 |
 |
| Korean J Intern Med > Volume 13(1); 1998 > Article |
|
Abstract
Behcet‚Äôs syndrome is a multi-systemic and chronic disorder that affects many organs. It has been suggested that the diagnosis was based on the presence of the ‚Äėmajor‚Äô and ‚Äėminor‚Äô clinical criteria. When thromobophlebitis, arthritis, central nervous system or gastrointestinal lesions are also present, Behcet‚Äôs syndrome will be thought to be present in the appropriate geographic area.
We report a case of superior vena cava syndrome caused by Behcet’s disease in a 40-year-oid man with recurrent oral aphthous ulcers and skin rashes on the anterior chest wall. There were multiple thrombosis of the superior vena cava, innominate and subclavian veins. This patient also had a solitary cecal ulcer with an ileocecal fistula and downhill varix. The chest CT, veno-cavography, pulmonary angiography and colon study were taken and follow-up was performed.
Behcet‚Äôs syndrome was described as a clinical syndrome with aphthous oral ulcers, genital ulceration and eye involvement. As it affects many other organs, Behcet‚Äôs syndrome is now recognized as a systemic disease1). The clinical characteristics of Behcet‚Äôs syndrome are thought to be caused by vasculitis that principally affects veins, venules and capillaries. But there were no pathognomic findings and the diagnosis was based on the presence of the ‚Äėmajor‚Äô and ‚Äėminor‚Äô criteria, e.g. oral ulcers, genital ulcers, ocular lesions, skin lesions, arthropathies, gastrointestinal lesions and cardiovascular lesions2). Although there were no definite symptoms of major criteria, thrombophlebitis, arthritis, central nervous system or gastrointestinal lesions are also present and Behcet‚Äôs syndrome is thought to be present in the appropriate geographic area3).
We report a patient with Behcet’s syndrome having recurrent oral ulceration, cutaneous pustules, as well as non-deforming arthritis and ileocecal fistula presenting SVC syndrome and down-hill esophageal varix.
In November 1995, a 40-year-old man presented with a four-month history of intermittent lower abdominal pain and neck vein engorgement. He had had recurrent oral ulcers for 7 years. His face was plethoric and the external jugular vein was markedly engorged. There were multiple maculoerythematous lesions, about 2‚Äď3mm in diameter, on his face, neck and anterior chest. There were distended collateral vessels on the anterior chest wall and abdomen. Chest radiograph revealed a slightly widened superior mediastinum, suggesting a pathologic problem of the supenor mediastinum(Fig. 1). Initial computed tomography(CT) scan, following a bolus injection of intravenous contrast medium, demonstrated obstruction of the superior vena cava with thrombosis and pleural effusion of the right hemithorax(Fig. 2). Abdominal sonogram did not show any sign of obstruction of the inferior vena cava or portal vein. Gastroscopy revealed a mild degree of esophageal varix(Form 1, white color, Locus superior, negative red color sign). Pleural tapping was not taken because the patient did not desire admission or further diagnostic studies to be done.
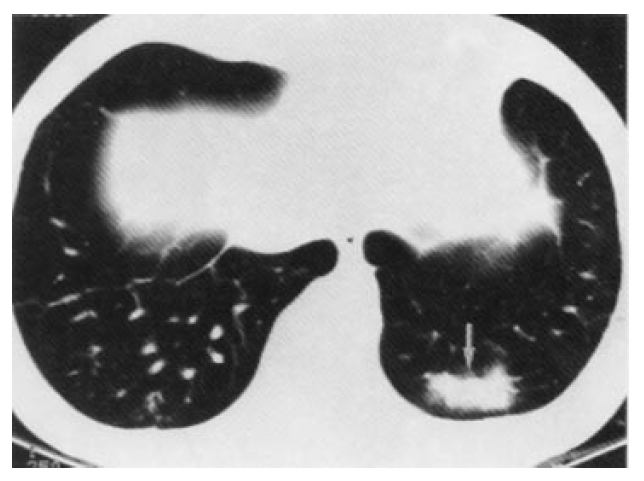
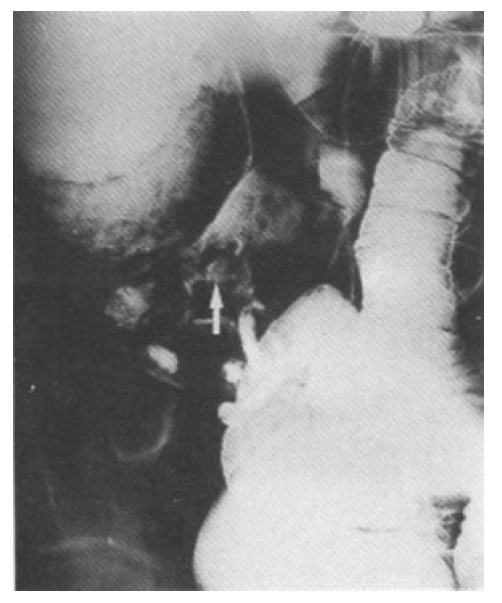
In February 1996, he was admitted to the hospital. In the ensuing twelve weeks, he felt less plethoric and had mildly collapsed neck vein engorgement. On examination, he had several painful aphthous oral ulcers, 2‚Äď8mm in diameter, with a central yellowish necrotic base on the tongue. Neck vein engorgement was somewhat decreased. He had painful swelling of the right knee joint. The pathergy skin test was negative. Hematological and biochemical tests were as follows: white blood cell count was 7,200/mm3, hemoglobin 12.6g/dL, platelet 320,000/mm3. Erythrocyte sedimentation rate was 39mm/hr and C-reactive protein was negative. The prothrombin time and activated partial thromboplastin time were normal. The rheumatoid factors, antinuclear antibody, antinuclear DNA, anticardiolipin antibody, lupus anticoagulant, antiphospholipid antibody and antineutophil cytoplasmic autoantibody were negative. There was no proteinuria and hematuria. Protein C, protein S, factor VIII, fibrinogen and antithrombin III were all normal. Follow-up CT scan demonstrated decreased pleural effusion, but newly developed consolidation at the left lower lobe territory, 3√ó1.5√ó3cm size in diameter, suggested focal pulmonary infarction(Fig. 3). SVC obstruction and many collateral vessels still persisted. Venocavogram was obtained after injection of 40ml contrast materials(Ultravist, Scherring, Germany) through both basilic veins. The venacavogram showed complete occlusion of the SVC, right subclavian vein and partial occlusion of the left subclavian vein. Subsequent pulmonary angiography was normal. The colon study and colonoscopy revealed solitary cecal ulcer with an irregular mucosal change of the ileocecal area and ileocecal fistula(Fig. 5).
A final diagnosis of Behcet’s syndrome with superior vena cava syndrome and ileocecal fistula was made and we started medical treatment with high dose sulfasalazine and azathioprine. He showed mild decompression of neck vein engorgement and disappearance of right abdominal pain, but still plethoric.
Behcet‚Äôs disease is a multi-systemic disorder having orogenital ulcers, uveitis, cutaneous, arthritic, neurological, vascular and intestinal manifestations. This syndrome affects mainly men aged 20‚Äď30 years. Because Behcet‚Äôs disease has various clinical manifestations, it is difficult to diagnose1,2). Diagnosis of Behcet‚Äôs disease is based only on clinical findings, because there are no pathognomonic laboratory or histopathological diagnostic criteria for this syndrome. The histological hallmark of Behcet‚Äôs disease is a nonspecific vasulitis of the arteries, veins, arterioles, venules, and capillaries of both systemic circulations4).
The pulmononary vasculitis characteristic of Behcet’s syndrome is usually necrotizing, and histopathologic findings indicate perivascular infiltration by lymhocytes. The syndrome characteristically affects the large vessels(mainly the veins such as SVC and innominate vein)5,6). In this case, obstruction of the SVC and subclavian veins were confirmed by the venocavogram. Right pleural effusion, which was not confirmed by pleural tapping, was thought to be transudate resulting from increased high venous pressure due to obstruction of the azygose vein and vena cava. Downhill esophageal varix with no evidence of portal hypertension is thought to arised by the same mechanism. Air space consolidation, which was seen on the CT scan, was thought to be an infarction or infiltration. But this was not identified by angiography. The previous post-mortem study reported that the pulmonary infact, with no evidence of peripheral venous thrombosis, resulted from an in situ thrombosis rather than an embolism. In situ venous thrombosis secondary to vasculitis probably plays an important role in the development of the major vessel venous thrombosis seen in Behcet’s syndrome5).
The gastrointestinal manifestation of this syndrome resembles that in Crohn’s disease, but granulomas are not found and free perforation is common7). Microscopically, vasculitis, often of the leucocytoclastic variety, is a common multi-systemic nature of Behcet’s disease usually allowing its recognition. In this case, there was a fistula between the cecum and terminal ileum. Endoscopic biopsy at the cecum shows massive infiltration of inflammatory cells without granuloma and crypt abscess.
There were some case reports about Behcet’s syndrome with SVC syndrome in Korea8), but there was no evidence of gastrointestinal involvement. This case has typical solitary ulcer with ileal fistula near the ileocecal valve. Also, there was downhill inverted superior esophageal varix. The downhill superior esophageal varix had been described in thoracic goiter, lung cancer, mediastinal fibrosis, tumors, superior vena cava ligation, subclavian vein thrombosis and congestive heart failure. There were few cases of downhill superior esophageal varix due to Behcet’s disease in the literature9).
Treatment of Behcet’s syndrome is symptomatic and empirical. Mucous membrane involvement may respond to topical corticosteroids. The arthritis responds to rest and analgesics. Thrombophlebitis is treated with antiplatelet agents. But uveitis, central nervous system involvement and pulmonary disease usually require corticosteroid and immunosuppressives. Other medications which have been tried include azathioprine, chlorambucil, colchicine, levamisole, cyclosporine and fibrinolysis-enhancing drugs, such as stanozolol and phenformin10).
However, there is little data on the use of the drugs specifically for the pulmonary disease of Behcet’s syndrome. Therapy for gastrointestinal Behcet’s disease is identical to that of inflammatory bowel disease, especially high dose sulfasalazine11).
Fig. 1.
Chest radiography shows slightly widened superior mediastinum and laterally shifted dome of the right hemidiaphragm.
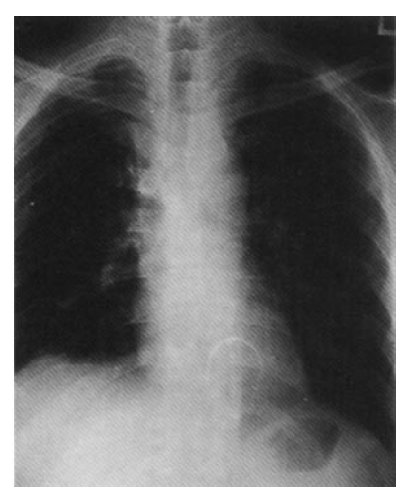
Fig. 2.
Initial contrast enhanced CT scan shows filling defect in the superior vena cava suggesting thrombosis (long arrow) and rich collateral veins in the mediastinum(arrow head) and chest wall. Pleural effusion is seen in the right hemithorax.
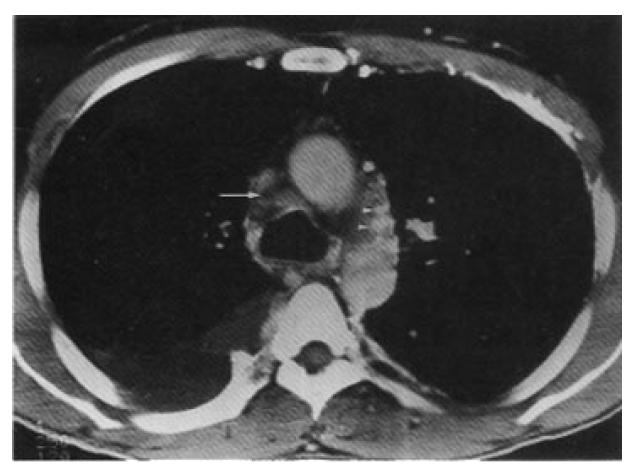
Fig. 3.
Follow-up CT after 3 months from initial CT scan shows newly developed consolidation in the left posterior basal segment(long arrow). The pleural effusion in the right hemithorax is decreased.
REFERENCES
1. Conn DL, Hunder GG, O‚ÄôDuffy JD. Behcet‚Äôs disease. In: Kelley WN, Harris ED, Ruddy S, Sledge CB, eds. Textbook of Rheumatology. 4th ed. Philadelphia: WB Saunders Co, 1993;1097‚Äď1099.
2. International study group for Behcet‚Äôs disease. criteria for diagnosis of Behcet‚Äôs disease. La‚Äôncet 1990;335:1078‚Äď1080.
3. Morita H, Kawai S, Kobori O, Ohya N, Sumiya M, Dohi T. Incomplete form of Behcet‚Äôs colitis in Japan: Is it a distinct entity. Am J Gastroenterol 1995;90:523‚Äď524.

4. Moutosopoulos HM. Behcet‚Äôs syndrome. In: Isselbacher KJ, Braunwald E, Wilson JD, Martin JB, Faauci AS, Kasper DL, eds. Harrison‚Äôs Principles of Internal Medicine. 13th ed. New York: McGraw-Hill Inc, 1994;1669‚Äď1670.
5. Tunaci A, Berkmen YM, Gokmen E. Thoracic involvement in Behcet‚Äôs disease: Pathologic, clinical and imaging features. ARJ 1995;164:51‚Äď56.

6. Ahn JM, Im JG, Ryoo JW, Kim SJ, Do YS, Choi YW, Oh YW, Yeon KM, Han MC. Thoracic manifestation of Behcet syndrome: radiographic and CT findings in nine patients. Radiology 1995;194:199‚Äď203.


7. Kasahara Y, Tanaka S, Nishino M, Umemura H, Shiraha S, Kuyama T. Intestinal involvement in Behcet‚Äôs disease: Review of 136 surgical cases in the Japanese literature. Dis Colon Rectum 1981;24:103‚Äď106.


8. Min Hee Won, Cho Seung Yun, Chun Sang Il, Bang Dong Sik. A case of Behcet‚Äôs syndrome with the superior vena cava syndrome. Kor J Int Med 1986;30:401‚Äď406(in Korean).
9. Ghayad E, Ghayad A. Inverted esophageal varices with superior vena cava obstruction secondary to Behcet‚Äôs disease. In: O‚ÄôDuffl JD, Kokmen E, eds. Behcet‚Äôs disease Basic and clinical aspects. 1st. New York: Marcel Dekker Inc, 1993;215‚Äď216.
10. Yazici H, Pazarli H, Barnes CG, Tuzun Y, Ozyazgan Y, Silman A, Serdaroglu S, Oguz V, Yurdakul S, Lovatt GE, Yazici B, Somani S, Muftuoglu A. A controlled trial of azathioprine in Behcet‚Äôs syndrome. N Engl J Med 1990;322:281‚Äď285.


11. Matsukawa M. Clinical Course and treatment for the intestinal Behcet and so called ‚Äúsimple ulcer‚ÄĚ. Stomach and Intestine 1992;303‚Äď311(In Japanese).
-
METRICS

- Related articles
-
Validation and reliability of a Behcet’s Syndrome Activity Scale in Korea 2016 January;31(1)
A Case of Cushing's Syndrome Presenting as Endometrial Hyperplasia2008 March;23(1)
A Case of McCune-Albright Syndrome with Associated Multiple Endocrinopathies2007 March;22(1)
A Case of Sweet`s Syndrome in Patient with Dermatomyositis1999 July;14(2)
A Case of Marfan Syndrome with Acute Monoblastic Leukemia1998 July;13(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


