 |
 |
| Korean J Intern Med > Volume 38(3); 2023 > Article |
|
Abstract
Although some clinical trials have demonstrated reduced incidence of cardiovascular disease with the use of omega-3 fatty acids, others have found an increased risk of atrial fibrillation (AF). AF is the most common sustained cardiac arrhythmia worldwide. It is associated with high morbidity and mortality rates and significant public health burden. Previous studies of the effect of omega-3 fatty acids on AF occurrence have reported contradictory results. Here we reviewed the effect of omega-3 fatty acids on the risk of AF.
A healthy diet requires fish intake at least twice a week. The popular belief that fish oil improves cardiovascular (CV) health has fueled a lucrative global market for omega-3 fatty acid supplements. However, it is unknown whether marine-based omega-3 fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), prevent cardiovascular disease (CVD). Omega-3 fatty acid supplementation is used to prevent CVD in patients with elevated plasma triglyceride level [1–3]. More than 20 clinical trials have confirmed the benefits of omega-3 fatty acid supplementation and have reported varying results. Therefore, although omega-3 fatty acids have promising health benefits, certain issues remain unresolved.
Atrial fibrillation (AF) is a common cardiac arrhythmia; its prevalence increases with age, from < 0.5% in individuals in their 40s to 10% in individuals aged > 80 years [4]. Strategies to prevent AF and its complications, such as stroke, are important because of the significant morbidity, mortality, and economic costs of AF. Previous animal studies showed that omega-3 fatty acids have anti-arrhythmic effects [5] and treatment with n-3 fatty acids reduces the AF risk in rabbit models [6]. Additionally, n-3 fatty acids suppress structural remodeling and atrial fibrosis in canine models, which reduce AF occurrence [7]. Although animal model studies have shown that n-3 polyunsaturated fatty acids (PUFAs) from seafood reduce the development of AF, human studies have shown conflicting results in this regard [8]. A meta-analysis of population-based cohort studies and randomized controlled trials (RCTs) showed no significant role of n-3 fatty acids in the secondary prevention of AF after cardioversion [9,10]. Additionally, accumulating evidence from recent large-scale RCTs suggests that the benefits of omega-3 fatty acid supplementation are at the expense of an increased risk of AF.
Because of the contradictory results of previous studies, the effect of omega-3 fatty acid supplementation on the risk of AF should be evaluated. Differences in the dose of omega-3 fatty acids used in previous studies might account for the apparent discrepancy in the effects on AF. Here we reviewed the effect of omega-3 fatty acids on the risk of AF. Given the increasing use of omega-3 fatty acids for the treatment of dyslipidemia and CVD prevention, a better understanding of their potential association with AF is important.
Omega-3 fatty acids supplementation reduces the occurrence of CVD and CVD-related mortality in patients at high-risk of CVD and in patients with elevated plasma triglyceride level. In the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT), high-dose omega-3 fatty acid supplementation with highly purified icosapent ethyl (4 g) was associated with a 25% lower risk of the primary composite CV endpoint; however, the CV benefits were inconsistent [11]. Based on the abovementioned findings, the 2019 dyslipidemia guidelines from the European Society of Cardiology and European Atherosclerosis Society recommend the use of 4 g of icosapent ethyl in individuals with established CVD and triglyceride levels of 135 to 499 mg despite statin use [12]. By contrast, the recent Statin Residual Risk with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia (STRENGTH) trial, which used the same dose of omega-3 fatty acids but used a combination of EPA and DHA, showed no significant effect of omega-3 fatty acids on the composite CV endpoint. Moreover, certain other clinical studies demonstrated that low-dose omega-3 fatty acid supplementation did not improve CV outcomes. Furthermore, these trials also raised concerns about the potential off-target adverse effects of omega-3 fatty acids on the AF risk. Furthermore, RCTs of the effects of omega-3 fatty acid treatment on atrial and ventricular arrhythmias have revealed inconsistent results. In the past 10 years, several RCTs have evaluated the AF risk with omega-3 fatty acid supplementation (Table 1). Low-dose omega-3 fatty acid supplementation (≤ 1 g/day) has been evaluated in the Vitamin D and Omega-3 Trial (VITAL) Rhythm study, A Study of Cardiovascular Events in Diabetes (ASCEND), and Risk and Prevention Study (RP).
In the VITAL Rhythm study, 12,542 participants were randomized to receive standard-dose omega-3 fatty acids (840 mg/day), containing EPA and DHA, and 12,557 were randomized to receive placebo. Cases of incident AF were identified through self-reported diagnoses and claims data from the Centers for Medicare and Medicaid Services. After a median of 5.3 years, the incidence of AF in individuls receiving omega-3 fatty acids and placebo was 7.2 per 1,000 person-years and 6.6 per 1,000 person-years, respectively (hazard ratio [HR], 1.09; 95% confidence interval [CI], 0.96 to 1.24; p = 0.19) [13].
ASCEND, a large-scale mail-based RCT, examined the effects of daily intake of 0.84 g of omega-3 fatty acids (comprising EPA and DHA) on the incidence of life-threatening CV events in individuals with diabetes mellitus but without known atherosclerotic cardiovascular disease (ASCVD). Additionally, in a 2 × 2 factorial design, the study also investigated the effects of low-dose aspirin [14]. The study did not show any significant association of omega-3 fatty acid supplementation with the primary outcome. Although the original ASCEND trial showed patient-reported adverse outcomes caused by AF in the overall study participants, the post hoc extensive analysis of the electronic health records of patients with no known AF (n = 15,374; 99% of the study population) showed no significant difference in AF risk between participants in the omega-3 fatty acid and placebo groups (7.7% and 7.6%, respectively; rate ratio [RR], 1.02; 95% CI, 0.91 to 1.15). A non-fatal ventricular arrhythmia was also observed, with no significant difference between the omega-3 fatty acid and placebo groups (81 and 54 cases, respectively; RR, 1.49; 95% CI, 1.06 to 2.09; p = 0.02).
In RP, AF events were included as a reported reason for CVD hospitalization [15]. In the study, AF occurred in 113 of 6,239 (1.8%) patients in the experimental arm and 92 of 6,266 (1.5%) patients in the placebo arm (HR, 1.22; 95% CI, 0.93 to 1.61; p = 0.15). The study showed that daily supplementation with 1 g of omega-3 fatty acids did not reduce CV mortality or morbidity in patients with multiple CV risk factors.
In Omega-3 Fatty Acids in Elderly With Myocardial Infarction (OMEMI) trial, 1,027 elderly patients with a recent myocardial infarction were randomized to treatment with intermediate dose omega-3 fatty acids (combination of EPA and DHA; 1.8 g/day) or corn oil. After 2 years, there was no significant difference in the primary composite CV endpoint between the two groups; however, 7.2% of patients treated with omega-3 fatty acids developed AF compared to 4% of patients treated with corn oil (HR, 1.84; 95% CI, 0.98 to 3.45; p = 0.06) [16].
In the STRENGTH trial, 13,078 patients at high-risk of CVD were randomized to treatment with high-dose carboxylic acid formulation of omega-3 fatty acids (combination of EPA and DHA; 4 g/day) or corn oil. At a median follow-up of 42 months, the major composite CV endpoint showed no significant difference between the two groups; however, the risk of AF was higher in the omega-3 fatty acid group compared to the corn oil group (2.2% and 1.3%, respectively; HR, 1.69; 95% CI, 1.29 to 2.21; p < 0.001) [17].
In the REDUCE-IT trial, 8,179 participants were randomized to treatment with high-dose omega-3 fatty acids (4 g/day; similar to that used in the STRENGTH trial), consisting of purified EPA (icosapent ethyl), or mineral oil. In line with the STRENGTH trial, there was a significant increase in the AF risk after treatment with omega-3 fatty acids compared to mineral oil (5.3% and 3.9%, respectively; p = 0.003). After a median follow-up of 4.9 years, icosapent ethyl reduced the primary composite CV outcome by 25% compared to mineral oil.
A recent meta-analysis of six RCTs showed that omega-3 fatty acids were associated with a higher risk of incident AF compared to placebo (incidence rate ratio [IRR], 1.29; 95% CI, 1.13 to 1.48; p = 0.0002; random effects model) [18]. The meta-analysis showed that individuals at high-risk of or with established CVD and elevated plasma triglyceride levels were at a higher risk of incident AF with omega-3 fatty acid supplementation than with placebo treatment. Another meta-analysis of six RCTs also found significantly increased risk of AF with omega-3 fatty acid supplementation (IRR, 1.31; 95% CI, 1.13 to 1.51; p = 0.04; random effects model) [19]. Similarly, a recent meta-analysis of seven RCTs and 81,210 patients found a 25% increase in AF risk in patients treated with omega-3 fatty acids compared to those treated with placebo [20].
Therefore, although previous studies have shown inconsistent positive effects of omega-3 fatty acid supplementation on the composite CV endpoints, most trials have found an increased risk of AF.
Recent clinical trials suggest, but do not prove, a dose-related risk of AF with omega-3 fatty acid supplementation. Curfman [21] found that treatment with 4.0 g/day of omega-3 fatty acid was associated with an almost 2-fold increased risk of AF, whereas treatment with an intermediate dose of 1.8 g/day was not associated with an increased risk of AF (HR, 1.84). Similarly, there was no apparent increase in AF risk at a standard daily dose of 840 mg/day in VITAL Rhythm study [21]. Therefore, the occurrence of AF appears to be independent of clinical outcomes, with increased incidence of AF in trials that did (e.g., REDUCE-IT) and did not (e.g., STRENGTH) demonstrate significant reductions in ASCVD occurrence.
A recent meta-analysis of seven RCTs found that the incremental risk of AF associated with omega-3 fatty acids was greater in trials that used > 1 g/day of omega-3 fatty acid supplementation [22]. The AF risk increases with omega-3 fatty acid dose, with HR of 1.12 (95% CI, 1.03 to 1.22; p = 0.024) for doses ≤ 1 g/day to 1.49 (95% CI, 1.04 to 2.15; p = 0.042) for doses > 1 g/day. In a meta-regression model of the linear association between omega-3 fatty acid dose and risk of AF, the HR for AF increased by 1.11 (95% CI, 1.06 to 1.15; p = 0.001) for every 1 g increase in the dose of omega-3 fatty acids (residual heterogeneity, I2 = 0.00%; p = 0.54) over a dose of 1 to 4 g/day. Sensitivity analysis with the constant term showed similar results (HR, 1.09; 95% CI, 1.01 to 1.18; p = 0.030 per 1 g increase of omega-3 fatty acid dose) (Fig. 1) [22].
The large-scale RCTs of CV outcomes showed that omega-3 fatty acid supplementation was associated with increased risk of AF, particularly in trials that used high-dose omega-3 fatty acids, as the AF risk is higher at doses > 1 g/day. Large-scale trials have shown that omega-3 fatty acid supplementation may have a dose-dependent protective effect against coronary events. Therefore, patients who take omega-3 fatty acids, particularly at high doses, should be informed about the AF risk and monitored for possible development of this common and dangerous arrhythmia.
The U.S. Food and Drug Administration has approved several prescription omega-3 fatty acid agents for the treatment of extremely high triglyceride levels [1]. The typical daily dose of the 1-g omega-3 fatty acid capules is 4 g, which provides > 3 g/day of EPA + DHA. The U.S. Food and Drug Administration has approved the n-3 fatty acid products Lovaza (n-3 acid-ethyl esters [O3AEE]; 0.465 g of EPA and 0.375 g of DHA per capsule; GlaxoSmithKline, Brentford, UK), Omtryg (similar to O3AEE but not available for clinical use; Beckloff Associates, Overland Park, KS, USA), and Vascepa (ethyl ester product consisting of EPA without DHA, chemically known as icosapent ethyl; Amarin Corp., Dublin, Ireland). The concentrations and ratios of EPA and DHA vary between the preparations. EPA and DHA are present in the form of ethyl esters rather than free fatty acids in the ethyl ester agents (O3AEE and icosapent ethyl). Notably, the bioavailability of EPA and DHA also varies between the chemical forms, as products containing ethyl esters have lower bioavailability than the free fatty acid forms. Omega-3 fatty acid supplements have varying effects on CVDs depending on the type of omega-3 fatty acid formulation [1], as EPA and DHA may have variable effects on lipids and lipoproteins or other metabolic parameters [23]. Based on the results of the REDUCE-IT and Japan EPA Lipid Intervention Study (JELIS) trials, EPA-alone formulations are increasingly being used to reduce the risk of ASCVD [11,24]. Therefore, the AF risk of AF would may vary according to the formulation of omega-3 fatty acids.
A recent meta-analysis of RCTs showed increased AF risk with omega-3 fatty acid treatment when the results of REDUCE-IT trial, which tested only EPA, were excluded [22]. However, it is possible that certain omega-3 fatty acid formulations used in the included trials may have different effects on the AF risk. In the OMEMI trial, greater increase in serum phospholipid EPAs was independently associated with a lower risk of incident major adverse CV events and a higher risk of new-onset AF [25]. A greater increase in EPA was associated with a higher probability of developing AF (adjusted HR, 1.36; 95% CI, 1.07 to 1.72; p = 0.011), with a linear relationship [16]. Similarly, the serum phospholipid EPA level was associated with new-onset AF (adjusted HR, 1.36; 95% CI, 1.07 to 1.72; p = 0.011). By contrast, changes in the DHA level were not significantly associated with incident AF after treatment with 1.8 g/day of EPA/DHA (adjusted HR, 1.29; 95% CI, 0.91 to 1.83; p = 0.16). Similarly, no associations were observed between the achieved DHA level and AF risk (adjusted HR, 1.29; 95% CI, 0.91 to 1.83; p = 0.15). The ratio of change in EPA level was associated with incident AF after treatment with 1.8 g/day of EPA/DHA (HR, 1.35; 95% CI, 1.06 to 1.72; p = 0.01); by contrast, change in the DHA level was not significantly associated with the incident AF risk. There was no significant correlation of changes in EPA and DHA levels with incident AF in patients randomized to the placebo group (corn oil).
A recent study found a consistent inverse association between the EPA content in adipose tissues and AF risk in both men and women [26]. The DHA content was inversely related to the AF risk in women, but not in men. Given the discrepancy between the results of previous studies, the association between types of prescription omega-3 fatty acids and AF risk should be further evaluated.
The risk-benefit ratio of omega-3 fatty acids for AF varies according to its dose and formulation as well as patient characteristics. The OMEMI trial showed that individuals aged ≥ 75 years had the highest absolute risk difference (> 3%) for developing AF in the omega-3 fatty acid supplement group. The OMEMI trial investigators used a monitor to record AF occurrences, which may have led to the greater identification of asymptomatic subclinical cases [16]. A known history of AF may also be a risk factor for AF recurrence after omega-3 fatty acid supplementation. Some previous studies have reported a higher risk of postoperative AF in patients with elevated omega-3 fatty acid level. However, sensitivity analysis of clinical trials that excluded patients with AF at baseline showed no difference in results compared to studies that did not exclude patients with AF at baseline, as well as no significant interaction [22].
Because most clinical trials of omega-3 fatty acids were conducted on Western populations, ethnic differences in the association between omega-3 fatty acids and AF should be considered. Notably, few studies have investigated the effects of omega-3 fatty acids supplementation on ventricular arrhythmias in the Asian population. In the JELIS trial, 18,645 Japanese patients with a history of coronary artery disease and hypercholesterolemia were randomly assigned to 1.8 g/day of EPA plus statin or statin-only groups; significantly fewer major coronary events were noted in the EPA plus statin group compared to the statin-only group. However, AF outcomes related to omega-3 fatty acids were not evaluated as a pre-specified endpoint of the trial. Considering the probable benefit of omega-3 fatty acids on AS-CVD in the Asian population, the AF risk associated with omega-3 fatty acid supplementation should be investigated in more detail.
To date, few reports have investigated the effects of omega-3 fatty acid supplementation on the AF risk according to the patient-level characteristics. Therefore, to understand more fully the risk-benefit ratio of omega-3 fatty acids in high-risk individuals (e.g., older and patients with CV comorbidities), future trials should include systematic, pre-specified ascertainment and adjudication of AF outcomes assisted by electrocardiogram monitoring via new smart devices and adjudication by independent clinicians.
It is unclear how omega-3 fatty acids increase the AF risk. Omega-3 fatty acids stabilize the cardiac membrane and protect against arrhythmias, including ventricular arrhythmias, through both direct electrophysiologic actions and positive effects on biological processes involved in atrial remodeling. However, omega-3 fatty acids have direct effects on the activity of cardiac calcium, sodium, and potassium ionic currents, which can alter the duration of the ventricular action potential [27]. Therefore, omega-3 fatty acids may promote re-entrant arrhythmias, despite being anti-arrhythmic in therapeutic conditions. Several previous studies have evaluated the electrical characteristics of the ventricular wall; few studies have evaluated how omega-3 fatty acids influence the atria.
Recently, some studies have found that PIEZO proteins mediate the effect of omega-3 fatty acids on AF [28]. The PIEZO1/2 proteins are two of the largest ion channels discovered to date, with over 2,500 amino acids arranged in 38 transmembrane helices. These proteins form homotrimeric dome-shaped structures with three upward curved propeller-like blades and a central pore during assembly. The blades flatten due to increased mechanical force, which causes the central pore to open, leading to non-selective influx of positively charged ions, such as calcium (Fig. 2). Lipids in the cell membranes regulate the conformational changes that result in the opening and closing of the PIEZO channel. Dietary omega-3 fatty acids are incorporated into the cardiomyocyte surface membranes, making them thinner and more compliant [29]. The impact of these changes varies according to the type of omega-3 fatty acid used. DHA slows down the inactivation of PIEZO1 while EPA speeds it up. Therefore, the DHA:EPA ratio might determine the overall effect. A DHA-predominant effect would result in PIEZO1 activation and increased influx of calcium and other cations (Fig. 2). Finally, these changes would prolong the action potential, increase the likelihood of delayed after-depolarizations and consequently AF, and promote calcium-dependent signaling. Other atrial cell types, such as fibroblasts, may also express mechanical stress-related signaling through the PIEZO1 channels. Atrial fibroblasts from patients with AF have higher PIEZO1 expression levels and activity than those from patients with sinus rhythm, indicating that PIEZO1 may promote atrial structural remodeling [30]. A pro-arrhythmic environment may result from changes in voltage-gated ion channels, ionic pumps, cell surface receptors, and extracellular matrix-cytoskeletal interactions. In addition, PIEZO1 channels, which are one of several cell membrane characteristics altered by omega-3 fatty acid ingestion, also create a pro-arrhythmic environment.
Further mechanistic clinical trials are required to understand the processes by which omega-3 fatty acid supplementation increases the AF risk and alters the electrophysiologic characteristics of the myocardium. This is particularly important because of the clinical use of omega-3 fatty acids for the treatment of hypertriglyceridemia and prevention of ASCVD. Furthermore, additional research is required to understand the role of active metabolites with unique characteristics produced by omega-3 fatty acid consumption.
Omega-3 fatty acids supplementation is associated with increased AF risk, particularly in trials that used high doses. Therefore, several factors should be considered before prescribing omega-3 fatty acids, including their dose, type, and formulation (fish, dietary fish oil supplements, and purified fatty acids), as well as patient-related factors and atrial mechanical milieu. Because the benefits of omega-3 fatty acids are dose-dependent, the associated AF risk should be balanced against the benefit for ASCVD. Patients who take omega-3 fatty acids, particularly at high doses, should be informed of the risk of AF and followed up for the possible development of this common and potentially hazardous arrhythmia.
Notes
Figure 1
Effects of omega-3 fatty acid supplementation on the risk of atrial fibrillation. HR, hazard ratio; CI, confidence interval; VITAL, Vitamin D and Omega-3 Trial; ASCEND, A Study of Cardiovascular Events in Diabetes; STRENGTH, Statin Residual Risk with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia; RP, Risk and Prevention Study; REDUCE-IT, Reduction of Cardiovascular Events With Icosapent Ethyl-Intervention Trial; GISSI-HF, Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca-Heart Failure; OMEMI, Omega-3 Fatty Acids in Elderly With Myocardial Infarction.
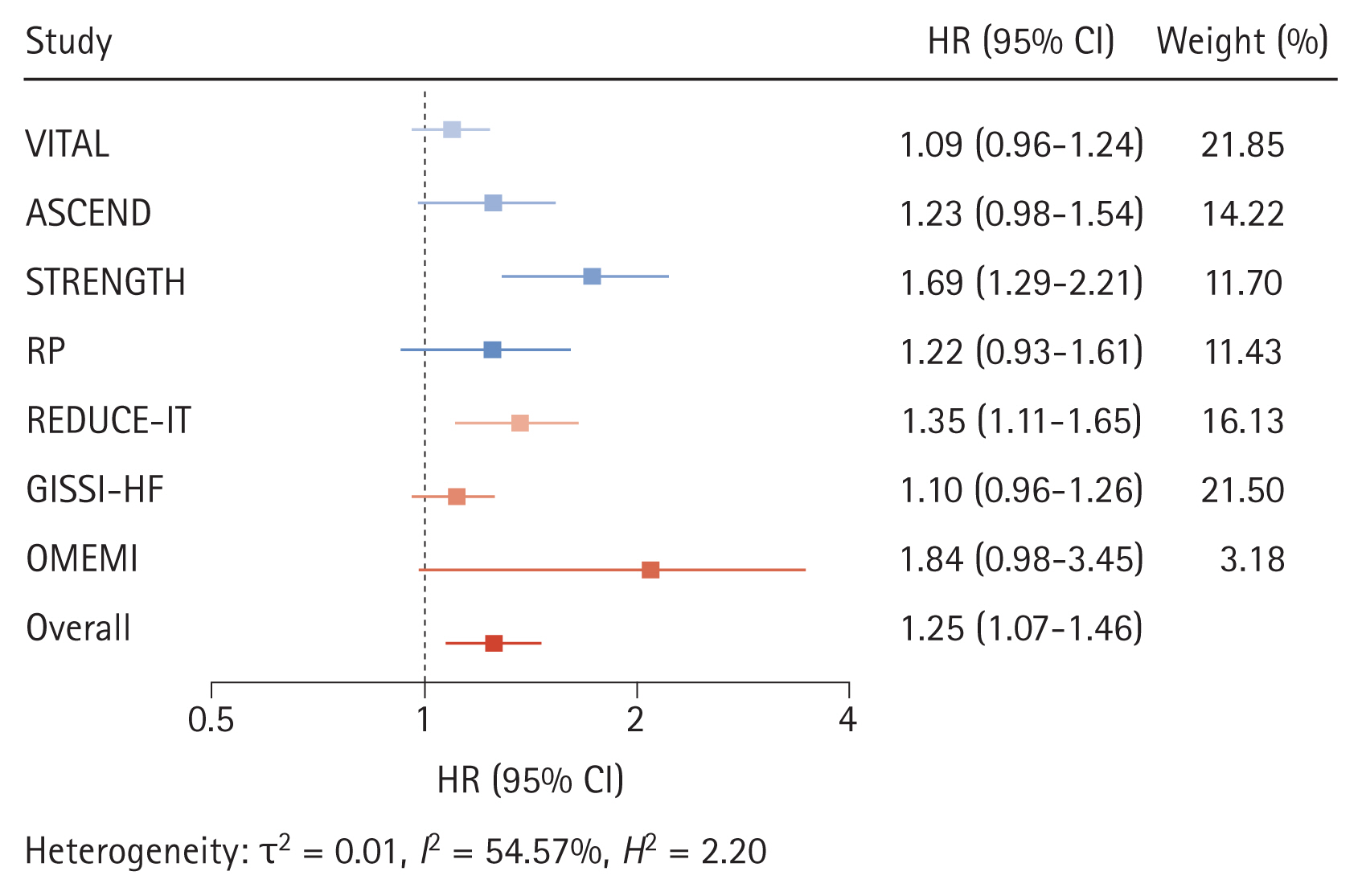
Figure 2
Possible mechanisms underlying the association between omega-3 fatty acid supplementation and atrial fibrillation.
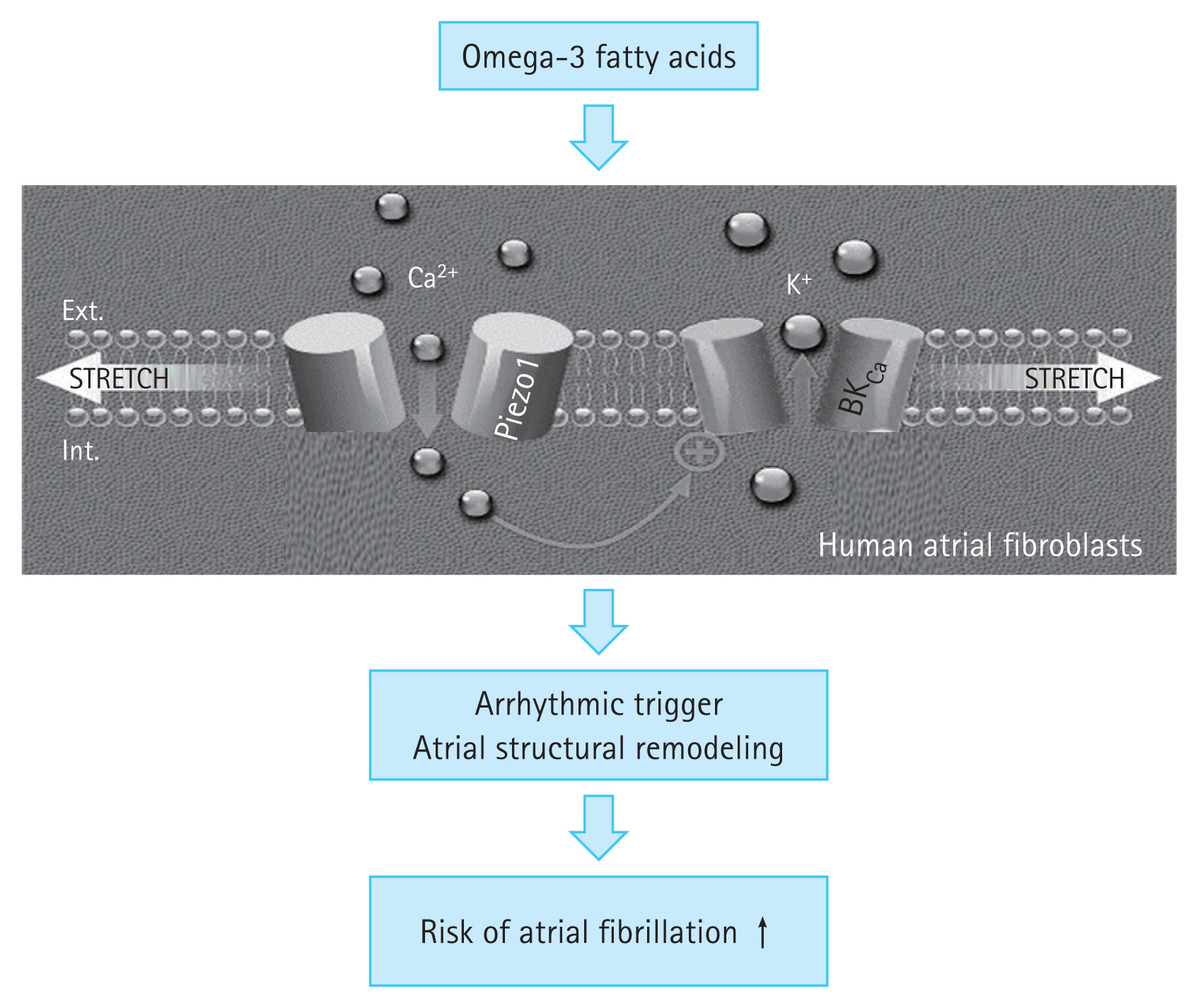
Table 1
Major clinical trials of the risk of atrial fibrillation with omega-3 fatty acid supplementation
| Trial | Number | Median follow-up, yr | AF at baseline included in the analysis | Study drug | Comparator | Total AF events, %/yr | Types of AF event | Risk of AF |
|---|---|---|---|---|---|---|---|---|
| VITAL (2020) | 25,119 | 5.3 | No | 1 g/day of ɷ-3 fatty acids (460 mg EPA and 380 mg DHA) | Olive oil | 900 (0.7) | New-onset AF | Hazard ratio was 1.09 (95% CI, 0.96–1.24; p = 0.19) |
| ASCENDa (2018) | 15,480 | 7.4 | Yes | 1 g/day of ɷ-3 fatty acids (460 mg EPA and 380 mg DHA) | Olive oil | 301 (0.3) | Main article: patient-reported AF (without excluding preexisting AF); research letter: new-onset AF |
Main article: Rate ratio was 1.23 (95% CI, 0.98–1.54). Research letter: 1.02 (95% CI, 0.91–1.15) |
| Risk and Prevention study (RP) (2013) | 12,505 | 5 | Yes | 1 g/day of ɷ-3 fatty acids (EPA and DHA < 85% in ratio from 0.9:1 to 1.5:1) | Olive oil | 205 (0.3) | Hospitalization for AF (without excluding preexisting AF) | Hazard ratio was 1.23 (95% CI, 0.94–1.62; p = 0.15) |
| OMEMI (2020) | 1,014 | 2b | No | 1.8 g/day of ɷ-3 fatty acids (930 mg EPA and 660 mg DHA) | Corn oil | 43 (2.1) | New-onset AF | Hazard ratio was 1.84 (95% CI, 0.98–3.45; p = 0.06) |
| STRENGTH (2020) | 13,078 | 3.5 | Yes | 4 g/day of ɷ-3 fatty acids (EPA and DHA) | Corn oil | 230 (0.5) | New-onset AF | Hazard ratio was 1.67 (95% CI, 1.28–2.18; p < 0.001) |
| REDUCE-IT (2019) | 8,179 | 4.9 | Yes | 4g/day of icosapent ethyl (ethyl ester of EPA) | Mineral oil | 374 (0.9) | New-onset or worsening AF events (without excluding preexisting AF) | The calculated risk ratio was 1.35 (95% CI, 1.11–1.65; p = 0.003) |
AF, atrial fibrillation; VITAL, Vitamin D and Omega-3 Trial; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; CI, confidence interval; ASCEND, A Study of Cardiovascular Events in Diabetes; OMEMI, Omega-3 Fatty Acids in Elderly With Myocardial Infarction; STRENGTH, Statin Residual Risk with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia; REDUCE-IT, Reduction of Cardiovascular Events With Icosapent Ethyl-Intervention Trial.
REFERENCES
1. Skulas-Ray AC, Wilson PW, Harris WS, et al. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation 2019;140:e673–e691.


2. Hu Y, Hu FB, Manson JE. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc 2019;8:e013543.



3. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 1999;354:447–455.


4. Patel NJ, Deshmukh A, Pant S, et al. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation 2014;129:2371–2379.


5. Kumar S, Kassotis JT. Do omega-3 polyunsaturated fatty acids prevent atrial fibrillation? Cardiology 2014;127:220–222.



6. Ninio DM, Murphy KJ, Howe PR, Saint DA. Dietary fish oil protects against stretch-induced vulnerability to atrial fibrillation in a rabbit model. J Cardiovasc Electrophysiol 2005;16:1189–1194.


7. Sakabe M, Shiroshita-Takeshita A, Maguy A, et al. Omega-3 polyunsaturated fatty acids prevent atrial fibrillation associated with heart failure but not atrial tachycardia remodeling. Circulation 2007;116:2101–2109.


8. Rix TA, Mortensen LM, Schmidt EB. Fish, marine n-3 fatty acids, and atrial fibrillation: experimental data and clinical effects. Front Physiol 2012;3:152.



9. Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA 2010;304:2363–2372.


10. Cao H, Wang X, Huang H, et al. Omega-3 fatty acids in the prevention of atrial fibrillation recurrences after cardioversion: a meta-analysis of randomized controlled trials. Intern Med 2012;51:2503–2508.


11. Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22.


12. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188.

13. Albert CM, Cook NR, Pester J, et al. Effect of marine omega-3 fatty acid and vitamin D supplementation on incident atrial fibrillation: a randomized clinical trial. JAMA 2021;325:1061–1073.



14. ASCEND Study Collaborative Group; Bowman L, et al.; Mafham M. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med 2018;379:1540–1550.


15. Risk and Prevention Study Collaborative Group; Roncaglioni MC, et al.; Tombesi M. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med 2013;368:1800–1808.


16. Kalstad AA, Myhre PL, Laake K, et al. Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction: a randomized, controlled trial. Circulation 2021;143:528–539.


17. Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA 2020;324:2268–2280.


18. Lombardi M, Carbone S, Del Buono MG, et al. Omega-3 fatty acids supplementation and risk of atrial fibrillation: an updated meta-analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother 2021;7:e69–e70.




19. Kow CS, Doi SAR, Hasan SS. The coincidence of increased risk of atrial fibrillation in randomized control trials of omega-3 fatty acids: a meta-analysis. Expert Rev Clin Pharmacol 2021;14:773–775.


20. Okereke OI, Vyas CM, Mischoulon D, et al. Effect of long-term supplementation with marine omega-3 fatty acids vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA 2021;326:2385–2394.



22. Gencer B, Djousse L, Al-Ramady OT, Cook NR, Manson JE, Albert CM. Effect of long-term marine ɷ-3 fatty acids supplementation on the risk of atrial fibrillation in randomized controlled trials of cardiovascular outcomes: a systematic review and meta-analysis. Circulation 2021;144:1981–1990.



23. Mozaffarian D, Wu JH. (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? J Nutr 2012;142:614S–625S.



24. Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090–1098.


25. Myhre PL, Kalstad AA, Tveit SH, et al. Changes in eicosapentaenoic acid and docosahexaenoic acid and risk of cardiovascular events and atrial fibrillation: a secondary analysis of the OMEMI trial. J Intern Med 2022;291:637–647.



26. Rix TA, Dinesen P, Lundbye-Christensen S, et al. Omega-3 fatty acids in adipose tissue and risk of atrial fibrillation. Eur J Clin Invest 2022;52:e13649.



27. Den Ruijter HM, Berecki G, Opthof T, Verkerk AO, Zock PL, Coronel R. Pro- and antiarrhythmic properties of a diet rich in fish oil. Cardiovasc Res 2007;73:316–325.


28. Fatkin D, Cox CD, Martinac B. Fishing for links between omega-3 fatty acids and atrial fibrillation. Circulation 2022;145:1037–1039.


-
METRICS

- Related articles
-
Exercise capacity and risk of incident atrial fibrillation in healthy adults2023 November;38(6)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


