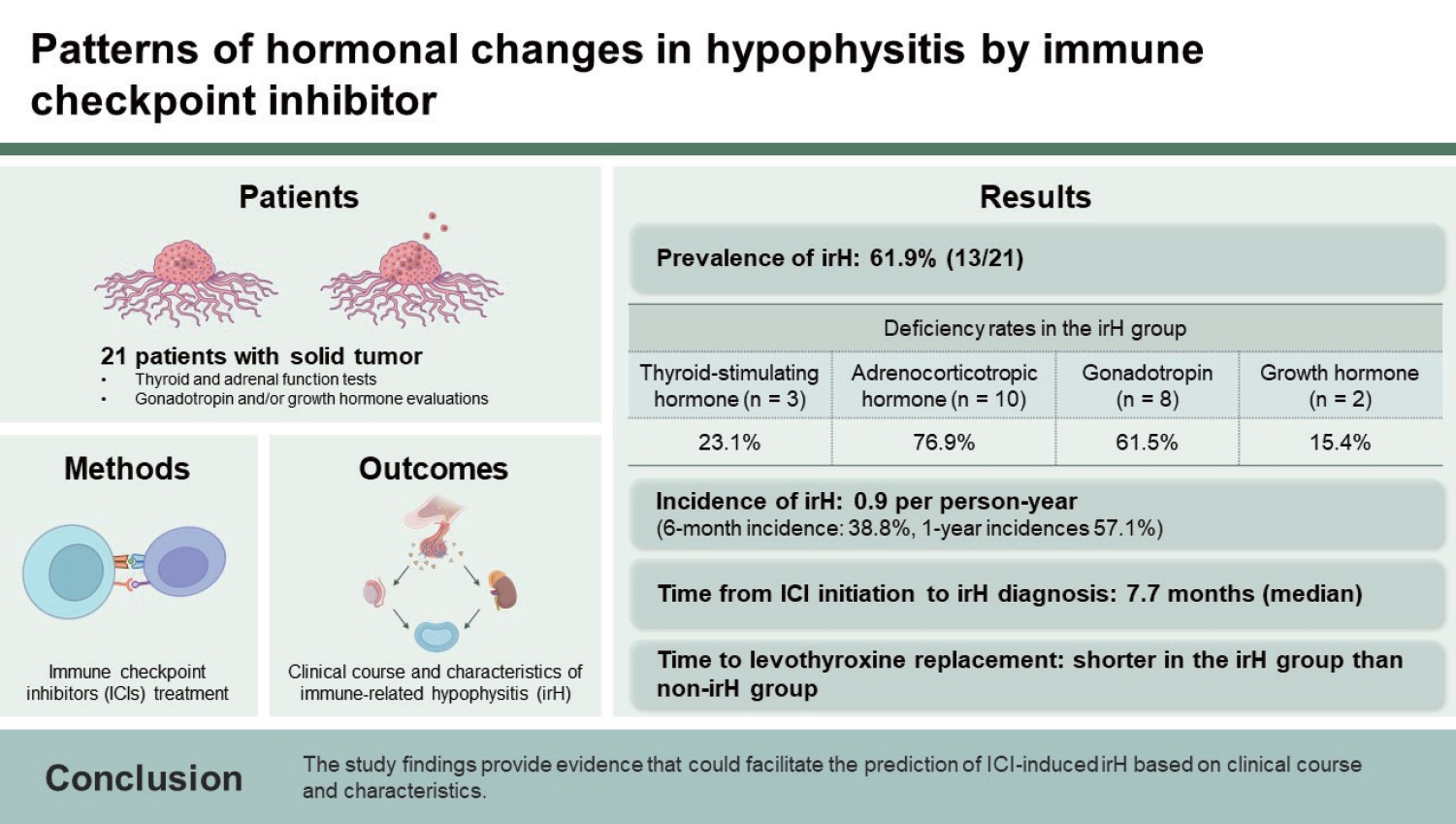
Background/Aims
Methods
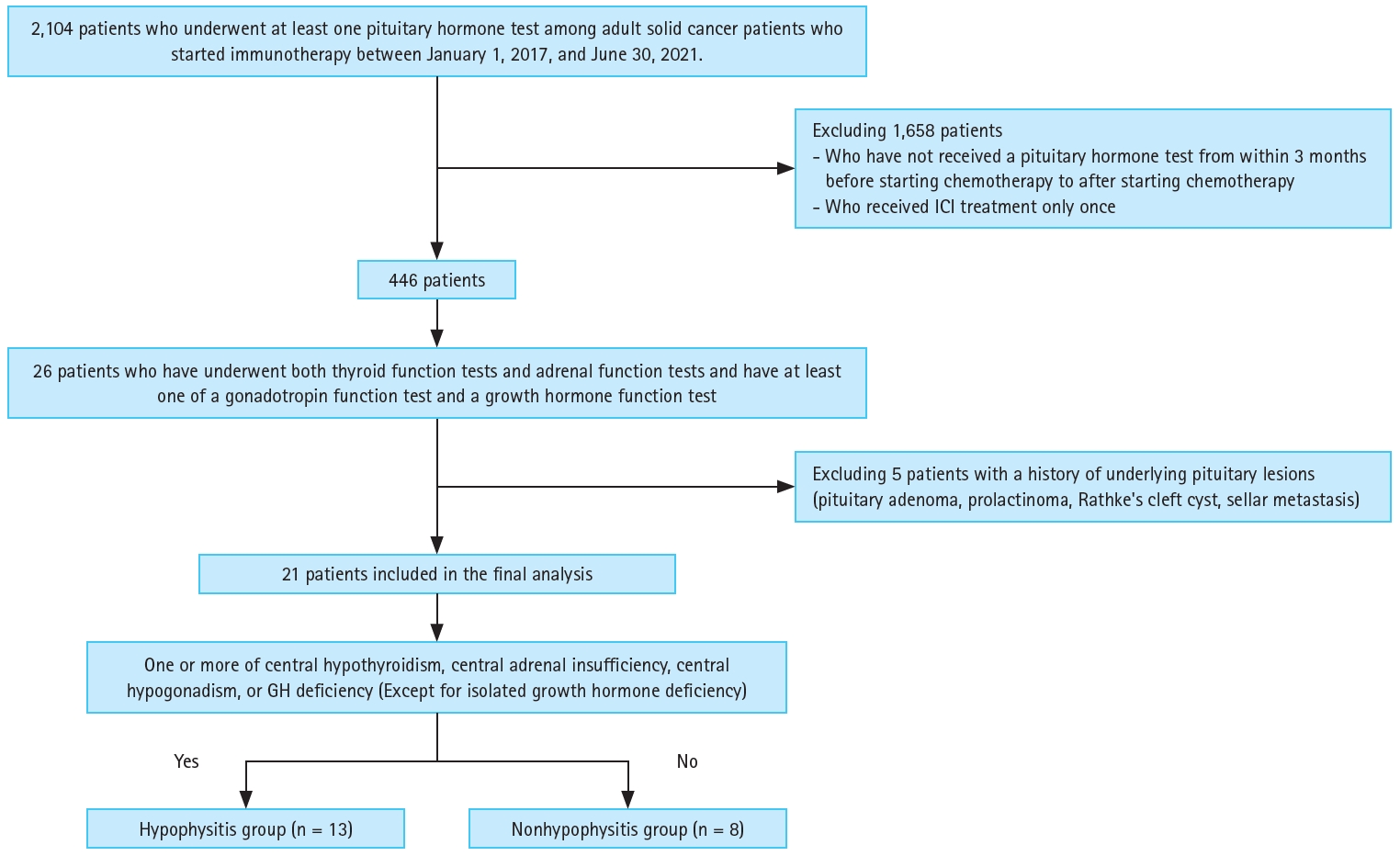
Results
Acknowledgments
Notes
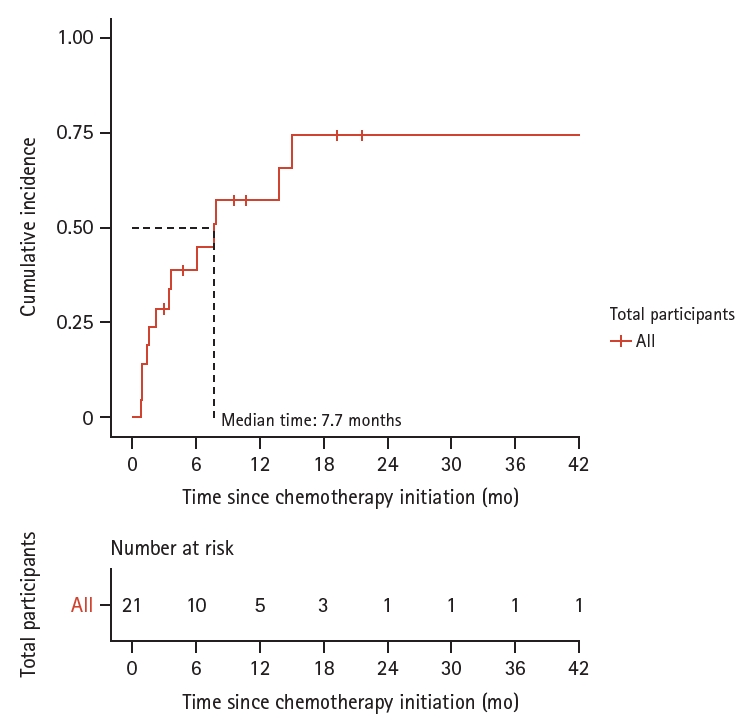
Figure┬Ā2.
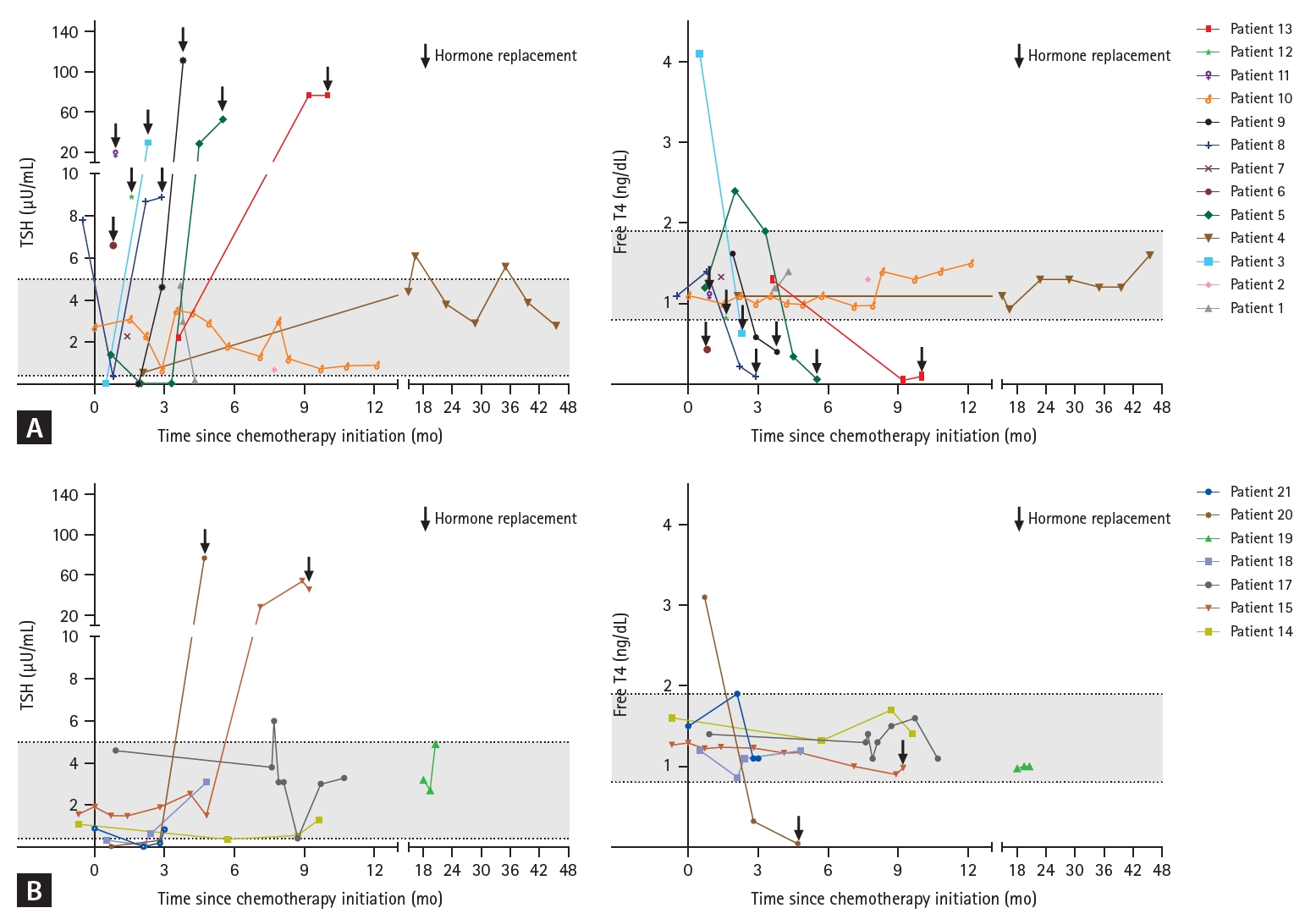
Figure┬Ā3.
Table┬Ā1.
| Patient# | Sex/age (yr) | Cancer type | ICI/dosage/interval/cyclesa) | Symptoms | Affected hormone axes | Non-hypophysitis endocrine irAEs | MRI findings | Other irAEs |
|---|---|---|---|---|---|---|---|---|
| 1 | M/76 | Esophageal cancerb) | Nivo/3 mg per kg/2 weeks/7 | Anorexia, nausea, fatigue, dizziness, weight loss, head- ache, general weakness | ACTH, Gonadotropin | None | CSF invagination into the sellar fossa | Not reported |
| 2 | F/55 | Cholangio-carcinoma | Pembro/200 mg/3 weeks/6 | Not reported | ACTH, Gonadotropin | None | No remarkable findings | Not reported |
| 3 | F/61 | NSCLC, ADC | Nivo/3 mg per kg/2 weeks/2 | Fatigue | ACTH, Gonadotropin | Thyroiditis (immune-related or HashimotoŌĆÖs thyroiditis) | Not done | Not reported |
| 4 | M/59 | NSCLC, ADC | Pembro/100 mg/3 weeks/20 | Anorexia, nausea, vomiting, general weakness | ACTH | None | No remarkable findings | Not reported |
| 5 | F/82 | Malignant peritoneal mesothelioma | Pembro/200 mg/3 weeks/7 | Not reported | ACTH, Gonadotropin | Thyroiditis | No remarkable findings | Arthritis |
| 6 | M/33 | NSCLC, SqCC | Nivo/3 mg per kg/2 weeks/2 | Not reported | TSH | Hyper-prolactinemia | Not done | Not reported |
| 7 | M/32 | Gastric cancer | Nivo/3 mg per kg/2 weeks/4 | Anorexia, fatigue | Gonadotropin | Hyper-prolactinemia | Normal | Not reported |
| 8 | M/71 | Kidney cancer | Nivo+Ipi/Nivo 3 mg per kg + Ipi 1 mg per kg/3 weeks/3 | Not reported | Panhypopituitarism | None | Tiny poorly enhancing lesion in the posterior pituitary gland | Type 1 diabetes mellitus |
| 9 | M/60 | Esophageal cancer | Nivo/3 mg per kg/2 weeks/7 | Fatigue | ACTH | Primary hypothyroidism, Hyper-prolactinemia | No remarkable findings | Pruritis, rash |
| 10 | M/75 | NSCLC, SqCC | Pembro/200 mg/3 weeks/11 | Anorexia, nausea, vomiting | ACTH | None | Normal | Colitis |
| 11 | F/24 | Gastric cancer | Nivo/3 mg per kg/2 weeks/2 | Anorexia, nausea, vomiting, fatigue, hyponatremia | Gonadotropin | Primary hypothyroidism | No remarkable findings | Not reported |
| 12 | M/58 | Gastric cancer | Nivo/3 mg per kg/2 weeks/3 | Hyponatremia | Panhypo- pituitarism | None | No remarkable finding | Arthralgia, myalgia, pruritis, rash |
| 13 | M/55 | NSCLC, ADC | Pembro/200 mg/3 weeks/13 | Anorexia | ACTH | Thyroiditis | No remarkable findings | Not reported |
a) The number of ICI cycles performed until the occurrence of hypophysitis or termination of immunochemotherapy.
ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; MRI, magnetic resonance imaging; Nivo, nivolumab; ACTH, adrenocorticotropic hormone; CSF, cerebrospinal fluid; Pembro, pembrolizumab; NSCLC, non-small cell lung cancer; ADC, adenocarcinoma; SqCC, squamous cell carcinoma; TSH, thyroid-stimulating hormone; Ipi, ipilimumab.
Table┬Ā2.
| Group | No | Sex/age (yr) |
TSH |
Free T4 |
ACTH |
Cortisol |
IGF-1 |
hGH |
FSHa) |
LHb) |
Testosterone |
Estradiol |
Prolactin |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | At Dx | 1st | At Dx | 1st | At Dx | 1st | At Dx | 1st | At Dx | 1st | At Dx | 1st | At Dx | 1st | At Dx | 1st | At Dx | 1st | At Dx | 1st | At Dx | |||
| irH | 1 | M/76 | - | 0.2 | - | 1.4 | - | 18.0 | - | 12.8 | - | 63.9 | - | 0.1 | - | 13.1 | - | 2.7 | - | 0.7 | - | - | - | - |
| 2 | F/55 | - | 0.7 | - | 1.3 | - | 18.2 | - | 12.6 | - | 163.0 | - | 0.1 | - | 16.6 | - | 1.3 | - | - | - | 4.0 | - | 11.1 | |
| 3 | F/61 | - | 29.4 | - | 0.6 | - | 8.9 | - | 6.0 | - | 348.0 | - | 0.6 | - | 6.8 | - | 0.8 | - | - | - | 4.0 | - | 14.8 | |
| 4 | M/59 | - | 2.8 | - | 1.6 | - | 18.5 | - | 5.1 | - | 53.1 | - | 7.6 | - | 4.9 | - | 5.5 | - | 5.8 | - | - | - | 10.0 | |
| 5 | F/82 | - | 28.1 | - | 0.3 | - | 15.8 | - | 17.3 | - | 136.0 | - | 0.8 | - | 27.3 | - | 6.4 | - | - | - | 17.7 | - | 15.2 | |
| 6 | M/33 | - | 6.6 | - | 0.4 | - | 17.6 | - | 94.0 | - | 38.2 | - | 0.6 | - | 0.4 | - | 1.5 | - | 3.1 | - | - | - | 47.3 | |
| 7 | M/32 | - | 2.3 | - | 1.3 | - | 35.9 | - | 39.6 | - | 13.6 | - | 1.0 | - | 4.6 | - | 2.8 | - | 0.4 | - | - | - | 37.0 | |
| 8 | M/71 | 7.8 | 8.7 | 1.1 | 0.2 | - | 40.1c) | - | 2.1c) | - | 86.5 | - | 2.4 | - | 3.5 | - | 5.3 | - | 1.2 | - | - | - | 4.5 | |
| 9 | M/60 | - | 111.3 | - | 0.4 | - | 27.7 | - | 2.2 | - | 112.0 | - | 0.8 | - | 14.4 | - | 5.0 | - | 4.1 | - | - | - | 21.8 | |
| 10 | M/75 | 2.7 | 0.9 | 1.1 | 1.5 | 43.6 | 32.0 | 8.2c) | 8.5 | - | - | - | - | - | 17.5 | - | 36.3 | - | 6.7 | - | - | - | 14.3 | |
| 11 | F/24 | - | 17.7 | - | 1.1 | - | 19.8 | - | 76.0 | - | 68.1 | - | 1.0 | - | 0.6 | - | 1.2 | - | - | - | 4.0 | - | 7.0 | |
| 12 | M/58 | - | 8.9 | - | 0.8 | - | 27.5 | - | 12.5 | - | 4.9 | - | 3.8 | - | 8.3 | - | 4.1 | - | 1.3 | - | - | - | 5.9 | |
| 13 | M/55 | - | 76.8 | - | 0.1 | - | 27.5 | - | 2.9 | - | 52.0 | - | 4.6 | - | 4.2 | - | 2.9 | - | 8.1 | - | - | - | 12.9 | |
| Non-irH | 14 | M/69 | 1.1 | 1.3 | 1.6 | 1.4 | - | 24.0 | 13.2c) | 24.4 | - | - | - | - | - | - | - | - | 5.2 | 0.2 | - | - | - | - |
| 15 | F/61 | 1.6 | 27.7 | 1.3 | 1.0 | - | 26.8 | - | 15.8d) | - | 93.8 | - | 0.4 | - | 23.5 | - | 25.5 | - | - | - | 4.0 | 7.5 | 11.9 | |
| 16 | M/63 | 1.2 | 15.7 | 1.6 | 1.4 | - | 23.9 | - | 1.7c,e) | - | - | - | - | - | 5.3 | - | 2.6 | - | 0.5 | - | - | - | 32.2 | |
| 17 | M/46 | - | 3.3 | - | 1.1 | - | 10.4c) | - | 2.6c) | - | - | - | - | - | - | - | - | - | - | - | - | - | 21.6 | |
| 18 | F/41 | - | 3.1 | - | 1.2 | - | 7.0 | - | 1.2f) | - | 76.3 | - | 0.6 | - | - | - | - | - | - | - | - | - | - | |
| 19 | M/42 | - | 4.9 | - | 1.0 | - | 28.9 | - | 11.4c) | - | - | - | - | - | 6.1 | - | 0.4 | - | 0.9 | - | - | - | 14.2 | |
| 20 | F/57 | - | 76.8 | - | 0.04 | - | 54.0 | - | 35.5 | - | 78.7 | - | 4.0 | - | 50.8 | - | 21.0 | - | - | - | 4.0 | - | - | |
| 21 | M/57 | 0.9 | 0.9 | 1.5 | 1.1 | - | 21.8 | - | 3.5c) | - | 145.0 | - | 0.1 | - | 8.3 | - | 4.4 | - | 4.4 | - | - | - | 3.5 | |
irAE, immune-related adverse event; irH, immune-related hypophysitis; TSH, thyroid-stimulating hormone; T4, thyroxine; ACTH, adrenocorticotropic hormone; IGF-1, insulin-like growth factor; hGH, human growth hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; At Dx: laboratory values at diagnosis of irH or last follow-up.
a) FSH reference range: 1.2ŌĆō33.1 mIU/mL (premenopausal), 27.7ŌĆō93.3 mIU/mL (postmenopausal), 1.3ŌĆō8.1 mIU/mL (male).
b) LH reference range: 0.5ŌĆō41.1 mIU/mL (premenopausal), 14.4ŌĆō52.8 mIU/mL (postmenopausal), 1.0ŌĆō5.3 mIU/mL(male).
c) ACTH and cortisol values were measured at 8:00 AM. The remaining values, which are unmarked, are the peak cortisol values at 60 minutes after corticotropin administration.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print



