 |
 |
| Korean J Intern Med > Volume 39(1); 2024 > Article |
|
Abstract
Background/Aims
We aimed to clarify the clinical characteristics of psoriatic arthritis (PsA) in Korean patients focusing on PsA with axial involvement.
Methods
A retrospective medical chart review was performed to identify PsA patients at a single tertiary center. Cases of AS patients with psoriasis were recruited from a prospective AS registry of the same center. Demographics, laboratory findings, and radiologic characteristics were assessed.
Results
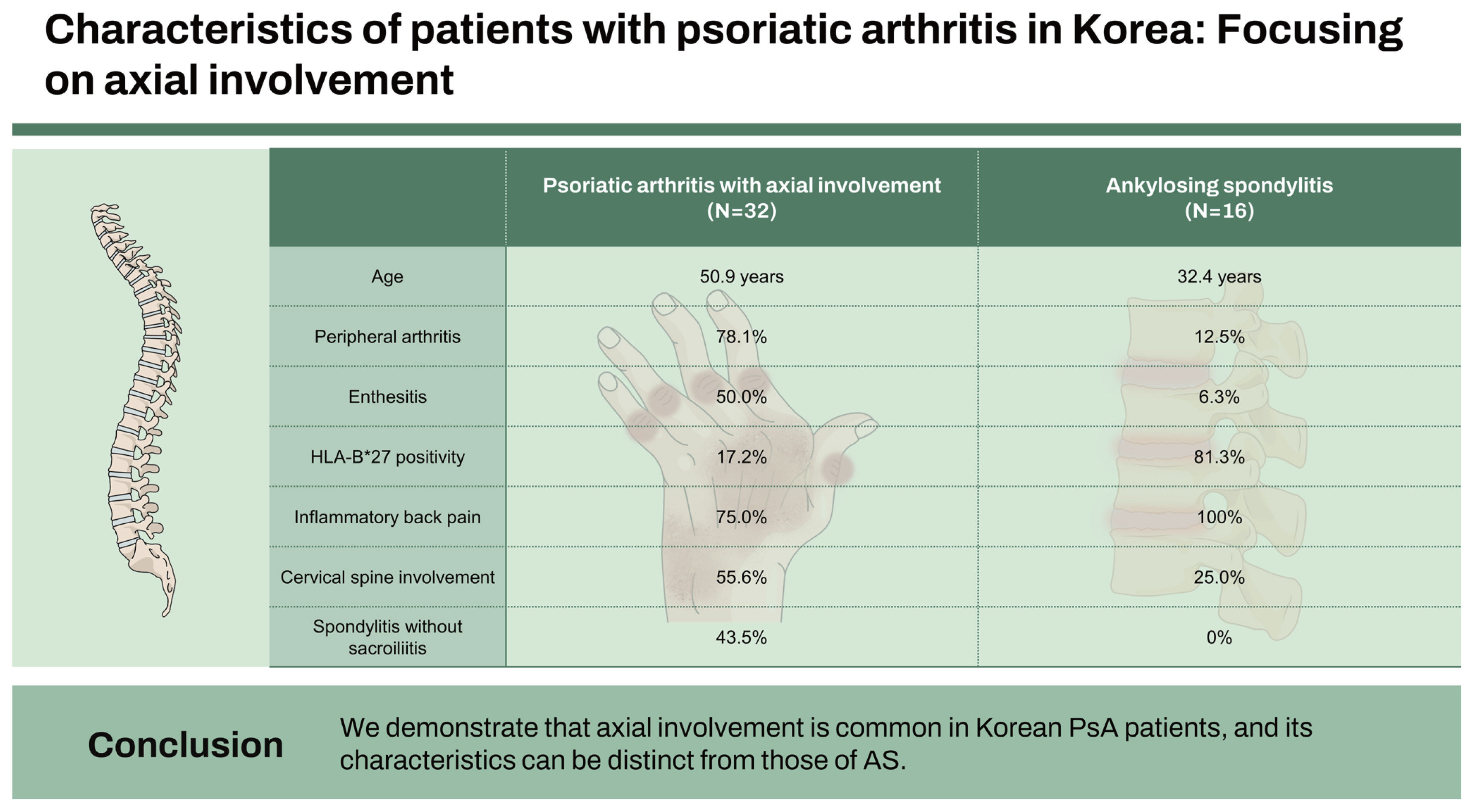
A total of 69 PsA patients were identified. In PsA patients, spondylitis (46.4%) was the most common form. Compared to AS patients with psoriasis, PsA patients with radiographic axial involvement were older (50.9 vs. 32.4 years; p < 0.001) and showed greater peripheral disease activity (peripheral arthritis 78.1 vs. 12.5%, p < 0.001; enthesitis 50.0 vs. 6.3%, p = 0.003). AS patients with psoriasis presented a higher rate of HLA-B*27 positivity (81.3 vs. 17.2%; p < 0.001) and a more frequent history of inflammatory back pain (100.0 vs. 75.0%; p = 0.039) than PsA patients with radiographic axial involvement. Significant proportions of PsA patients with radiographic axial involvement had cervical spine involvement (10/18, 55.6%) and spondylitis without sacroiliitis (10/23, 43.5%).
Psoriatic arthritis (PsA) is an inflammatory arthritis with a complex clinical presentation [1,2]. Originally, Moll and Wright described five clinical subtypes—oligoarthritis, polyarthritis, distal interphalangeal (DIP) joint arthritis, spondylitis, and arthritis mutilans—with the most common clinical pattern being oligoarthritis [1]. However, later series have suggested polyarthritis is the most frequent subgroup, affecting about 60% of PsA patients [3]. Recently, Gladman et al. [4] demonstrated polyarthritis occurs in 53% of patients with PsA, and its frequency was similar to that of oligoarticular disease (47%). Therefore, most clinical trials for biologics have included patients with polyarthritis [5]. Recently, interest in AxPsA has increased; for example, there has been a clinical trial focused on managing axial manifestations in PsA patients [6]. In Korea, the prevalence of PsA has been reported as 9–14% among patients with psoriasis, which is much lower than that in Western countries [7]. Because of the low incidence of PsA nationwide, only a few studies with small numbers of patients have been conducted in Korea, but the authors of these investigations consistently reported that spondylitis is the most common pattern [7–9].
The pathogenesis of PsA, which includes genetics, environmental factors, and immune-mediated inflammation, is complex. Evidence for the role of pathogenic CD8+ T-cells and activation of tumor necrosis factor and interleukin (IL)-23–T helper 17 pathways has led to the development of effective biologics and small-molecular targeting components of these pathways [10].
PsA is diagnosed according to the classification criteria for psoriatic arthritis (CASPAR) criteria, which are primarily based on clinical phenotype. To meet the CASPAR criteria, a patient must have inflammatory articular disease (joint, spine, or enthesitis) [11]. Therefore, spondylitis can be considered one of the conditions facilitating adherence to the CASPAR criteria. Meanwhile, the spondylitis form of PsA is currently known as axial psoriatic arthritis (AxPsA).
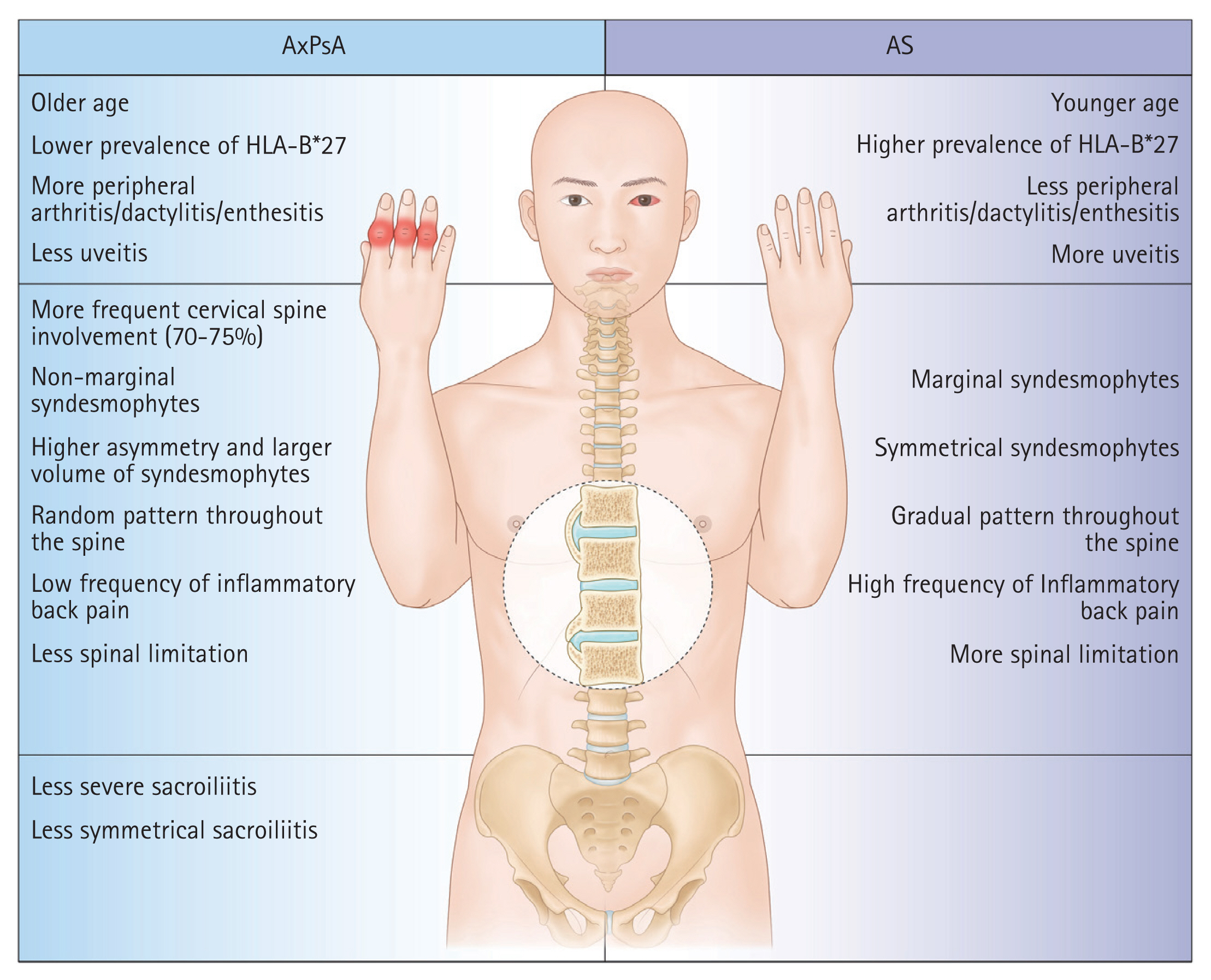
Both AxPsA and ankylosing spondylitis (AS) involve the axial skeleton, but they differ from one another clinically. Compared to AS, AxPsA occurs less frequently in men, has more peripheral joint involvement, and shows a reduced association with human leukocyte antigen (HLA)-B*27. Also, there are radiological characteristics that differ between the two diseases: patients with AxPsA tend to have a larger number of coarse, non-marginal syndesmophytes; more involvement of the cervical spine; and less involvement of the sacroiliac joints [12,13]. In this study, we aimed to clarify the clinical characteristics of Korean patients with PsA, focusing on distinct features of the most common form (AxPsA) and comparing them to the clinical and radiographic characteristics of AS.
A retrospective medical chart review was conducted to identify PsA patients who have been followed up with at The Catholic University of Korea, Seoul St. Mary’s Hospital from January 1, 2015 to Sep 30, 2021. A diagnosis of PsA was made on the basis of the CASPAR criteria [11]. With regard to identifying AS patients with psoriasis, data from the Catholic Axial Spondyloarthritis Cohort (CASCO), an ongoing prospective cohort that follows AS patients with annual data acquisition, were used. Patients who were enrolled in CASCO fulfilled the 1984 modified New York (mNY) diagnostic criteria for AS [14].
The following clinical features were evaluated: body mass index (BMI), age at diagnosis, nail changes, enthesitis, dactylitis, uveitis, affected peripheral joints, and inflammatory back pain (IBP). In particular, CASCO patients were evaluated in detail for spondyloarthritis (SpA) features. Laboratory data, including the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level, and the presence of HLA-B*27 were also evaluated. In patients with PsA, we identified the involved joints and classified the manifestation pattern of arthritis using one or more of the following descriptive terms: spondylitis, oligoarthritis, polyarthritis, DIP only, and arthritis mutilans. A spondylitis pattern was classified as that having axial involvement, and the presence of axial involvement was determined based on a positive imaging finding suggestive of sacroiliitis and/or spondylitis. Sacroiliitis was scored according to the mNY scoring method (grades 0–4), where a grade ≥ 2 was considered indicative of axial involvement [14]. Spondylitis was evaluated by applying the modified stoke ankylosing spondylitis spinal score (mSASSS) scoring method to cervical and lumbar spine images and determining the presence of syndesmophytes on thoracic spine images [15]. An mSASSS score of ≥ 1 point(s) on cervical and/or lumbar spine images or the presence of syndesmophytes in the thoracic spine was considered to suggest spondylitis. Therefore, axial involvement in PsA patients was determined based on radiographic evidence of spondylitis or sacroiliitis regardless of the presence of symptoms, and these patients were classified as PsA with radiographic axial involvement in the current study. The radiographs at the time of diagnosis of PsA with radiographic axial involvement or AS were evaluated by two experienced rheumatologists, who were blinded to the clinical data, by consensus. The need for informed consent was waived due to the retrospective nature of the study. This study was approved by the Institutional Review Board of The Catholic University of Korea, Seoul St. Mary’s Hospital (approval number: KC22RISI0003).
Bivariate associations between the groups were determined using the t-test with a normal distribution for continuous variables. The Mann–Whitney U test was used as an alternative to the t-test when the data were not normally distributed. The chi-squared test or Fisher’s exact test was used for differences between proportions. The statistical analyses were performed using the SPSS version 24 (IBM Corp., Armonk, NY, USA). All significance tests were conducted at the 0.05 significance level.
A total of 69 PsA patients were identified (Table 1). The male ratio was 55.1%, and the mean BMI was 24.7 kg/m2. The mean age at the time of PsA diagnosis was 46.7 years. HLA-B*27 positivity was confirmed in 22.0% (13/59) of PsA patients. Nail changes, enthesitis, dactylitis, and uveitis were observed in 43.5%, 39.1%, 31.9%, and 4.3% of patients with PsA, respectively. According to the Moll–Wright classification, spondylitis was the most common form, affecting 46.4% of patients, followed by oligoarthritis (44.9%) and polyarthritis (42.0%). There were 2 (2.9%) patients with DIP-only involvement, and no patient had arthritis mutilans [1]. Most of the patients with spondylitis also had peripheral involvement (25 [oligoarthritis 13 and polyarthritis 12]/32, 78.1%), but 7 (21.9%) patients had only spondylitis.
The clinical features of 32 PsA patients with axial involvement were compared to those of 37 PsA patients without axial involvement (Table 2). There was no significant difference in the ratio of men to women or the BMI between the two groups. The mean age at PsA diagnoses among the PsA patients with axial involvement was younger than that of those without axial involvement (43.2 vs. 50.9 yr; p = 0.023). The CRP level was higher in the PsA patients with axial involvement than in those without axial involvement (6.2 vs. 1.4 mg/L; p = 0.003). There was no significant difference between PsA patients with axial involvement and those without axial involvement in terms of HLA-B*27 prevalence or the frequency of extra-articular manifestations.
For comparison with PsA with radiographic axial involvement, a total of 349 patients with AS at baseline were analyzed, and 16 patients with psoriasis were identified. Different characteristics between the two groups were observed (Table 3). Compared to AS patients with psoriasis, PsA patients with radiographic axial involvement were significantly older at the points of diagnosis (50.9 vs. 32.4 yr; p < 0.001). There were no significant differences between the two groups in the ratio of men to women, BMI, and inflammatory markers (ESR, CRP). PsA patients with radiographic axial involvement were significantly more likely to have affected peripheral joints. (78.1 vs. 12.5%; p < 0.001). The frequently involved joints in axial PsA were proximal interphalangeal joints of the hands (32%), knees (28%), and ankles (28%) (Fig. 1). Nail changes (50.0 vs. 0.0%; p = 0.001) and enthesitis (50.0 vs. 6.3%; p = 0.003) were more frequent in PsA patients with radiographic axial involvement than in AS patients with psoriasis. On the contrary, uveitis was less common in PsA patients with radiographic axial involvement compared to AS patients with psoriasis (6.3 vs. 31.3%; p = 0.033). HLA-B*27 was more prevalent in AS patients with psoriasis compared to PsA patients with radiographic axial involvement (81.3 vs. 17.2%; p < 0.001). AS patients with psoriasis were significantly more likely to report IBP than PsA patients with radiographic axial involvement (100.0 vs. 75.0%, p = 0.039).
All of the identified AS with psoriasis cases fulfilled the radiographic criteria of the mNY criteria. Comparison of radiologic findings between PsA with radiographic axial involvement and AS with psoriasis cases is summarized in Table 4. Among PsA with radiographic axial involvement cases, sacroiliitis was seen in 24/32 (75.0%) cases. There was no significant difference between the two groups in the occurrence of spondylitis. The predominant pattern of sacroiliitis in both AS with psoriasis (15/16, 93.8%) and PsA with radiographic axial involvement (17/24, 70.8%) cases was bilateral, and there was more bilateral involvement in AS with psoriasis cases, but the difference was not statistically significant. Both sacroiliac joints were evaluated in all patients with sacroiliitis. AS cases with psoriasis were more likely to have higher grade sacroiliitis than PsA with radiographic axial involvement cases. Sacroiliitis of grade 3 or 4 was observed in 45.8% of joints assessed in PsA patients with radiographic axial involvement compared to 71.9% in AS patients. Of 23 cases classified as PsA with spondylitis, 10 (43.5%) had spondylitis alone without sacroiliitis. Among 18 PsA with radiographic axial involvement patients with cervical radiographs available, 10 (55.6%) had cervical spine involvement. Among the PsA with radiographic axial involvement cases, non-marginal syndesmophytes (10/23, 43.5%) were more common than marginal syndesmophytes (4/23, 17.4%). Although not statistically significant, PsA with radiographic axial involvement cases were more likely to have cervical spine involvement and non-marginal syndesmophytes than AS with psoriasis cases. We summarized the differences between PsA with radiographic axial involvement and AS in Figure 2.
PsA is remarkably diverse in its presentation and course, with different subtypes. Moll and Wright divided PsA patients into the following five clinical subtypes: oligoarthritis, polyarthritis, spondylitis, DIP only, and arthritis mutilans [1]. Although there have been conflicting reports on the prevalence of each pattern, many studies conducted in Western countries have reported that oligoarthritis (25–65%) or polyarthritis (39–68%) are the most common patterns of PsA [4,16]. In contrast, spondylitis was the most common pattern of PsA (46.4%) in our study, and previous studies in Korea and other Asian countries have concurred that spondylitis is the predominant pattern of PsA [7–9,17]. The total number of patients with peripheral arthritis combining oligoarthritis and polyarthritis that are indistinguishable throughout the disease course was greater than that of spondylitis. Therefore, our results suggest that axial involvement is common in Korean PsA patients, showing similar proportions of oligoarthritis or polyarthritis subtypes to that of Western countries. Also, the variation in the prevalence of each pattern seems to be due to using different definitions of PsA patterns and different evolutions of the arthritic pattern with the duration of arthritis.
The radiographic definition of axial involvement in PsA used in the previous studies has varied from unilateral grade 2 sacroiliitis to radiographic criteria of the mNY criteria; sacroiliitis of grade ≥ 2 or unilateral grade 3 or 4 sacroiliitis [2]. Additionally, there have been studies where the presence of IBP was used to define the presence of axial involvement. In previous studies in Korea, IBP was a criterion for defining spondylitis [8,9]. One study suggested that AxPsA defined by IBP might result in underdiagnosis because radiographic findings of AxPsA can be identified regardless of IBP [18]. To avoid that, we used the definition of PsA with radiographic axial involvement which only required a positive radiographic finding suggestive of sacroiliitis and/or spondylitis regardless of IBP. Therefore, 25% of patients with PsA with radiographic axial involvement had radiographically present but asymptomatic axial disease. Further studies in large cohorts of patients with PsA addressing both radiologic findings and clinical symptoms will be needed to elucidate the best definition of axial involvement of PsA.
Depending on the definition used, about 25 to 75% of patients with PsA have axial involvement [19–21]. Previous studies have found that factors associated with an increased risk of axial involvement in the PsA disease course include male sex, HLA-B*27 positivity, nail dystrophy, greater radiographic damage to peripheral joints, higher ESR, and longer disease duration [12]. The results of a 2018 study showed that PsA patients who exhibited axial involvement at baseline were associated with worse disease, more skin manifestations, more severe joint disease, more enthesitis, and higher CRP levels [22]. Predictors of AxPsA occurrence in our study included a younger age at PsA diagnosis and higher CRP level.
Jadon et al. [23] reported that 24% of each group fulfilled the classification criteria for both conditions. In our study, all of the AS with psoriasis cases (all of which fulfilled the mNY criteria) also fulfilled the CASPAR criteria for PsA. Since these patients all had current psoriasis or a history of psoriasis and RF-negative, so they scored ≥ 3 points in the criterion. Fourteen of 32 (43.8%) AxPsA cases (all fulfilling the CASPAR criteria) also fulfilled the mNY criteria. Two patients were included in both groups. We suggest a high degree of overlap exists between AxPsA and AS, and we sought to define the similarities and differences between them.
HLA-B*27, the cardinal genetic variant associated with AS, is also associated with PsA [24]. However, the prevalence of HLA-B*27 is much lower in PsA (20%) than in AS (80–90%) [12]. To our knowledge, the HLA-B*27 status of patients with AS accompanied by psoriasis has only been reported in two small European cohorts (44 and 80%) [19,25]. Data are needed from larger cohorts with patients of different ethnicities to confirm these findings. HLA-B*39, HLA-B*08, and HLA-B*38 also have been linked to axial disease in patients with PsA, and HLA-Cw*07:02 was associated with AxPsA in a Spanish cohort [12]. In our study, the prevalence of HLA-B*27 was lower in the PsA with radiographic axial involvement group (17.2%) than in the AS with psoriasis group (81.3%). Other HLA loci could not be identified. HLA-B*27 seems to be the main area of genetic overlap between AS and PsA. However, so far, only a small part of the heritability of these diseases has been discovered.
A limited number of studies exist on the incidence of extra-articular manifestations in AS and AxPsA. Rodolfo et al. found that patients with AxPsA more frequently had dactylitis and enthesitis than patients with AS [26]. According to a study by Bengtsson et al. [27], the incidence rate for anterior uveitis was 14.4 events per 1,000 person-years at risk in a AS cohort and 1.7 events per 1,000 person-years at risk in a PsA cohort, i.e., higher in the AS group than the PsA group. Our estimates concerning AS and AxPsA are consistent with those of other studies. Among PsA with radiographic axial involvement cases, dactylitis and enthesitis were more frequent and uveitis was less frequent compared to among AS with psoriasis cases. Although a presumably higher proportion of HLA-B*27 existing in the AS group relative to the PsA group could further explain the difference found, confirmatory data are lacking.
The cardinal manifestation of spinal inflammation is IBP. While IBP is essential for the diagnosis of AS, previous studies found that 45–75% of patients with AxPsA have a history of IBP [12]. Battisone et al. [20] also found that sacroiliitis can occur in the absence of spinal symptoms in PsA. In our study, 8 of 32 (25.0%) PsA patients with radiographic axial involvement had no history of IBP. Axial involvement in PsA is a marker of severity, and those with axial disease often have worse outcomes compared to those with peripheral arthritis alone [2]. Therefore, even in the absence of IBP in patients with PsA, imaging modalities should be used to rule out axial involvement.
There are different radiological characteristics between AxPsA and AS. In AS, syndesmophytes are distributed symmetrically, progress caudally to cranially, and have a classical marginal shape. In AxPsA, syndesmophytes seem to be less symmetrical than in AS and appear more frequently with a coarse, non-marginal shape [12,23]. Similarly, in patients with PsA, syndesmophytes progress randomly along the spine in AxPsA, and 70–75% have cervical radiographic changes [28]. Consistent with this, in our study, PsA with radiographic axial involvement cases had more cervical spine involvement (55.6%) and non-marginal syndesmophytes than AS cases did. Sacroiliitis is more commonly bilateral in AS patients and is either unilateral or bilateral in AxPsA [2]. Gladman et al. [29] reported a higher frequency of grade 4 sacroiliitis in AS patients compared to PsA patients, and grade 3 sacroiliitis was more frequent in our data. Radiographic severity appears to be worse in AS than in AxPsA. Differences in the cytokine profile and the demographics of patients with AxPsA and AS, respectively, are expected to explain the different radiological characteristics of the two diseases [12,30]. As underdiagnosed AxPsA could have impacts on outcomes and therapies, the importance of performing routine screening using imaging methods is emphasized.
Recommendations for the treatment of PsA have been made by both the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the European Alliance of Associations for Rheumatology (EULAR) [31,32]. Each suggests that patients with peripheral disease are responsive to conventional disease-modifying anti-rheumatic drug (DMARD) therapies after trying non-steroidal anti-inflammatory drugs (NSAIDs). In contrast, for axial disease, biological therapies constitute the next medical treatment step after NSAIDs. Tumor necrosis factor inhibitors are recommended, along with conditional recommendations for the use of IL-17 and IL-12/−23 inhibitors. In Korea, PsA is classified as peripheral arthritis, so swelling and tenderness of the peripheral joints must be confirmed to be reimbursed for biologics. Although spondylitis is the most common pattern of PsA in Korea, its evaluation is not included in the reimbursement standards, so it is difficult to administer biologic therapy to patients with AxPsA. Also, in our study, the rate of biologics use in AxPsA patients was lower than that in AS patients (data not presented).
Metrics for axial disease activity and function in AS, including the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Ankylosing Spondylitis Disease Activity Score, and the Bath Ankylosing Spondylitis Functional Index, have been borrowed for the assessment of AxPsA [12]. Although BASDAI could reliably be used in peripheral PsA, it was not sufficient for evaluating axial involvement [33]. Radiographic scoring methods used for axial SpA may be reliable for AxPsA, such as the Bath Ankylosing Spondylitis Radiology Index, mSASSS, and Psoriatic Arthritis Spondylitis Radiology Index (PASRI), the latter of which was developed for AxPsA. PASRI was found to be superior for assessing structural damage in AxPsA [34]. Unfortunately, in our study, disease activity assessment using the above methods was not performed.
Our study has some limitations. First, this was a single-center and retrospective observational study, which might have generated biased data. Second, our study included a single case of synoivits, acne, pustulosis, hyperostosis, and osteitis syndrome and a single case of diffuse idiopathic skeletal hyperostosis, respectively, which may be considered as another type of PsA. Third, because our study relaxed the radiographic criteria compared to previous studies in judging axial involvement, PsA with radiographic axial involvement may have been overdiagnosed. Especially, the assessment of degenerative changes may have led to false-positive evaluations suggesting axial involvement. Despite these limitations, this is the first study to compare PsA with radiographic axial involvement and AS in Asia.
In conclusion, in Asian countries, the prevalence of PsA is lower than in Western countries, and the spondylitis subtype is the most common. AxPsA should not be underdiagnosed because the treatment approach is different from those for other subtypes. Thus, in order to avoid a poor prognosis, axial disease needs to be ruled out using imaging modalities in all patients with PsA regardless of the presence or the nature of back pain.
Notes
CRedit authorship contributions
Hanna Park: data curation, formal analysis, writing - original draft; Ji Hyeon Lee: data curation, writing - review & editing; Seung-Ki Kwok: data curation, writing - review & editing; Ji Hyeon Ju: data curation, writing - review & editing; Wan-Uk Kim: data curation, writing - review & editing; Sung-Hwan Park: data curation, writing - review & editing; Jennifer Jooha Lee: conceptualization, methodology, resources, investigation, data curation, formal analysis, validation, writing - original draft, writing - review & editing, supervision, funding acquisition
Figure 1
Sites of concomitant peripheral involvement in axial psoriatic arthritis. DIP, distal interphalangeal; MCP, metacarpophalangeal; MTP, metatarsophalangeal; PIP, proximal interphalangeal.
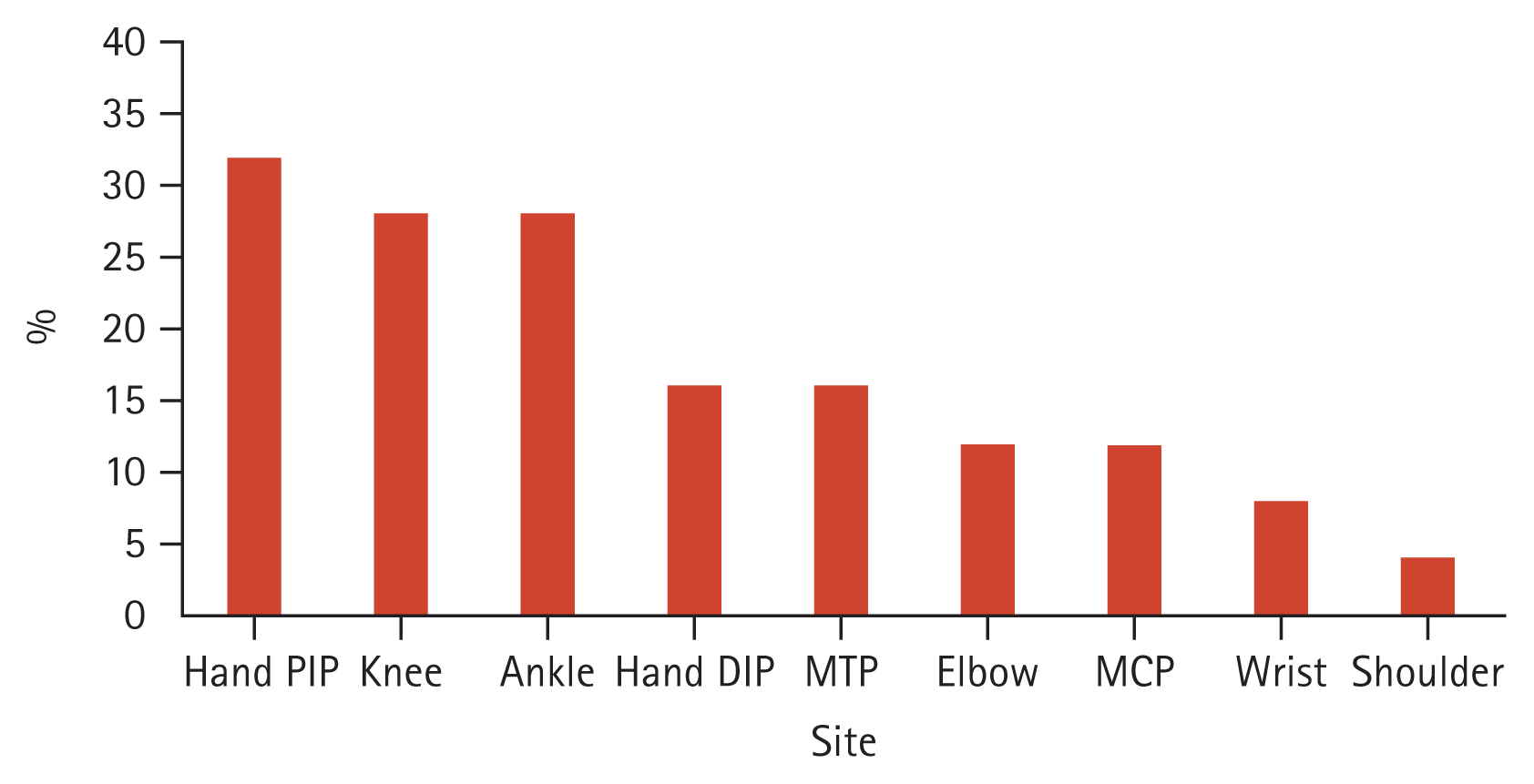
Table 1
Clinical characteristics of patients with PsA
Table 2
Comparison of clinical characteristics of patients with psoriatic arthritis according to axial involvement
| Variable | With axial involvement (n = 32) | Without axial involvement (n = 37) | p value |
|---|---|---|---|
| Sex, male | 19 (59.4) | 19 (51.4) | 0.504 |
| Body mass index (kg/m2) | 25.0 ± 3.3 | 24.5 ± 4.0 | 0.577 |
| Age of diagnosis (yr) | 43.2 ± 13.3 | 50.9 ± 14.0 | 0.023* |
| ESR (mm/h) | 12.5 (9.3–30.3) | 11.0 (3.0–20.0) | 0.114 |
| CRP (mg/L) | 6.2 (1.0–17.5) | 1.4 (0.4–3.2) | 0.003* |
| HLA-B*27 | 5/29 (17.2) | 8/30 (26.7) | 0.363 |
| Nail change | 16 (50.0) | 14 (37.8) | 0.309 |
| Enthesitis | 16 (50.0) | 11 (29.7) | 0.085 |
| Dactylitis | 9 (28.1) | 13 (35.1) | 0.533 |
| Uveitis | 2 (6.3) | 1 (2.7) | 0.593 |
Table 3
Comparison of clinical characteristics between psoriatic arthritis with radiographic axial involvement and ankylosing spondylitis with psoriasis
| Variable | PsA with radiographic axial involvement (n = 32) | AS with psoriasis (n = 16) | p value |
|---|---|---|---|
| Age at diagnosis (yr) | 50.9 ± 14.0 | 32.4 ± 11.8 | < 0.001* |
| Sex, male | 19 (59.4) | 12 (75.0) | 0.286 |
| Body mass index | 25.0 ± 3.3 | 23.2 ± 4.1 | 0.124 |
| ESR (mm/h) | 12.5 (9.3–30.3) | 12.5 (5.0–24.0) | 0.684 |
| CRP (mg/L) | 6.2 (1.0–17.5) | 1.7 (0.5–5.2) | 0.081 |
| Peripheral joints affected | 25 (78.1) | 2 (12.5) | < 0.001* |
| Nail change | 16 (50.0) | 0 (0.0) | 0.001* |
| Dactylitis | 9 (28.1) | 2 (12.5) | 0.293 |
| Enthesitis | 16 (50.0) | 1 (6.3) | 0.003* |
| Uveitis | 2 (6.3) | 5 (31.3) | 0.033* |
| HLA-B*27 | 5/29 (17.2) | 13 (81.3) | < 0.001* |
| Inflammatory back pain | 24 (75.0) | 16 (100.0) | 0.039* |
Table 4
Comparison of radiologic findings between PsA with radiographic axial involvement and AS with psoriasis
| Variable | PsA with radiographic axial involvement (%) | AS with psoriasis (%) | p value |
|---|---|---|---|
| Radiographic | |||
| Sacroiliitis | 24/32 (75.0) | 16/16 (100.0) | 0.039* |
| Spondylitis | 23/32 (71.9) | 12/16 (75.0) | > 0.999 |
| Sacroiliac joint | |||
| Bilateral involvement | 17/24 (70.8) | 15/16 (93.8) | 0.114 |
| Symmetrical grade | 14/24 (58.3) | 10/16 (62.5) | 0.792 |
| Grade 2 | 19/48 (39.6) | 9/32 (28.1) | 0.004* |
| Grade 3 | 17/48 (35.4) | 20/32 (62.5) | 0.017* |
| Grade 4 | 5/48 (10.4) | 3/32 (9.4) | > 0.999 |
| Vertebral | |||
| Without sacroilitis | 10/23 (43.5) | 0/12 (0.0) | 0.007* |
| Cervical spine involvement | 10/18 (55.6) | 3/12 (25.0) | 0.098 |
| Marginal syndesmophyte | 4/23 (17.4) | 2/12 (16.7) | > 0.999 |
| Non-marginal syndesmophyte | 10/23 (43.5) | 1/12 (8.3) | 0.055 |
REFERENCES
2. Yap KS, Ye JY, Li S, Gladman DD, Chandran V. Back pain in psoriatic arthritis: defining prevalence, characteristics and performance of inflammatory back pain criteria in psoriatic arthritis. Ann Rheum Dis 2018;77:1573–1577.


3. Helliwell PS, Porter G, Taylor WJ, CASPAR Study Group. Polyarticular psoriatic arthritis is more like oligoarticular psoriatic arthritis, than rheumatoid arthritis. Ann Rheum Dis 2007;66:113–117.



4. Gladman DD, Ye JY, Chandran V, Lee KA, Cook RJ. Oligoarticular vs polyarticular psoriatic arthritis: a longitudinal study showing similar characteristics. J Rheumatol 2021;48:1824–1829.


5. McInnes IB, Anderson JK, Magrey M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med 2021;384:1227–1239.


6. Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis 2021;80:582–590.



7. Shin D, Kim HJ, Kim DS, et al. Clinical features of psoriatic arthritis in Korean patients with psoriasis: a cross-sectional observational study of 196 patients with psoriasis using psoriatic arthritis screening questionnaires. Rheumatol Int 2016;36:207–212.



8. Baek HJ, Yoo CD, Shin KC, et al. Spondylitis is the most common pattern of psoriatic arthritis in Korea. Rheumatol Int 2000;19:89–94.



9. Choi HJ, Lee YJ, Park JJ, et al. Clinical features of Korean patients with psoriatic arthritis. Korean J Med 2008;74:418–425.
11. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–2673.


12. Feld J, Chandran V, Haroon N, Inman R, Gladman D. Axial disease in psoriatic arthritis and ankylosing spondylitis: a critical comparison. Nat Rev Rheumatol 2018;14:363–371.



14. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–368.

15. van der Heijde D, Braun J, Deodhar A, et al. Modified stoke ankylosing spondylitis spinal score as an outcome measure to assess the impact of treatment on structural progression in ankylosing spondylitis. Rheumatology (Oxford) 2019;58:388–400.



16. Helliwell P, Marchesoni A, Peters M, Barker M, Wright V. A re-evaluation of the osteoarticular manifestations of psoriasis. Br J Rheumatol 1991;30:339–345.


17. Tsai YG, Chang DM, Kuo SY, Wang WM, Chen YC, Lai JH. Relationship between human lymphocyte antigen-B27 and clinical features of psoriatic arthritis. J Microbiol Immunol Infect 2003;36:101–104.

18. Aydin SZ, Kucuksahin O, Kilic L, et al. Axial psoriatic arthritis: the impact of underdiagnosed disease on outcomes in real life. Clin Rheumatol 2018;37:3443–3448.



19. Hanly JG, Russell ML, Gladman DD. Psoriatic spondyloarthropathy: a long term prospective study. Ann Rheum Dis 1988;47:386–393.



20. Battistone MJ, Manaster BJ, Reda DJ, Clegg DO. The prevalence of sacroilitis in psoriatic arthritis: new perspectives from a large, multicenter cohort. A Department of Veterans Affairs Cooperative Study. Skeletal Radiol 1999;28:196–201.

21. Taylor WJ, Zmierczak HG, Helliwell PS. Problems with the definition of axial and peripheral disease patterns in psoriatic arthritis. J Rheumatol 2005;32:974–977.

22. Mease PJ, Palmer JB, Liu M, et al. Influence of axial involvement on clinical characteristics of psoriatic arthritis: analysis from the corrona psoriatic arthritis/spondyloarthritis registry. J Rheumatol 2018;45:1389–1396.


23. Jadon DR, Sengupta R, Nightingale A, et al. Axial Disease in Psoriatic Arthritis study: defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann Rheum Dis 2017;76:701–707.



24. Chandran V, Bull SB, Pellett FJ, Ayearst R, Rahman P, Gladman DD. Human leukocyte antigen alleles and susceptibility to psoriatic arthritis. Hum Immunol 2013;74:1333–1338.


25. Machado P, Landewé R, Braun J, et al. Ankylosing spondylitis patients with and without psoriasis do not differ in disease phenotype. Ann Rheum Dis 2013;72:1104–1107.


26. Pérez Alamino R, Maldonado Cocco JA, Citera G, et al.; RESPONDIA Group. Differential features between primary ankylosing spondylitis and spondylitis associated with psoriasis and inflammatory bowel disease. J Rheumatol 2011;38:1656–1660.


27. Bengtsson K, Forsblad-d’Elia H, Deminger A, et al. Incidence of extra-articular manifestations in ankylosing spondylitis, psoriatic arthritis and undifferentiated spondyloarthritis: results from a national register-based cohort study. Rheumatology (Oxford) 2021;60:2725–2734.




29. Gladman DD, Brubacher B, Buskila D, Langevitz P, Farewell VT. Differences in the expression of spondyloarthropathy: a comparison between ankylosing spondylitis and psoriatic arthritis. Clin Invest Med 1993;16:1–7.

30. Turkiewicz AM, Moreland LW. Psoriatic arthritis: current concepts on pathogenesis-oriented therapeutic options. Arthritis Rheum 2007;56:1051–1066.


31. Coates LC, Kavanaugh A, Mease PJ, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–1071.

32. Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–712.

-
METRICS

-
- 0 Crossref
- 0 Scopus
- 62 View
- 115 Download
- Related articles
-
Clinical Characteristics of Colonic Diverticulosis in Korea: A Prospective Study2010 June;25(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


