 |
 |
| Korean J Intern Med > Volume 28(5); 2013 > Article |
|
Abstract
Background/Aims
We investigated the clinical characteristics and follow-up findings of subjects with adrenal incidentalomas in a single, tertiary-care hospital in South Korea.
Methods
The study consisted of a retrospective analysis of 282 adrenal incidentaloma patients who underwent radiographic and endocrinological evaluations at Samsung Medical Center in Seoul, South Korea, between January 2004 and July 2011.
Results
Most (86.2%) of the subjects were found to have nonfunctioning tumors. Functioning tumors were seen in 39 patients (13.8%). Among them, 28 (9.9%) had subclinical Cushing syndrome (SCS), six (2.1%) had pheochromocytoma, and five (1.8%) had primary hyperaldosteronism. Malignant adrenal tumors were discovered in three cases: two (0.7%) were primary adrenal cancers, and one (0.4%) was a secondary metastasis from a lung cancer. Significant risk factors for functional tumors were female gender (odds ratio [OR], 3.386; 95% confidence interval [CI], 1.611 to 7.117; p = 0.0013) and a noncontrast attenuation value of > 10 Hounsfield units (OR, 2.806; 95% CI, 1.231 to 6.397; p = 0.0141). During follow-up (mean, 22.5 months) of 72 of the patients, three (4.2%) developed hormonal changes due to functional tumors. One was confirmed as pheochromocytoma by histopathology, and the others were diagnosed with SCS and followed routinely without surgical intervention. No malignant transformation was found in these patients.
Adrenal incidentalomas are defined as clinically nonapparent adrenal masses that are discovered during diagnostic testing or treatment for nonadrenal disease. Adrenal masses are one of the most prevalent tumors in humans, and the reported prevalence is increasing with the continued advances in imaging technology, making the management of adrenal incidentalomas a challenge for modern medicine [1].
Based on clinical studies, the prevalence of adrenal incidentaloma is ~4% overall, with nearly 80% of these masses found to be benign [1-5]. Although functional or malignant adrenal masses represent only a small proportion of adrenal incidentalomas, these cases require immediate management [6,7]. There have been numerous studies regarding adrenal incidentalomas since they were first described [8,9]. Most of the large-scale studies were conducted in Western patients [4,5]. Few studies with small numbers of patients have reported the clinical findings of adrenal incidentalomas in Asians [10-13]. One previous Korean report showed a prevalence of pheochromocytoma of up to 20% [13]. This was a significantly higher prevalence than in Western data (range, 1.5% to 14%) [4,5,14]. Additionally, follow-up strategies for adrenal incidentaloma are still under debate.
Thus, this study was designed to investigate the clinical characteristics and follow-up findings of 282 patients with adrenal incidentalomas who presented at a tertiary-care hospital in South Korea. To our knowledge, this is the largest reported study of adrenal incidentaloma data in a Korean population.
We performed a retrospective review of consecutive patients aged 18 years or older with adrenal lesions discovered via computed tomography (CT) at Samsung Medical Center in Seoul, South Korea, between January 2004 and July 2011. Of 448 patients, 166 were excluded due to: 1) signs or symptoms of adrenal disease (i.e., severe or paroxysmal hypertension, frank hypokalemia, Cushingoid features, or referral from another hospital for adrenal examination, n = 93); 2) adrenal tumor smaller than 1.0 cm (n = 13); 3) adrenal thickening or hyperplasia (n = 32); or 4) missing baseline characteristics or an incomplete hormonal evaluation (n = 28). After applying these exclusion criteria, the total number of subjects eligible for the study was 282 (172 males, 110 females). The subjects underwent radiographic and hormonal evaluations. Among them, 147 were followed for more than 6 months.
We performed a retrospective review of the medical records of each patient, examining the medical history, anthropometric data, blood pressure, fasting glucose level, and lipid profile. The pathology database at Samsung Medical Center was used to provide information on all patients who had undergone adrenalectomies or biopsies. All patients were initially diagnosed and followed-up by CT. A GE 64 VCT Lightspeed (General Electric Co., Fairfield, CT, USA) CT scanner was used for adrenal imaging. The CT findings were reported by radiologists who were experts at abdominal imaging. If the adrenal lesions contained multiple masses, all measurable lesions were described in the formal report, and the sum of all tumor volumes was used for analysis.
The hormonal evaluation included 8:00 AM. serum basal cortisol level, urinary free cortisol level, overnight 1 mg dexamethasone suppression test (DST) result, plasma renin activity, serum aldosterone level, urinary vanillylmandelic acid (VMA), metanephrine, and normetanephrine levels. Blood cortisol and urinary free cortisol were measured using a radioimmunoassay (RIA) cortisol kit (Beckman Coulter Inc., Brea, CA, USA). Plasma renin activity was measured using a plasma renin activity RIA kit (DiaSorin Inc., Stillwater, MN, USA), and serum aldosterone was measured using an aldosterone RIA kit. Urinary VMA and metanephrines were measured using high performance liquid chromatography (Agilent Technologies, Santa Clara, CA, USA).
Autonomous cortisol secretion was excluded when post-DST cortisol levels fell below 2.0 µg/dL, as recommended by the National Institutes of Health (NIH), based on its higher sensitivity for detecting subclinical Cushing syndrome (SCS) [3]. Patients were considered to have SCS if post-DST cortisol level remained above 2.0 µg/dL. Pheochromocytoma was defined as an elevated urinary VMA, metanephrine, and/or normetanephrine level. Aldosterone/plasma renin activity ratio (ARR) was the screening measure for primary hyperaldosteronism, and patients with ARR > 30 were suspected of having primary hyperaldosteronism; a saline infusion test was performed to confirm this diagnosis.
Adrenalectomy was recommended in patients with overt hormonal hypersecretion, a significantly large (diameter > 4 cm) or growing mass, or radiographic features suspicious of malignancy. Follow-up radiographic evaluations were performed by CT at 6 and 12 months after the initial visit and annually in subsequent visits. Hormonal evaluations were performed at 6 months after the initial visit and annually at subsequent visits.
Statistical analyses were performed using the SAS software version 9.1.3 (SAS Institute Inc., Cary, NC, USA). All data are summarized as means ± SD or as numbers with a percentage. For all statistical analyses, a two-sided p < 0.05 was considered to indicate statistical significance. All variables that resulted in a p < 0.05 in univariate analyses were entered into a logistic regression analysis to assess independent associations between risk factors and functional adrenal tumors.
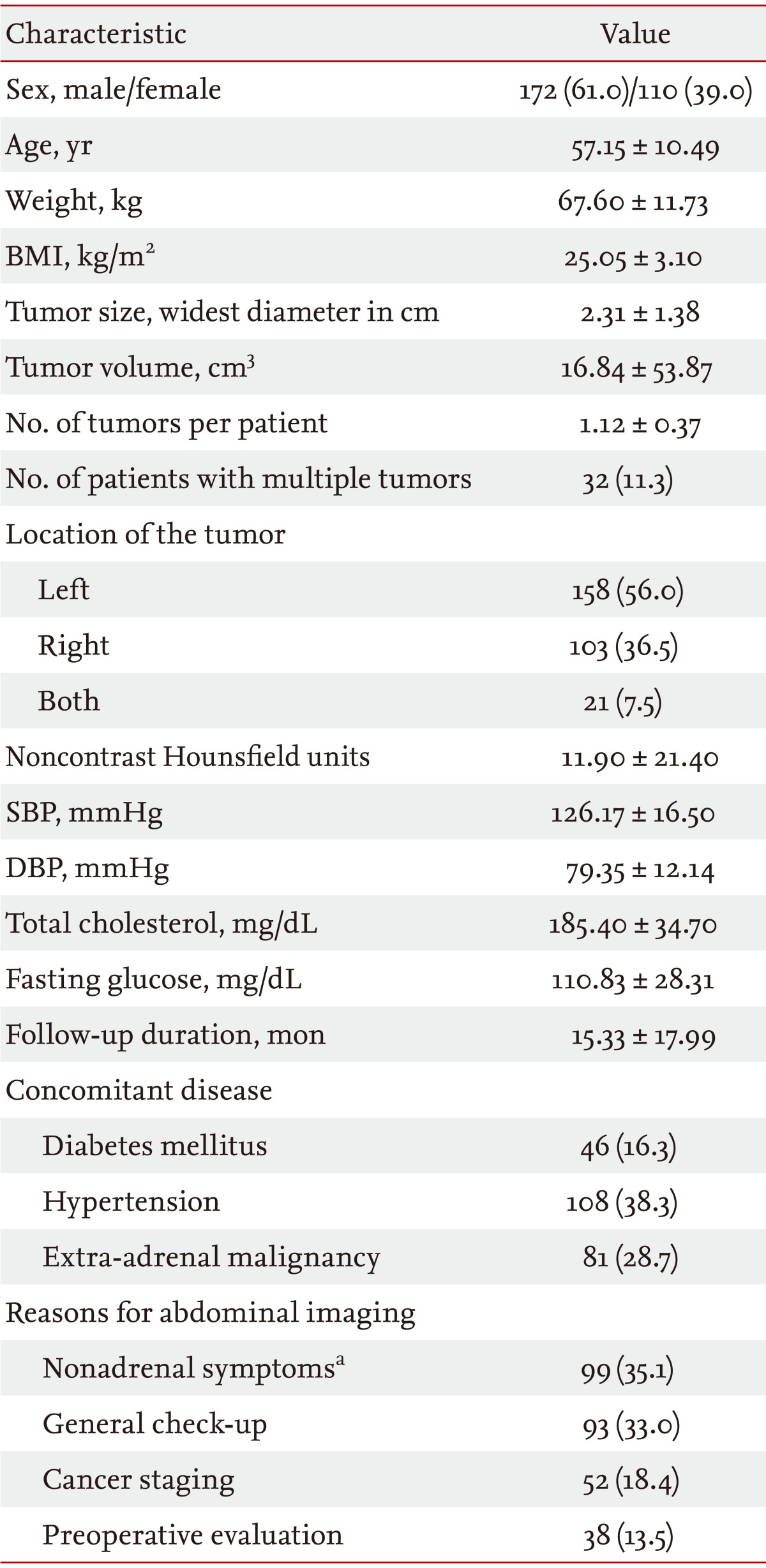
From January 2004 to July 2011, 448 patients with adrenal masses were treated in our institution, with 282 patients deemed suitable for this study. The most common cause of abdominal imaging was nonadrenal symptoms (35.1%), including abdominal pain, fever, and hematuria. Other causes were general check-ups (33.0%), cancer staging (18.4%), and preoperative evaluations (13.5%).
Table 1 shows the clinical characteristics of the patients with adrenal incidentalomas. Of the 282 patients, 172 (61.0%) were males. The mean age of the participants was 57.2 years. Most (88.7%) of the adrenal incidentalomas were single masses. The adrenal tumors were located on the left adrenal gland in 158 patients (56.0%), right adrenal gland in 103 (36.5%), and bilaterally in 21 (7.5%). Concomitant extra-adrenal malignancies were found in 81 patients (28.7%). The mean noncontrast Hounsf ield units (HU) of the adrenal masses was 11.9. The primary site of metastatic tumor was the gastrointestinal tract in 39 patients (48.1%), genitourinary tract in 19 (23.5%), lung in seven (8.6%), thyroid in five (6.2%), and various other locations in the remaining 11 patients (13.6%).
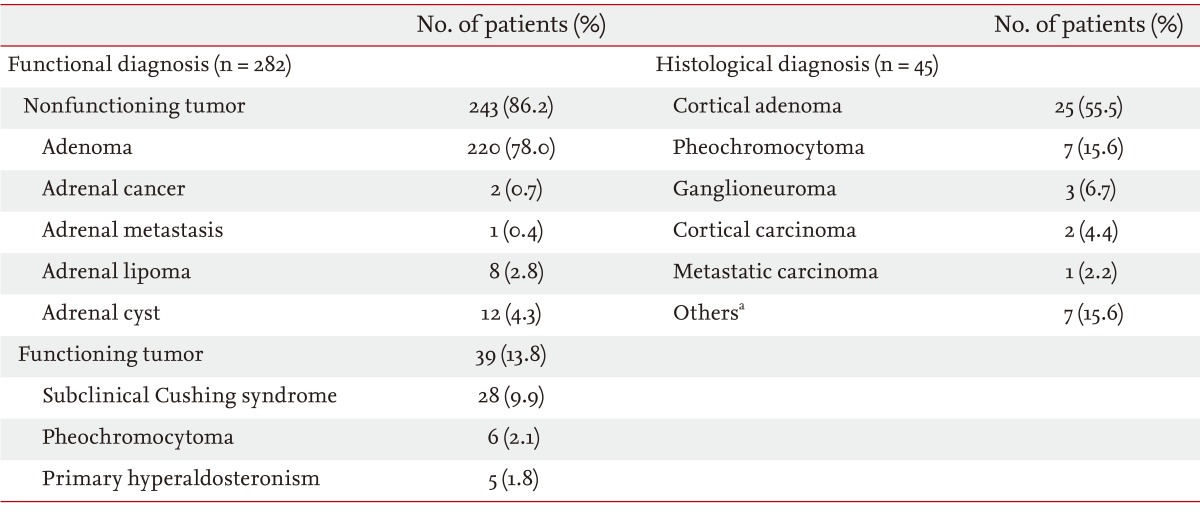
Table 2 shows the functional and histological diagnoses of patients with adrenal incidentalomas. Most (n = 243, 86.2%) cases were nonfunctioning tumors. Of the 39 patients with functional tumors (13.8%), 28 (9.9%) were diagnosed with SCS, six (2.1%) with pheochromocytoma, and five (1.8%) with primary hyperaldosteronism. Histological diagnoses were made in 45 patients (44 underwent adrenalectomies, and one patient had an ultrasound-guided biopsy). Primary adrenal cancer (both adrenocortical carcinomas) was discovered in two patients (0.7%). Adrenal metastasis was detected in one patient (0.4%) who had been previously diagnosed with primary lung cancer. Reasons for adrenalectomies were functional tumors (n = 23, 52.3%), large mass (> 4 cm in diameter; n = 11, 25.0%), growing mass (n = 4, 9.1%), primary adrenal cancer (n = 2, 4.5%), adrenal metastasis (n = 1, 2.3%), and resection during another abdominal surgery (n = 3, 6.8%).
Among the patients with functional tumors, 18 did not undergo an adrenalectomy (17 with SCS, one with hyperaldosteronism). Of them, 11 refused surgical treatment, one was at high risk for complications of general anesthesia, four showed normal post-DST cortisol levels at follow-up, one had been treated medically for bilateral adrenal hyperplasia with hyperaldosteronism, and one was lost to follow-up.
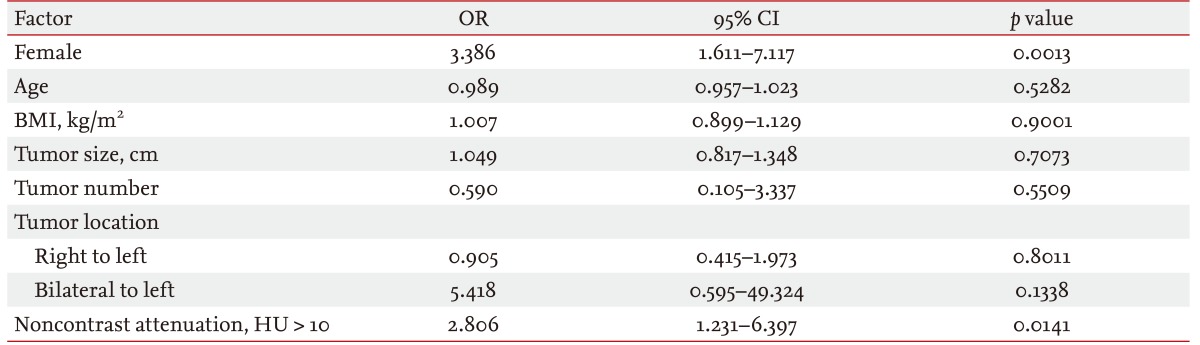
Table 3 shows the risk factors for a functional adrenal tumor. Female gender (odds ratio [OR], 3.386; 95% confidence interval [CI], 1.611 to 7.117; p = 0.0013) and a noncontrast attenuation value of > 10 HU (OR, 2.806; 95% CI, 1.231 to 6.397; p = 0.0141) were independent risk factors for functional adrenal incidentalomas. Age, BMI, tumor size, number, and location were not statistically significant factors for this tumor type.
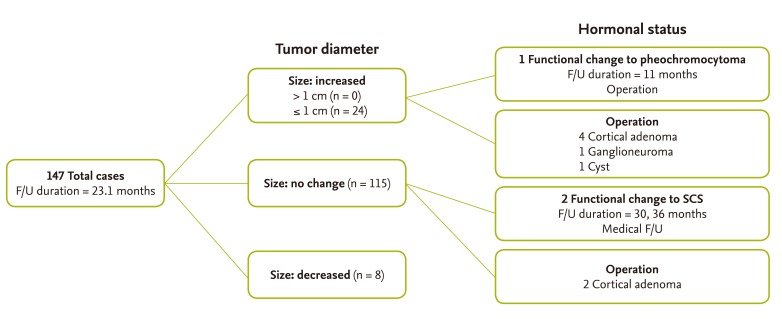
Fig. 1 shows the radiographic and hormonal follow-up of the adrenal incidentalomas. The mean duration of follow-up was 23.1 months. Of the 147 patients who were re-evaluated, 147 received only adrenal CT and 72 were re-evaluated with both adrenal CT and hormonal studies. Most patients showed no change in the diameter of the adrenal mass. Among patients with an increase in size, all experienced an increase less than 1.0 cm. In our data, age, gender, BMI, tumor size, and the attenuation value of noncontrast HU were not significant factors for patients who showed an increase in the diameter of the masses (data not shown). Hormonal changes in a functional tumor were detected in three patients. One was a 48-year-old male patient, initially diagnosed as having a nonfunctioning tumor during a general check-up. At the 9-month follow-up, the patient was found to have slightly elevated urine metanephrines and a small increasing atypical adrenal mass (mass diameter from 2.0 to 2.1 cm). This patient was histopathologically confirmed to have pheochromocytoma after an adrenalectomy. The two other patients were diagnosed with SCS at 30 and 36 months after the initial visit. The first was a 67-year-old male who had been treated previously for stomach cancer. On routine follow-up CT, multiple adrenal masses were detected and the initial hormonal evaluation showed nonfunctioning adrenal lesions. At the 30-month follow-up, the post-DST cortisol level showed 2.4 µg/dL and the repeated post-DST cortisol was not suppressed. The second subject was a previously healthy 63-year-old male who was diagnosed with a nonfunctioning adrenal mass during a general check-up. The diameter of the adrenal mass was unchanged; however, post-DST cortisol level was elevated, up to 4.0 µg/dL at 36 months after the initial visit. These two patients were followed medically for SCS due to patient preference. In this study, no malignant transformation occurred.
This study involved a retrospective review of the clinical characteristics and follow-up of 282 patients with adrenal incidentalomas. Most of the tumors were nonfunctioning, benign adrenal masses. In our data, female gender and noncontrast HU value more than 10 were found to be significant risk factors associated with a functional tumor. During follow-up (mean, 22.5 months) of 72 subjects, only three showed a change in hormone levels.
In this study, 243 patients (86.2%) were diagnosed with nonfunctioning tumors, 28 patients (9.9%) with SCS, six (2.1%) with pheochromocytoma, and five (1.8%) with primary hyperaldosteronism. The composition of the adrenal tumors was similar to that in Western studies [4,5,14]. In a previous Korean study, pheochromocytoma was seen at a much higher incidence, of up to 20%, of adrenal incidentalomas [13]. However, a separate Korean study and our data suggest that the incidence of pheochromocytoma was much lower, similar to that seen in Western data [11].
In the present study, more than half (56.0%) of the adrenal incidentalomas were found on the left side. Mantero et al. [5] reported adrenal incidentalomas to be more frequent on the right side, although this was likely due to the diagnostic modality used. Mantero et al. [5] used ultrasonography (US) imaging as their diagnostic technique in most cases, which might allow for greater visualization of the right adrenal gland than the left [15]. Other studies using US scan have shown a right-sided predominance (50% to 60% of cases) of adrenal incidentalomas [16,17]. However, no difference in location was apparent in studies evaluated using CT scans [18,19] or at autopsy [14].
In our series, more males were included in the total group (172 males, 110 females). Many studies have reported that adrenal incidentalomas are found more frequently in females (female to male ratio, 1.3 to 1.5:1), although this could also be due to a generally higher rate of abdominal imaging in females than in males [5,18,20-22]. The gender difference in our series could also be related to reliance on data from a single, tertiary-care hospital. Studies of autopsies have demonstrated no gender preferences in adrenal incidentalomas [3,23].
Our data also revealed that females were more likely to have functional tumors. The female predominance of functional tumors may be a consequence of the type of tumor itself. The most frequent hormonal imbalance in this study was SCS, which has been shown in previous studies to have a female predominance [24,25]. The higher attenuation value of noncontrast HU was another risk factor of functional tumors. It is known that up to 70% of adrenal adenomas contain intracellular fat [1,3]. Lipid-rich adrenal masses that have an attenuation of ≤ 10 HU suggest adrenal adenomas, with a sensitivity of 96% to 100% and a specificity of 50% to 100% [26-28]. In this study, the mean ± SD noncontrast HU values of functioning tumors was 20.4 ± 16.6 and that of nonfunctioning tumors was 10.5 ± 21.8. In the multivariate analysis with a 10-HU cutoff value, functioning tumors tended to have a density over 10 HU (OR, 2.806; 95% CI, 1.231 to 6.397; p = 0.0141). This might be because most nonfunctioning tumors consisted of adrenal adenomas.
In the present study, age, BMI, tumor size, number, and location were not independent risk factors for functional tumors. One series reported that older patients were more likely to develop hormonal hyperfunction, although the association was not significant [25]. Several retrospective or cross-sectional studies have reported increased frequencies of hypertension, dyslipidemia, osteoporosis, and obesity in patients with SCS [29,30]. However, some authors have reported no significant difference in the BMI of patients with SCS and nonfunctioning tumors [5,31]. Several studies demonstrated that tumor size greater than 3 cm at diagnosis was related to the occurrence of hormonal hyperfunction [25,32]. One Korean study reported that age, tumor size, number, and location showed no statistical significance as risk factors for functioning tumors [13]. Interpretation of these data must be considered with caution because of the possibility of confounding and referral biases related to the limitations in the design of these studies.
Among 72 subjects, three cases (4.2%) showed hormonal changes during a mean follow-up of 22.5 months. Two of them were diagnosed with SCS and agreed to be routinely followed without surgical intervention. The other was suspicious for pheochromocytoma at 9 months after the initial visit and underwent surgical intervention at 11 months. None of the adrenal tumors became malignant during the follow-up period. Several studies have also found that most adrenal lesions remain stable, with relatively few patients developing malignancy or clinically apparent functional lesions [25,33]. Barzon et al. [20] reported that functional change developed in about 1.7% of cases during follow-up, with SCS being the most common form.
There is to date no consensus as to the optimum strategy for follow-up of adrenal incidentaloma. The NIH consensus statement suggests repeating overnight 1-mg DST and urinary catecholamine and metabolite levels at annual intervals for 4 years because the risk of overt hyperfunction appears to plateau after that period [3]. In patients without surgical removal of the adrenal lesion, CT follow-up is recommended at 6 to 12 months after initial evaluation. If the lesions are not increasing, further radiographic evaluation is not needed [3]. Young recommended repeated CT imaging at 6, 12, and 24 months, combined with annual repeat hormone tests for 4 years [34]. Our hospital has conducted an evaluation of patients with adrenal incidentalomas according to the NIH consensus recommendations. A recent article suggested finding an optimal balance in the assessment and management of adrenal incidentalomas, given the significant diagnostic costs, cancer risk related to radiation exposure, and high rate of false-positive diagnoses [35]. Our data support this recommendation.
This study is limited in its ability to establish any causal relationship due to the retrospective design. Another weakness is that all patients were diagnosed at a single, tertiary-care medical center. These data may therefore not be representative of the entire Korean population. However, this study offers the advantage of being the largest source of adrenal incidentaloma data for Korean patients.
In conclusion, initial hormonal and radiographic evaluation for adrenal incidentaloma is more important than follow-up evaluations due to the low rate of development of malignant or functional tumors.
1. Most of the adrenal tumors were nonfunctioning and benign in 282 Korean patients with adrenal incidentalomas.
2. Female gender and noncontrast Hounsf ield units value of > 10 were significant risk factors for functional adrenal tumors.
3. During follow-up (mean, 22.5 months) of 72 subjects, development of malignant or functional tumors was rare.
References
1. Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev 2004;25:309–340PMID : 15082524.


2. Bovio S, Cataldi A, Reimondo G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest 2006;29:298–302PMID : 16699294.


3. Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass ("incidentaloma"). Ann Intern Med 2003;138:424–429PMID : 12614096.


4. Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol 2003;149:273–285PMID : 14514341.


5. Mantero F, Terzolo M, Arnaldi G, et al. A survey on adrenal incidentaloma in Italy: Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab 2000;85:637–644PMID : 10690869.


6. Allolio B, Fassnacht M. Clinical review: adrenocortical carcinoma: clinical update. J Clin Endocrinol Metab 2006;91:2027–2037PMID : 16551738.


7. Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet 2005;366:665–675PMID : 16112304.


8. Prinz RA, Brooks MH, Churchill R, et al. Incidental asymptomatic adrenal masses detected by computed tomographic scanning: is operation required? JAMA 1982;248:701–704PMID : 7097921.


9. Geelhoed GW, Druy EM. Management of the adrenal "incidentaloma". Surgery 1982;92:866–874PMID : 7135206.

10. Ng VW, Ma RC, So WY, et al. Evaluation of functional and malignant adrenal incidentalomas. Arch Intern Med 2010;170:2017–2020PMID : 21149760.


11. Jeong HS, Kim HJ, Kim HS, et al. Clinical characteristics for 132 patients with adrenal incidentaloma. J Korean Endocr Soc 2007;22:260–265.

12. Murai M, Baba S, Nakashima J, Tachibana M. Management of incidentally discovered adrenal masses. World J Urol 1999;17:9–14PMID : 10096145.


13. Kim HY, Kim SG, Lee KW, et al. Clinical study of adrenal incidentaloma in Korea. Korean J Intern Med 2005;20:303–309PMID : 16491828.



14. Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev 1995;16:460–484PMID : 8521790.


15. Yeh HC. Sonography of the adrenal glands: normal glands and small masses. AJR Am J Roentgenol 1980;135:1167–1177PMID : 6779521.


16. Barzon L, Scaroni C, Sonino N, et al. Incidentally discovered adrenal tumors: endocrine and scintigraphic correlates. J Clin Endocrinol Metab 1998;83:55–62PMID : 9435416.


17. Flecchia D, Mazza E, Carlini M, et al. Reduced serum levels of dehydroepiandrosterone sulphate in adrenal incidentalomas: a marker of adrenocortical tumour. Clin Endocrinol (Oxf) 1995;42:129–134PMID : 7704956.


18. Herrera MF, Grant CS, van Heerden JA, Sheedy PF, Ilstrup DM. Incidentally discovered adrenal tumors: an institutional perspective. Surgery 1991;110:1014–1021PMID : 1745970.

19. Abecassis M, McLoughlin MJ, Langer B, Kudlow JE. Serendipitous adrenal masses: prevalence, significance, and management. Am J Surg 1985;149:783–788PMID : 4014556.


20. Barzon L, Fallo F, Sonino N, Boscaro M. Development of overt Cushing's syndrome in patients with adrenal incidentaloma. Eur J Endocrinol 2002;146:61–66PMID : 11751069.


21. Bulow B, Ahren B. Swedish Research Council Study Group of Endocrine Abdominal Tumours. Adrenal incidentaloma: experience of a standardized diagnostic programme in the Swedish prospective study. J Intern Med 2002;252:239–246PMID : 12270004.


22. Kasperlik-Zeluska AA, Roslonowska E, Slowinska-Srzednicka J, et al. Incidentally discovered adrenal mass (incidentaloma): investigation and management of 208 patients. Clin Endocrinol (Oxf) 1997;46:29–37PMID : 9059555.


23. Russell RP, Masi AT, Richter ED. Adrenal cortical adenomas and hypertension: a clinical pathologic analysis of 690 cases with matched controls and a review of the literature. Medicine (Baltimore) 1972;51:211–225PMID : 5021770.


24. Comlekci A, Yener S, Ertilav S, et al. Adrenal incidentaloma, clinical, metabolic, follow-up aspects: single centre experience. Endocrine 2010;37:40–46PMID : 19882253.


25. Barzon L, Scaroni C, Sonino N, Fallo F, Paoletta A, Boscaro M. Risk factors and long-term follow-up of adrenal incidentalomas. J Clin Endocrinol Metab 1999;84:520–526PMID : 10022410.


26. Hamrahian AH, Ioachimescu AG, Remer EM, et al. Clinical utility of noncontrast computed tomography attenuation value (hounsf ield units) to differentiate adrenal adenomas/hyperplasias from nonadenomas: Cleveland Clinic experience. J Clin Endocrinol Metab 2005;90:871–877PMID : 15572420.


27. Korobkin M, Brodeur FJ, Yutzy GG, et al. Differentiation of adrenal adenomas from nonadenomas using CT attenuation values. AJR Am J Roentgenol 1996;166:531–536PMID : 8623622.


28. Cirillo RL Jr, Bennett WF, Vitellas KM, Poulos AG, Bova JG. Pathology of the adrenal gland: imaging features. AJR Am J Roentgenol 1998;170:429–435PMID : 9456959.


29. Terzolo M, Bovio S, Pia A, et al. Midnight serum cortisol as a marker of increased cardiovascular risk in patients with a clinically inapparent adrenal adenoma. Eur J Endocrinol 2005;153:307–315PMID : 16061838.


30. Tauchmanova L, Rossi R, Biondi B, et al. Patients with subclinical Cushing's syndrome due to adrenal adenoma have increased cardiovascular risk. J Clin Endocrinol Metab 2002;87:4872–4878PMID : 12414841.


31. Rossi R, Tauchmanova L, Luciano A, et al. Subclinical Cushing's syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J Clin Endocrinol Metab 2000;85:1440–1448PMID : 10770179.


32. Libe R, Dall'Asta C, Barbetta L, Baccarelli A, Beck-Peccoz P, Ambrosi B. Long-term follow-up study of patients with adrenal incidentalomas. Eur J Endocrinol 2002;147:489–494PMID : 12370111.


33. Song JH, Chaudhry FS, Mayo-Smith WW. The incidental indeterminate adrenal mass on CT (> 10 H) in patients without cancer: is further imaging necessary? Follow-up of 321 consecutive indeterminate adrenal masses. AJR Am J Roentgenol 2007;189:1119–1123PMID : 17954649.


34. Young WF Jr. Clinical practice: the incidentally discovered adrenal mass. N Engl J Med 2007;356:601–610PMID : 17287480.


35. Cawood TJ, Hunt PJ, O'Shea D, Cole D, Soule S. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant: time for a rethink? Eur J Endocrinol 2009;161:513–527PMID : 19439510.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



