INTRODUCTION
Diagnostic biomarkers have been successfully implemented in several fields of medicine (e.g., D-dimers in pulmonary embolism, natriuretic peptides in acute heart failure, and troponin in myocardial infarction). However, the timely diagnosis of bacterial infections remains a challenge [1]. Reliable clinical and/or microbiological parameters from easy to obtain specimens that may be used to diagnose bacterial infections and rule out other infections are mostly lacking. The main disadvantages of many current microbiological methods are diagnostic delays, such as those that occur with culture methods, suboptimal sensitivity for samples like blood cultures, and low specificity due to contamination in samples like sputum cultures, whereas others, such as lung biopsies, are not amenable to routine diagnostics due to their invasive nature. Furthermore, inflammatory markers, including C-reactive protein (CRP) and white blood cells (WBC), lack specificity for bacterial infections [2].
Recent advances in biotechnology and the sequencing of the human genome offer unprecedented opportunities to increase our understanding of critical illness and injury [3]. The early detection of infection has both physiologic and clinical importance. The earlier an injurious process can be identified, the more timely additional preventive (i.e., removal of a stimulus for injury) and potential therapeutic measures can be initiated [4].
Procalcitonin (PCT) has emerged as a promising marker for the diagnosis of bacterial infections because higher levels of PCT are found in severe bacterial infections relative to viral infections and nonspecific inflammatory diseases. Hence, PCT may be used to support clinical decisions regarding the initiation or discontinuation of antibiotic therapy [5,6]. The clinical utility of serum PCT levels continues to evolve [7]. PCT is regarded as a promising candidate marker for making a diagnosis and antibiotic stewardship in patients with systemic infections [1].
Importantly, as with any diagnostic tool, PCT use should be embedded in clinical algorithms adapted to the type of infection and the clinical context and setting. While optimal PCT level cutoffs have been established for some types of infections and clinical settings and their safety and efficacy shown in randomized-controlled intervention trials, for other types of infection only observational studies are available. Thus, the clinical benefit and safety of using PCT remains undefined [8].
The aim of this review is to summarize the current evidence for PCT in different infections and clinical settings, to discuss the strengths and limitations of PCT, and to summarize the reliability of this marker when used with validated diagnostic algorithms.
PCT BIOLOGY
Although PCT is the prohormone for calcitonin, its biologic activities are distinctly different [9]. In the C cells of the thyroid gland and K cells of the lung, elevated serum calcium concentrations or neoplastic changes result in transcription of the PCT gene. Subsequently, ribosomal synthesis of the 116-amino-acid PCT molecule occurs, with subsequent cleavage of amino acids 60 to 91 yielding calcitonin. Calcitonin's only recognized biologic activity is to lower the serum calcium concentration by inhibiting bone resorption [7]. Hormones are exclusively produced in endocrine cells and act systemically. Cytokines are produced by multiple cells and have local effects.
Hormokines can exhibit either classical hormonal expression, or, upon inflammatory stimulation, show more cytokine-like behavior [10,11]. Ubiquitous inflammatory release can be induced directly via microbial toxins (e.g., endotoxin), or indirectly via a humoral or cell-mediated host response (e.g., interleukin [IL]-1β, tumor necrosis factor [TNF]-α, and IL-6). Parenchymal cells, including liver cells, kidney cells, adipocytes, and muscle cells, provide the largest tissue mass and principal source of circulating hormokines in sepsis [11].
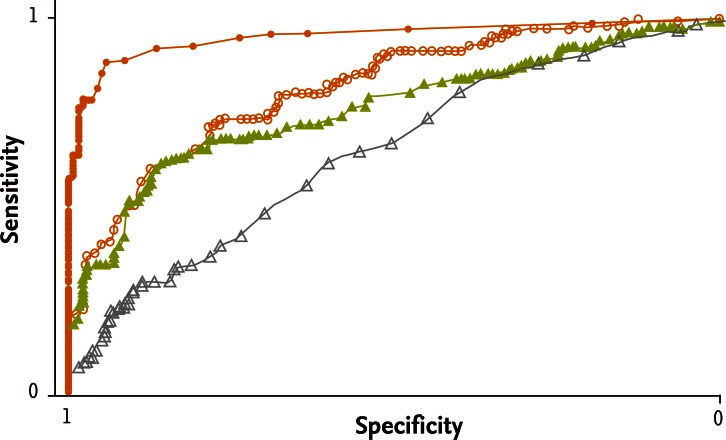
PCT is markedly elevated (up to 5,000-fold) within 2 to 4 hours in severe forms of systemic inflammation or in bacterial infections, and the level persists until recovery [7,12,13]. The biological half-life of PCT is 22 to 26 hours, an advantageous time point compared with CRP and other acute-phase reactants [14]. Distinct from CRP and other acute-phase reactants, existing data suggest that PCT levels rarely increase in response to viral infections, indicating that PCT may be useful for discrimination between bacterial and viral infections. The lack of viral response is postulated to result from the virus-stimulated synthesis of α-interferon by macrophages, which, in turn, inhibits TNF synthesis. The predictive value of PCT has been tested in several studies and in a recent prospective, multicenter study (Fig. 1) [15]. PCT is useful not only for monitoring bacterial infections but also for the differential diagnosis of systemic inflammatory response syndrome, which is a serious medical condition [15,16]. There are likely other biologic roles for PCT, beyond signaling bacterial invasion, which require further study [7,12].
PCT ASSAYS
Although it would be highly desirable, there is no research or clinical assay that exclusively detects the 116-kDa PCT peptide. Depending on the type of assay, all tests detect various portions of several computed tomography precursors (Table 1). Based on a highly sensitive research assay, the normal level of PCT in an uninfected individual is 0.033 ± 0.003 ng/mL. The first commercial PCT assay (LUMI test) has a functional lower limit of sensitivity of 0.5 ng/mL. The second-generation Food and Drug Administration (FDA)-approved PCT assay is technically a time-resolved cryptate emission immunoassay. The assay quantifies both PCT and part of the N-terminal end of the PCT molecule. The functional lower limit of sensitivity is 0.05 ng/mL, and the assay has reliable linear quantitation to 1,000 ng/mL. Either serum or plasma is used, and results are available in 1 hour or less [7,17].
CLINICAL USEFULNESS OF PCT IN INFECTIOUS DISEASES
The clinical evaluation of PCT levels continues. At present, there are four common uses of PCT levels. First, the current immunoassay was approved by the FDA for establishing the likelihood of mortality in critically ill septic patients [15]. Second, PCT levels have been used to guide empirical antibacterial therapy in patients with acute exacerbations of chronic bronchitis, community-acquired pneumonia (CAP), and sepsis [1,18]. Third, PCT levels, along with standard clinical parameters, can assist in determining whether the patient's empirical antibacterial therapy is effective [1]. Finally, the most useful application is the use of sequential PCT levels to determine when there is no longer a need for antibacterial therapy [7,19].
Correlation of PCT with the severity of sepsis
PCT has been demonstrated to be most useful clinically and superior to commonly obtained clinical variables and other laboratory tests in the diagnosis of sepsis, and it is correlated with the extent and severity of microbial invasion [20-22]. Unlike other diagnostic biomarkers, including CRP, there is strong evidence from animal studies that PCT plays a pathophysiological role in the development of severe sepsis and associated mortality [23]. PCT correlates with the extent and severity of infection and has prognostic implications, as the course of PCT predicts the risk of mortality in critically ill patients with infections and in patients with ventilator-associated pneumonia [16]. Furthermore, the production of PCT, in contrast to other biomarkers, including CRP, seems not to be significantly attenuated by nonsteroidal and steroidal anti-inflammatory drugs [24].
PCT for the guidance of antibiotic therapy in respiratory tract infections
As PCT levels increase upon bacterial infection and decrease upon recovery, they can be used to guide antibiotic therapy in individual patients as a surrogate biomarker [25,26]. Highly sensitive PCT assays are needed to reliably diagnose CAP and non-CAP lower respiratory tract infections (RTIs) [1,17]. Two low PCT measurements, over the first 4 to 6 hours of hospital admission, resulted in fewer patients started on empirical antibacterials. Low PCT levels over the first 4 hours of inpatient care have an excellent negative predictive value for bacterial infection [18].
PCT for antibiotic guidance in other infections
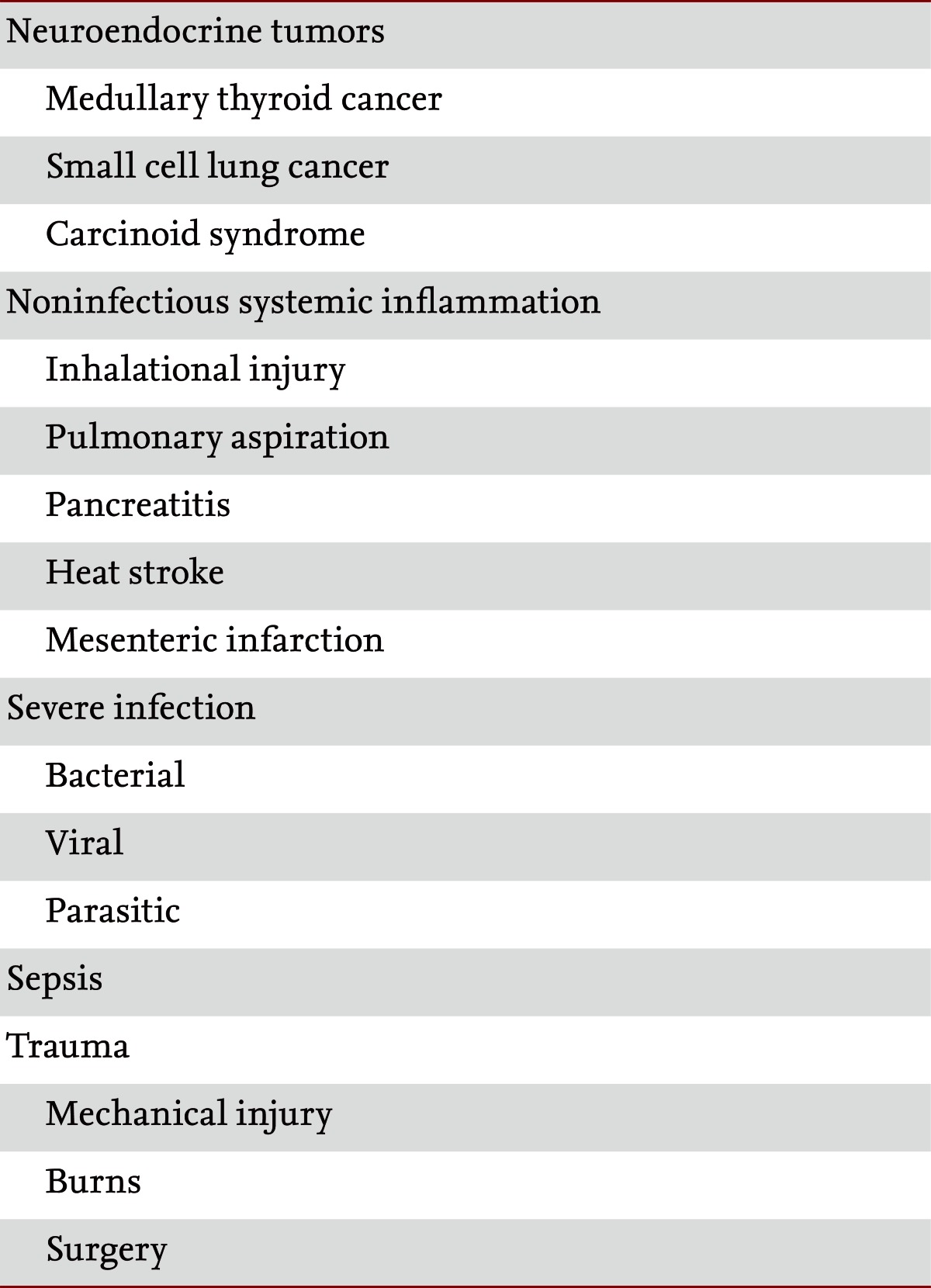
PCT has been proposed to be a promising marker for reliably distinguishing bacterial infections from other types of infections, including neutropenic fever, fungal infections, postoperative fever, arthritis, and suspected bloodstream infections [27-29]. Importantly, with the exception of RTIs, meningitis, and sepsis within the intensive care unit, all published studies were observational and it remains uncertain whether PCT can be safely used for antibiotic guidance in different settings [30,31]. For some infections, PCT may not be sensitive enough for routine clinical use. In patients with subacute endocarditis, PCT levels may remain low and cannot be used to discriminate infected from uninfected patients. Similarly, in patients with a Mycoplasma or viral infection, PCT levels may remain low, while with other atypical pathogens such as Legionella pneumophila PCT shows a prominent increase upon infection. Conversely, in patients with hypothermia after cardiac arrest, high initial levels of PCT were found independent of an underlying infection. This increase in PCT was nonspecific and mirrored an inflammatory reaction rather than true infection, limiting the diagnostic potential for early antibiotic stewardship in these high-risk patients [7,18].
Antibiotic stewardship with PCT
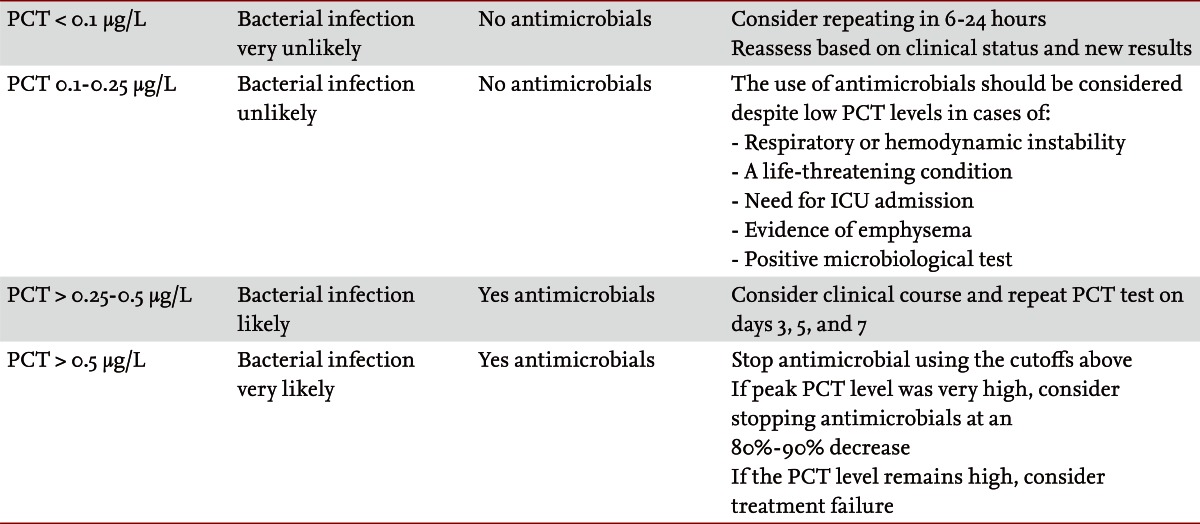
Antimicrobial resistance has emerged as a major factor affecting patient outcomes and overall resources. This calls for more stringent efforts to reduce antibiotic overuse [23]. A variety of cutoffs have been reported [32]. When PCT is used to guide diagnostic and therapeutic decisions in patients with infections in medical practice, two important issues need to be considered in order to optimize diagnostic accuracy and patient safety: the functional assay sensitivity and cutoff ranges. All published studies on antibiotic stewardship used similar clinical algorithms with recommendations for and against antibiotic treatment based on PCT cutoff ranges. The algorithms specified one of four antibiotic recommendations, ranging from 'strongly discourage' and 'discourage' to 'recommend' and 'strongly recommend,' respectively. The PCT cutoff ranges were derived from multilevel-likelihood ratio calculations obtained in observational studies and reflect the likelihood of a bacterial infection. The feasibility and safety of these algorithms was prospectively investigated and repetitively validated in multiple randomized control trials by independent groups (Table 2). In the intensive care unit at our institution, a fall in the level of PCT to ≤ 0.1 ng/mL is used to signify the end of bacterial invasion and that it is safe to discontinue antibiotic therapy [33]. Several studies have successfully adopted this approach, rather than an arbitrary one size fits all duration of therapy. Virtually every study to date in patients with sepsis or pneumonia has demonstrated substantive decreases in the duration of antibacterial therapy when it is guided by sequential PCT levels [34-36].
OTHER POTENTIAL USES OF PCT
PCT levels may help resolve the etiology of fever in patients with fever of unknown origin (FUO) syndrome, in that PCT levels do not increase in some disease entities that cause FUO syndrome (e.g., Still's disease, systemic lupus erythematosus, and inflammatory bowel disease) [18,37,38].
Initial data indicate that PCT levels are unaffected by patient use of nonsteroidal anti-inflammatory agents or glucocorticoids. If so, PCT levels remain a valuable marker of the host inflammatory response even when nonsteroidal anti-inflammatory drugs and pharmacologic doses of corticosteroids have altered the patient's temperature curve, WBC count, and WBC differential [24].
LIMITATIONS OF PCT
A number of limitations exist in using PCT as a marker of infection and sepsis. Nonspecific elevations in PCT levels in the absence of a bacterial infection can occur in situations of massive stress, such as after severe trauma and surgery, or in patients with cardiac shock [1,17]. This is the reason why the power of PCT to discriminate between sepsis and sterile inflammation is better for medical than for surgical patients Also, various other causes of nonbacterial systemic inflammation have been reported, including birth stress in newborns, heat shock, and acute graft-versus-host disease, as well as different types of immunotherapy, such as granulocyte transfusions, the administration of antilymphocyte globulin or anti-CD3 antibodies, and therapy with cytokines or related antibodies (IL-2 or TNF-α) [39]. Some autoimmune diseases such as Kawasaki disease or different types of vasculitis and paraneoplastic syndromes are also associated with elevated PCT levels [24].
CONCLUSIONS
Among the numerous markers of sepsis and infection that have been proposed, PCT is by far the most widely evaluated. This marker may help physicians make a diagnosis earlier, differentiate infectious from sterile causes of severe systemic inflammation, and assess the severity of systemic inflammation caused by bacterial infections.
There is a lot more to learn about PCT. The use of PCT, like any biomarker, should be considered within the context of the clinical workup and should take into account all patient related and therapy related factors that may interfere with the initial magnitude and course of this parameter.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





