 |
 |
| Korean J Intern Med > Volume 30(2); 2015 > Article |
|
Abstract
Background/Aims
Serum procalcitonin (PCT) levels are low in healthy individuals but are elevated in patients with a serious bacterial infection or sepsis. In this study, we examined the ability of serum PCT concentration to diagnose infections in end-stage renal disease (ESRD) patients, and sought to determine an appropriate threshold level.
Methods
Serum PCT levels were measured in ESRD patients on antibiotic therapy for a suspected bacterial infection (ESRD infection [iESRD] group, n = 21), and compared with those of ESRD patients on hemodialysis with no sign of infection (ESRD control [cESRD] group, n = 20).
Results
The mean serum PCT concentration of the iESRD group was significantly higher than in the cESRD group (2.95 ┬▒ 3.67 ng/mL vs. 0.50 ┬▒ 0.49 ng/mL, p = 0.006), but serum PCT concentrations did not correlate with severity of infection. The optimized threshold level derived for serum PCT was 0.75 ng/mL, rather than the currently used 0.5 ng/mL; this threshold demonstrated a sensitivity and specificity of 76.2% and 80.0% for infection and 100% and 60.6% for systemic inflammatory response syndrome, respectively, compared with the cutoff of 0.5 ng/mL.
Procalcitonin (PCT), the precursor of calcitonin, consists of 116 amino acids and it barely detectable in serum under normal conditions [1]. However, in the presence of a bacterial or fungal infection or sepsis, PCT is produced by various organs, included the thyroid, and released into blood [2]. Measurements of serum PCT concentration are useful for diagnosing bacterial infections and serum PCT levels can aid in the differential diagnosis of systemic inflammatory response syndrome (SIRS) [3]. Furthermore, the rapidity of PCT testing can facilitate diagnosis and prompt treatment [4].
PCT is elevated at 3 to 6 hours after systemic bacterial infection, but not by local infection or viral infection, non-infectious inflammatory responses, autoimmune disease, or sepsis. Thus, it would appear that PCT testing could increase the specificity of the diagnosis of a bacterial infection more than testing for other inflammatory markers, such as C-reactive protein (CRP) or leukocyte counts [5,6,7]. However, other conditions, not associated with infections, such as early postoperative state, trauma, severe burns, leukopenia, and renal dysfunction can also increase serum PCT [8,9]. Additionally, it has been shown that serum PCT levels are high in patients with reduced renal function and that these levels are reduced after dialysis [10,11]. Accordingly, it seems unreasonable to apply the same PCT cutoff value to all patients.
The aims of this study were to assess the potential use of serum PCT as a diagnostic biomarker of bacterial infection in end-stage renal disease (ESRD) patients and to determine an appropriate cutoff level.
The study included 41 patients who underwent hemodialysis (HD) or peritoneal dialysis (PD) between October 2010 and April 2012. Serum PCT levels were measured in 21 ESRD patients on dialysis suspected of having a bacterial infection with 24 hours of commencing antibiotic treatment (ESRD infection [iESRD] group), and in 20 ESRD patients on HD with no sign of infection (ESRD control [cESRD] group). No patient showed increased serum CRP during the month prior to study commencement and patients were not limited in terms of age or gender. HD treatment was performed using bicarbonate-buffered dialysate and heparin as the anticoagulant. Hemodialyser membranes were high permeability (polyamide Polyflux 170H, Gambro, Lakewood, CO, USA) and were not reused.
Vital signs and laboratory testing were conducted to determine the presence of infection. Body temperature, respiratory rate, pulse rate, serum PCT, albumin, blood leukocytes, hemoglobin, hematocrit (Hct), total calcium, ferritin, CRP, blood urea nitrogen, and creatinine were included. SIRS was defined as the presence of at least two of the following criteria: (1) a body temperature of < 36Ōäā or > 38Ōäā; (2) a heart rate of > 90 beats per minute; (3) a respiratory rate of > 20 breaths per minute or a PaCO2 of < 32 mmHg; and (4) white blood cell (WBC) of > 12,000/mm3 or < 4,000/mm3. Blood samples were obtained within a day of initiating antibiotic therapy in the iESRD group, and just prior to the start of dialysis in cESRD group. Serum PCT was measured before dialysis using an enzyme fluorescence assay kit (Enzyme-linked Fluorescent Assay, bioMerieux Co., Lyon, France).
The study design was approved by the Ethics Committee of Chosun University Hospital, Korea (IRB No. CHOSUN 2014-05-002).
The statistical analysis was performed using the SPSS version 20.0 (IBM Co., Armonk, NY, USA). The significance of differences between groups was assessed using Pearson chi-square test. Pearson correlation coefficients were used to identify linear correlations between continuous variables. The sensitivity and specificity of PCT for determining the presence of infection were confirmed by receiver operating characteristic (ROC) analysis. Data are expressed as means ┬▒ SD. Statistical significance was set at p < 0.05.
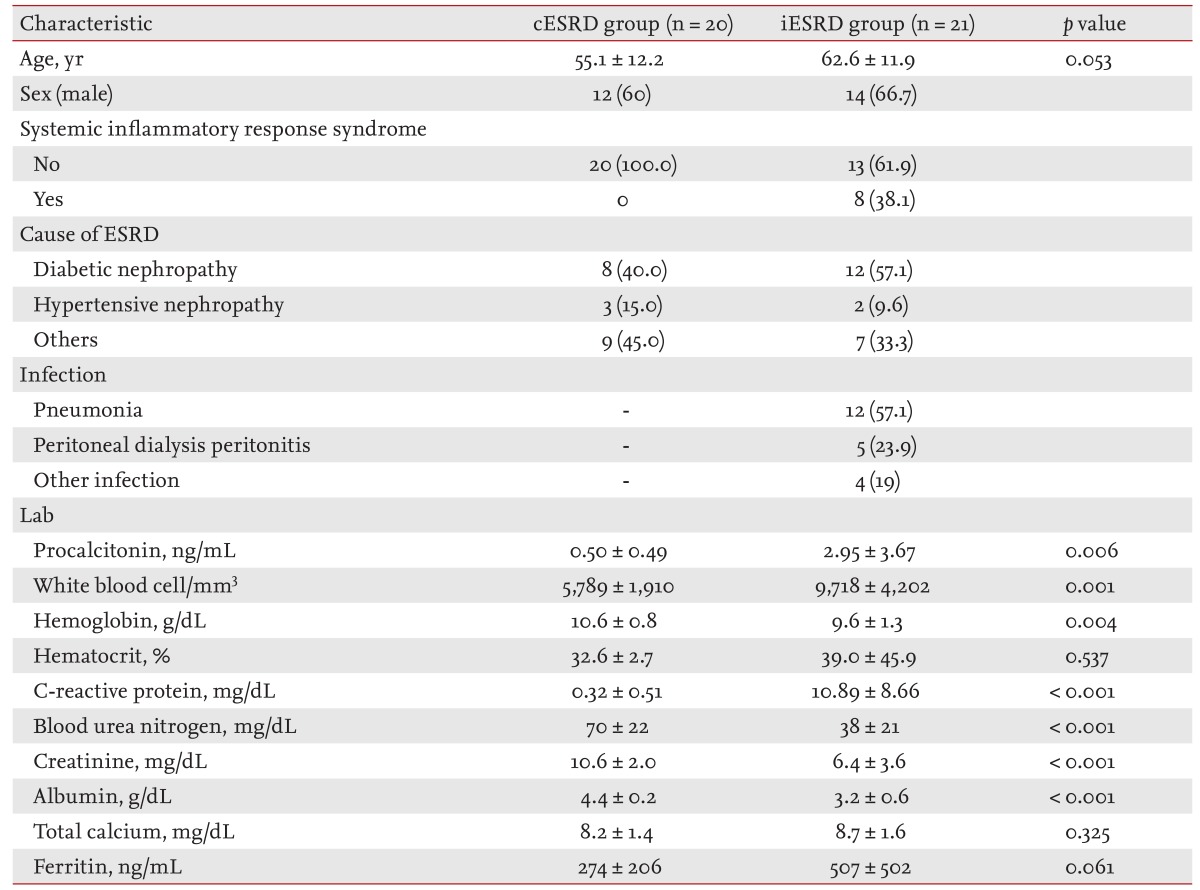
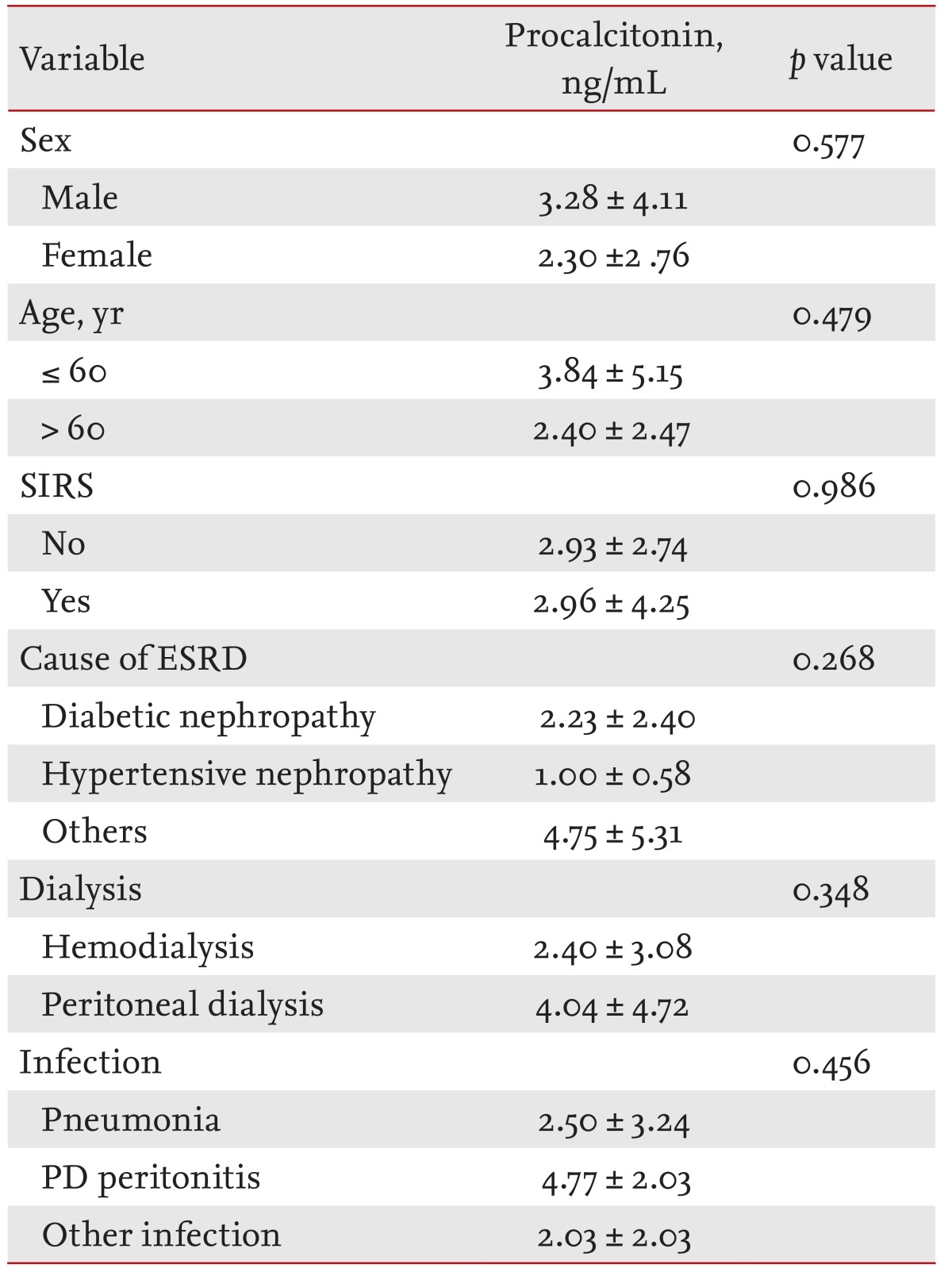
Of the 41 study subjects, 26 were males (63.4%) and the mean age was 59.0 years. The cause of ESRD was diabetic nephropathy in 20, hypertensive nephropathy in five, and another glomerulonephropathy in 16 patients. In the iESRD group (n = 21), 12 patients (57.1%) had pneumonia and five (23.8%) had PD-related peritonitis. Three soft-tissue infections and one infectious gastroenteritis were classified into the other infectious disease group. In the iESRD group, eight patients (38.1%) met the SIRS criteria (Table 1).
Serum PCT in the iESRD group (2.95 ┬▒ 3.67 ng/mL) was significantly higher than in the cESRD group (0.50 ┬▒ 0.49 ng/mL, p = 0.006). WBC counts, an indicator of the inflammatory response, were 9,718 ┬▒ 4,202 and 5,789 ┬▒ 1,910/mm3 in the iESRD and cESRD groups, respectively (p = 0.001). CRP was also significantly different in the two groups (10.89 ┬▒ 8.66 mg/dL vs. 0.32 ┬▒ 0.51 mg/dL, respectively; p < 0.001) (Table 1).
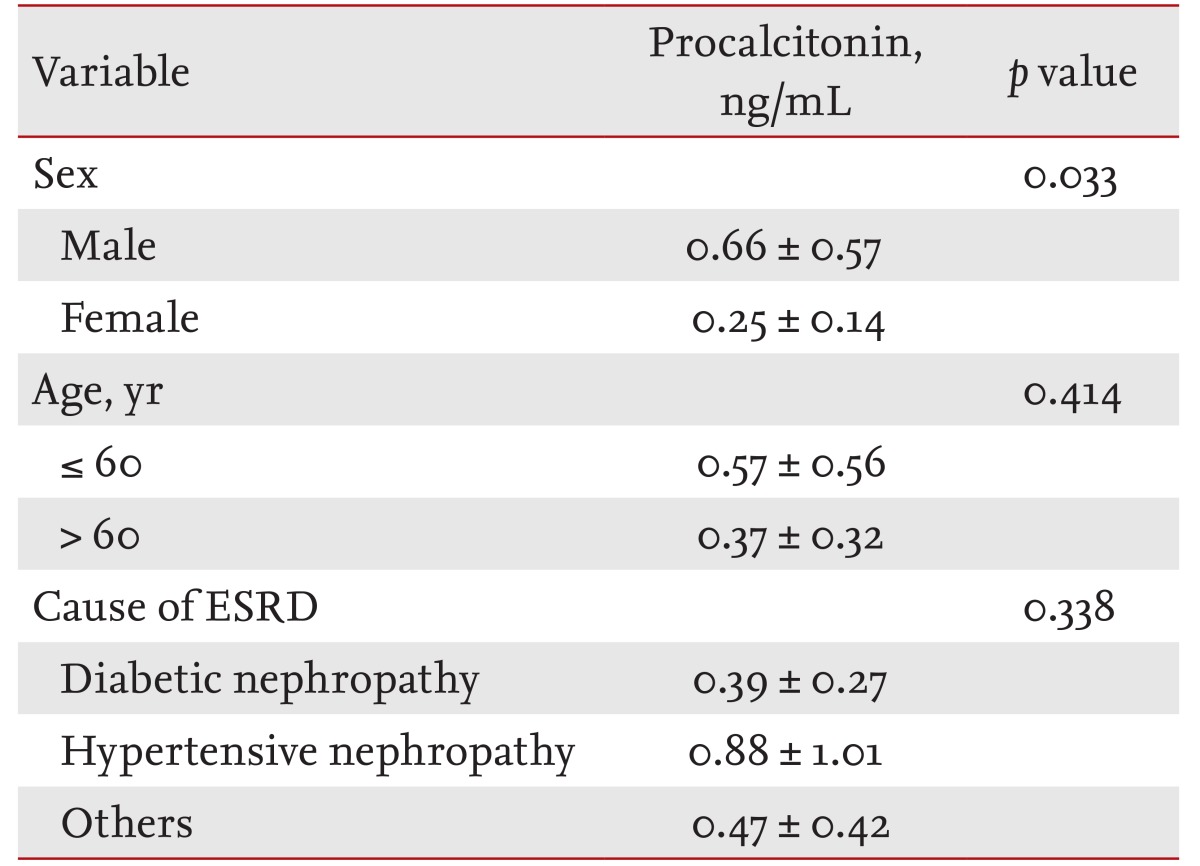
In the cESRD group, serum PCT was 0.66 ┬▒ 0.57 ng/mL in males and 0.25 ┬▒ 0.14 ng/mL in females (p = 0.033). No significant difference was found between PCT levels in those younger or older than 60 years (0.57 ┬▒ 0.56 and 0.37 ┬▒ 0.32 ng/mL, p = 0.414). For etiologic classes, no significant difference in serum PCT levels was observed diabetic nephropathy (0.39 ┬▒ 0.27 ng/mL), hypertensive nephropathy (0.88 ┬▒ 1.01 ng/mL), and other glomerulonephropathies (0.47 ┬▒ 0.42 ng/mL, p = 0.338) (Table 2).
In the iESRD group, serum PCT was non-significantly higher in those aged > 60 years and in men. Serum PCT in PD patients in the iESRD group was 2.40 ┬▒ 3.08 ng/mL, which was non-significantly higher than in HD patients (p = 0.348). No significant difference was found serum PCT levels in SIRS, by cause of ESRD, or by type of infection (p > 0.05) (Table 3).
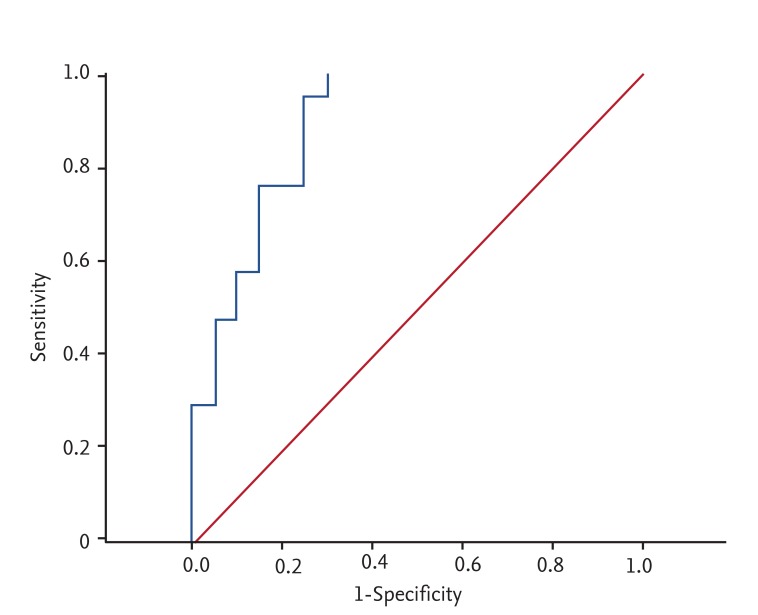
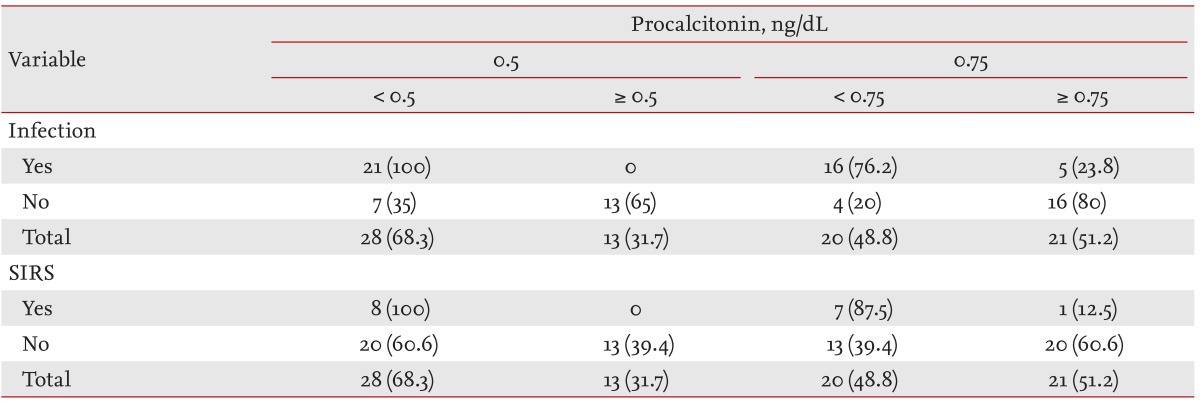
Mean serum PCT levels were significantly higher in the iESRD group than in the cESRD group. ROC curve analysis was used to evaluate the ability of PCT levels to predict the presence of infection (Fig. 1). At a serum PCT cutoff of 0.5 ng/mL, the sensitivity and specificity for infection in iESRD group were 100.0% and 65.0%, and for SIRS were 100.0% and 39.4%, respectively (Table 4). At a serum PCT level cutoff of 0.75 ng/mL corresponding values were 76.2% and 80.0% for infection, and 100.0% and 60.6% for SIRS (Table 4).
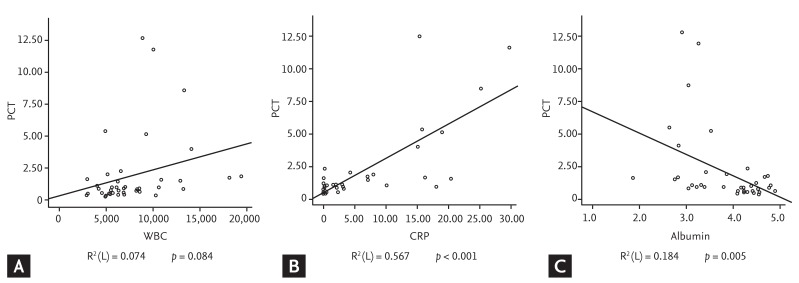
Of the variables that differed significantly in the cESRD and iESRD groups, WBC and CRP showed positive correlations with serum PCT (R2 = 0.074, p = 0.084 and R2 = 0.567, p < 0.01, respectively) and albumin showed a negative correlation (R2 = 0.184, p = 0.005) (Fig. 2).
The inflammatory response manifests as symptoms of fever, redness, swelling, and pain due to a localized reaction against body tissue damage or infection and proceeds with inflammatory mediators and monocytes and neutrophils within 30 to 60 minutes after inflammation development [5,12]. The degree of inflammation is assessed by measuring levels of an acute phase reactant in blood. CRP is often used as an indicator clinically, but it is difficult to differentiate between the signs and symptoms of inflammation and infection. Serum PCT levels increase from 3 to 6 hours after a bacterial infection or sepsis, but do not increase significantly in patients with viral infections, local infections, non-infectious inflammation, or autoimmune diseases. Thus, PCT aids in the diagnosis of bacterial infections, and has higher sensitivity and specificity than CRP [4,5,7].
Furthermore, serum PCT can aid in the differentiation of bacterial pneumonia from infections caused by viruses and atypical pathogens, and from tuberculosis and also provides guidance regarding antibiotic treatment [13,14,15]. When the serum PCT level is Ōēż 0.5 ng/mL in SIRS patients, a systemic bacterial infection can be excluded, but if > 2.0 ng/mL, a systemic infectious process is strongly suggested. A level of > 10 ng/mL indicates critical sepsis or septic shock, and a level of Ōēż 2.0 ng/mL, indicates the need for re-examination after 6 to 24 hours even if bacterial infection or sepsis is suspected [15]. In the present study, the iESRD and cESRD group had significantly different mean PCT levels (0.50 ┬▒ 0.49 ng/mL vs. 2.95 ┬▒ 3.67 ng/mL).
Serum PCT concentrations were measured in the cESRD group because serum PCT levels depend on the severity of infection. Serum PCT levels were increased significantly in males compared to females, but further larger studies are needed to confirm this. Serum PCT was slightly higher in those older than 60 years and with hypertensive renal disease patients, but no significant difference was found by age or renal failure etiology. The mean serum PCT levels in SIRS and non-SIRS patients were similar at 2.96 ┬▒ 4.25 and 2.93 ┬▒ 2.74 ng/mL, respectively. Thus, a higher serum PCT level does indicate the presence of SIRS (p = 0.986). Kim et al. [15] concluded that serum PCT was unable to determine SIRS severity: that is, sepsis, severe sepsis, or septic shock. However, it could differentiate sepsis and severe sepsis [15]. However, we did not separate sepsis and severe sepsis for the analysis in the present study.
Serum PCT is a recognized indicator of bacterial infection, but in patients with multiple injuries, in patients in the early stages after a critical surgery, and in severe burn cases, PCT levels can be elevated regardless of infection. Levels can also increase after a severe cardiogenic shock or during an organ perfusion disorder [8,9]. Likewise, patients with chronic renal insufficiency have elevated PCT levels that decrease after dialysis [10,11,16]. Thus, the use of a PCT cutoff for differentiating healthy individuals from patient is impractical.
One study on serum PCT in ESRD patients on dialysis obtained a mean value of 0.69 ┬▒ 0.81 ng/mL before dialysis, and a level higher than the standard value of 0.5 ng/mL in 57% of patients [17]. Dahaba et al. [16] measured the PCT values of terminal renal failure patients that had not reached a state of septicemia. Measurements were taken before dialysis, and PCT levels were also higher than those of normal healthy controls. The expected lowest value for serum PCT levels was 1.5 ng/mL. In a recent study, it was reported that mean serum PCT decreased to 83% ┬▒ 25% after high-flux dialysis, but did not decrease significantly after low-flux dialysis [18]. Furthermore, PCT levels differed according to the method of dialysis [10]. In many studies, dialysis patients without infection had PCT levels slightly higher than normal people due to the presence of procedure-related inflammation (for example, an invasive procedure for HD or PD), decreased renal clearance, or an increase in peripheral blood mononuclear cell counts [19].
Regarding the reference value of serum PCT in healthy individuals, at a cutoff of 0.5 ng/mL, PCT levels had a sensitivity and specificity of 100.0% and 65.0%, respectively, for infection and 100.0% and % for SIRS. Herget-Rosenthal et al. [19] used serum PCT to diagnose severe infection or septicemia. PCT was measured before dialysis and its sensitivity and specificity were 89% and 81%, respectively. Specificity was relatively low in the present study. We used ROC curve analysis to evaluate the ability of PCT to determine the presence of infection. When a cutoff of 0.75 ng/mL was applied, its sensitivity and specificity were 76.2% and 80.0% for infection and 100.0% and 60.6% for SIRS, respectively.
Several variables differed significantly in the iESRD group and correlations were found between WBC and CRP and serum PCT. WBC and CRP are known indices of infection and were positively correlated with PCT, whereas serum albumin, a known indicator of nutritional status, was negatively correlated with serum PCT [20]. The present study had some limitations. Dialysis patients without infection were chosen as controls but there was no healthy normal control group. Moreover, the sample size was relatively small.
In summary, serum PCT levels were significantly higher in dialysis patients with a bacterial infection, demonstrating the utility of serum PCT as an index of infection. Additionally, dialysis patients without infection had higher PCT levels than the 'standard' cutoff of 0.5 ng/mL. Thus, it appears to be inappropriate to apply this cutoff to patients with renal dysfunction. The present study shows that a serum PCT cutoff of 0.75 ng/mL is more appropriate for the diagnosis of infection in ESRD patients.
References
1. Becker KL, Nylen ES, White JC, Muller B, Snider RH Jr. Clinical review 167: procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab 2004;89:1512ŌĆō1525PMID : 15070906.


2. Lu XL, Xiao ZH, Yang MY, Zhu YM. Diagnostic value of serum procalcitonin in patients with chronic renal insufficiency: a systematic review and meta-analysis. Nephrol Dial Transplant 2013;28:122ŌĆō129PMID : 23045429.


3. Tang H, Huang T, Jing J, Shen H, Cui W. Effect of procalcitonin-guided treatment in patients with infections: a systematic review and meta-analysis. Infection 2009;37:497ŌĆō507PMID : 19826761.


4. Hur M, Moon HW, Yun YM, Kim KH, Kim HS, Lee KM. Comparison of diagnostic utility between procalcitonin and C-reactive protein for the patients with blood culture-positive sepsis. Korean J Lab Med 2009;29:529ŌĆō535PMID : 20046084.


5. Mitaka C. Clinical laboratory differentiation of infectious versus non-infectious systemic inflammatory response syndrome. Clin Chim Acta 2005;351:17ŌĆō29PMID : 15563869.


6. Pfafflin A, Schleicher E. Inflammation markers in point-of-care testing (POCT). Anal Bioanal Chem 2009;393:1473ŌĆō1480PMID : 19104782.


7. Whicher J, Bienvenu J, Monneret G. Procalcitonin as an acute phase marker. Ann Clin Biochem 2001;38(Pt 5):483ŌĆō493PMID : 11587126.


8. Boucher BA. Procalcitonin: clinical tool or laboratory curiosity? Crit Care Med 2000;28:1224ŌĆō1225PMID : 10809313.


9. Kuse ER, Langefeld I, Jaeger K, Kulpmann WR. Procalcitonin in fever of unknown origin after liver transplantation: a variable to differentiate acute rejection from infection. Crit Care Med 2000;28:555ŌĆō559PMID : 10708199.


10. Herget-Rosenthal S, Marggraf G, Pietruck F, et al. Procalcitonin for accurate detection of infection in haemodialysis. Nephrol Dial Transplant 2001;16:975ŌĆō979PMID : 11328903.


11. Lavin-Gomez BA, Palomar-Fontanet R, Gago-Fraile M, et al. Inflammation markers, chronic kidney disease, and renal replacement therapy. Adv Perit Dial 2011;27:33ŌĆō37PMID : 22073825.

12. Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 2004;39:206ŌĆō217PMID : 15307030.


13. Hedlund J, Hansson LO. Procalcitonin and C-reactive protein levels in community-acquired pneumonia: correlation with etiology and prognosis. Infection 2000;28:68ŌĆō73PMID : 10782390.


14. Kang YA, Kwon SY, Yoon HI, Lee JH, Lee CT. Role of C-reactive protein and procalcitonin in differentiation of tuberculosis from bacterial community acquired pneumonia. Korean J Intern Med 2009;24:337ŌĆō342PMID : 19949732.



15. Kim SW, Oh YM, Choe SM, Choe GH, Park KN, Oh JS. Practical application of semiquantitative procalcitonin test in emergency department. J Korean Soc Emerg Med 2008;19:665ŌĆō671.
16. Dahaba AA, Rehak PH, List WF. Procalcitonin and C-reactive protein plasma concentrations in nonseptic uremic patients undergoing hemodialysis. Intensive Care Med 2003;29:579ŌĆō583PMID : 12652350.


17. Level C, Chauveau P, Delmas Y, et al. Procalcitonin: a new marker of inflammation in haemodialysis patients? Nephrol Dial Transplant 2001;16:980ŌĆō986PMID : 11328904.


18. Montagnana M, Lippi G, Tessitore N, et al. Procalcitonin values after dialysis is closely related to type of dialysis membrane. Scand J Clin Lab Invest 2009;69:703ŌĆō707PMID : 19484659.


Figure┬Ā2
Correlation between serum procalcitonin (PCT), (A) white blood cell (WBC), (B) C-reactive protein (CRP), and (C) albumin. Serum PCT had a positive correlation with WBC and CRP, whereas serum PCT has a negative correlation with albumin.
-
METRICS

- Related articles
-
Procalcitonin as a biomarker of infectious diseases2013 May;28(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


