 |
 |
|
|
|
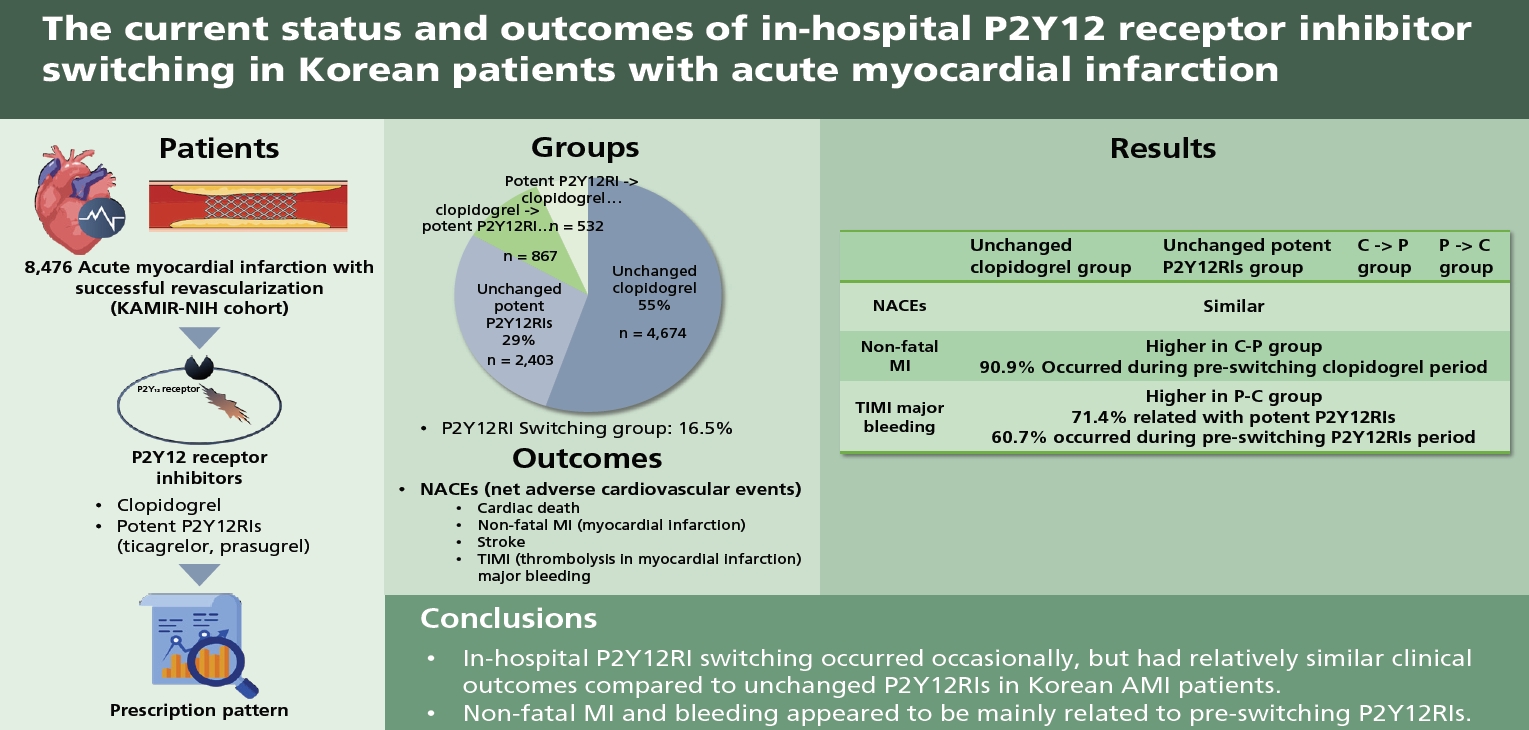
Abstract
Background/Aims
Methods
Results
Acknowledgments
Figure 1
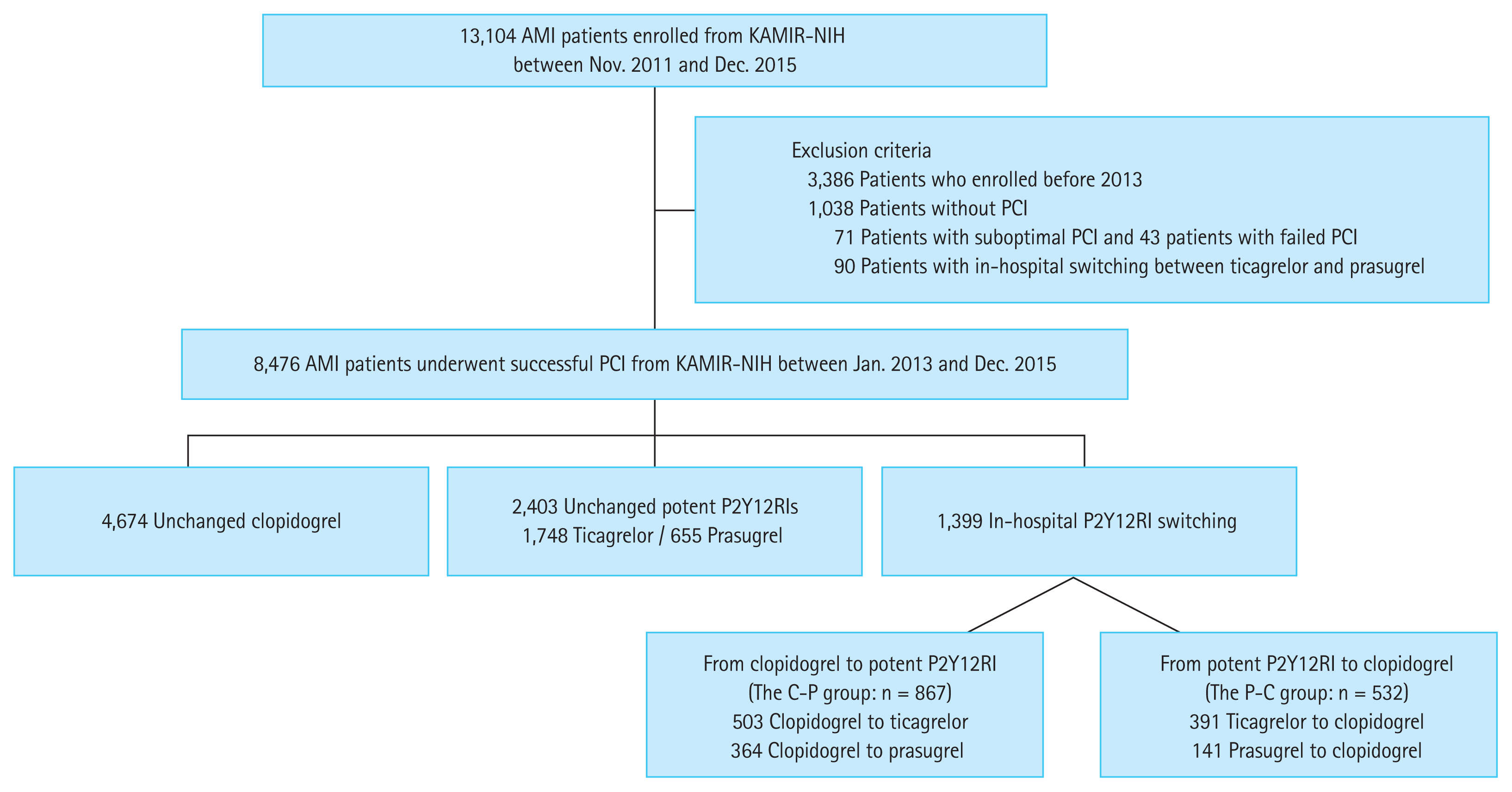
Figure 2
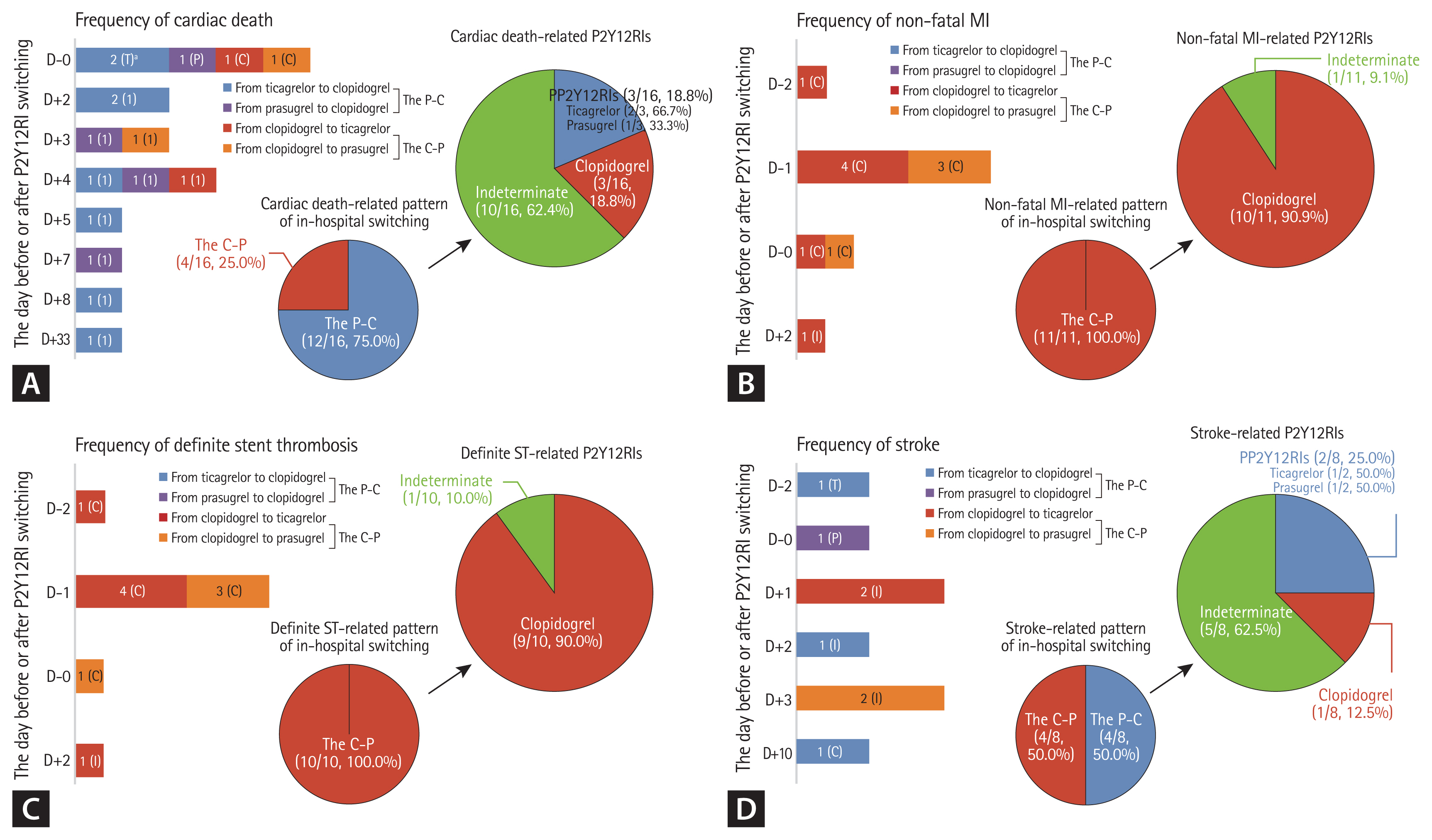
Figure 3
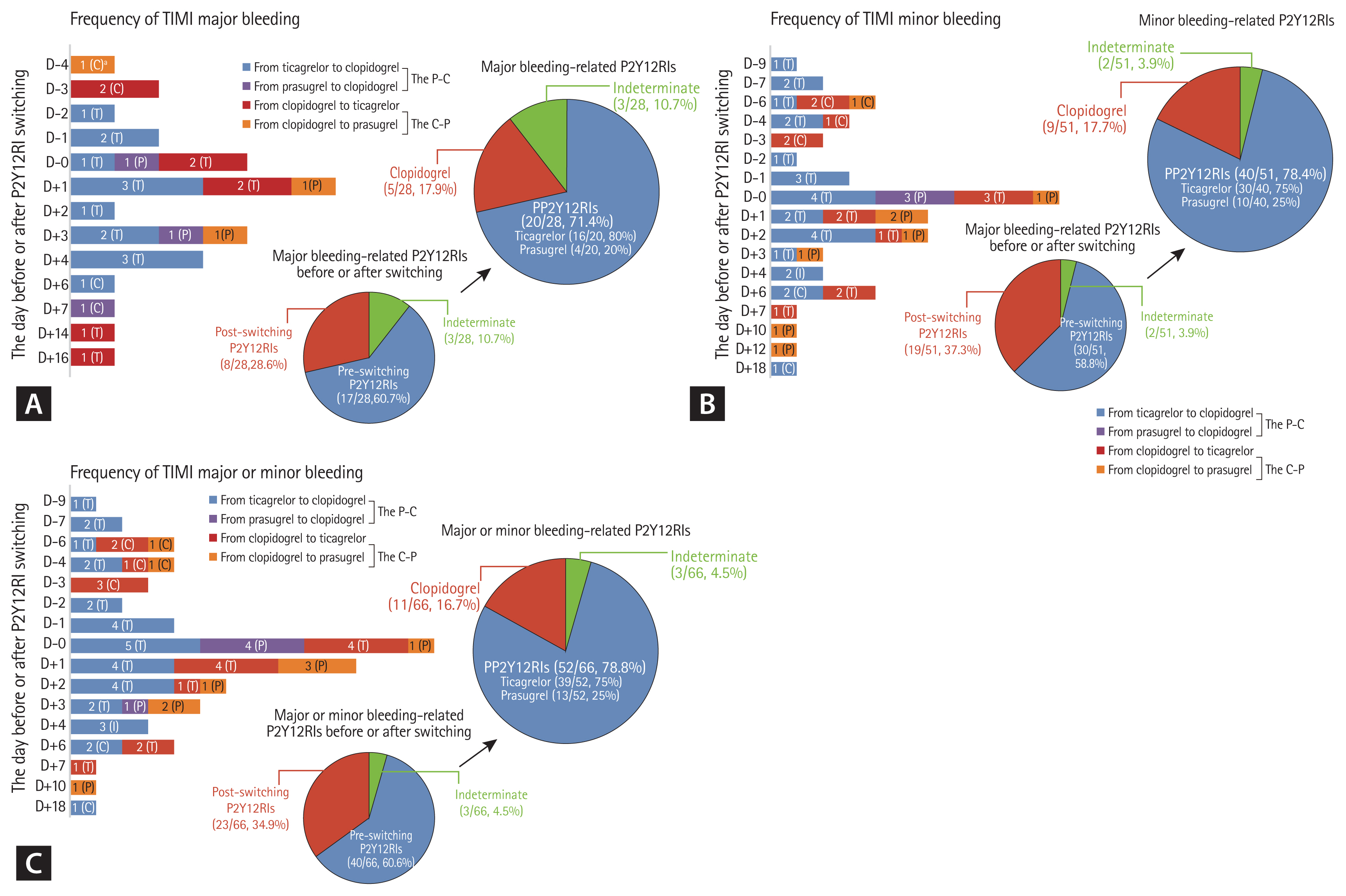
Table 1
| Variable | Unchanged clopidogrel (n = 4,674) | Unchanged potent P2Y12RIs (n = 2,403) | In-hospital P2Y12RI switching | p valuea | |||
|---|---|---|---|---|---|---|---|
| All patients (n = 1,399) | The C-P (n = 867) | The P-C (n = 532) | p value | ||||
| Age, yr | 65.86 ± 0.19 | 60.34 ± 0.24 | 62.10 ± 0.33 | 60.82 ± 0.40 | 64.20 ± 0.55 | < 0.001 | < 0.001 |
| Age ≥ 75 years | 1,348 (28.8) | 314 (13.1) | 243 (17.4) | 115 (13.3) | 128 (24.1) | < 0.001 | < 0.001 |
| Male gender | 3,339 (71.4) | 1980 (82.4) | 1,086 (77.6) | 703 (81.1) | 383 (72.0) | < 0.001 | < 0.001 |
| Hypertension | 2,494 (53.4) | 1.062 (44.2) | 680 (48.6) | 434 (50.1) | 246 (46.2) | 0.169 | < 0.001 |
| Diabetes | 1,390 (29.7) | 587 (24.4) | 360 (25.7) | 222 (25.6) | 138 (25.9) | 0.900 | < 0.001 |
| Dyslipidemia | 559 (12.0) | 267 (11.1) | 141 (10.1) | 96 (11.1) | 45 (8.5) | 0.121 | 0.131 |
| Current smoker | 1,624 (34.7) | 1,131 (47.1) | 595 (42.5) | 392 (45.2) | 203 (38.2) | 0.010 | < 0.001 |
| Family history of coronary artery disease | 284 (6.1) | 170 (7.1) | 82 (5.9) | 49 (5.7) | 33 (6.2) | 0.725 | 0.194 |
| Previous history of angina | 505 (10.8) | 135 (5.6) | 119 (8.5) | 78 (9.0) | 41 (7.7) | 0.431 | < 0.001 |
| Previous history of heart failure | 73 (1.6) | 16 (0.7) | 25 (1.8) | 17 (2.0) | 8 (1.5) | 0.679 | 0.002 |
| Previous history of MI | 339 (7.3) | 116 (4.8) | 128 (9.1) | 95 (11.0) | 33 (6.2) | 0.003 | < 0.001 |
| Previous history of CVA/TIA | 364 (7.8) | 83 (3.5) | 65 (4.6) | 40 (4.6) | 25 (4.7) | 1.000 | < 0.001 |
| Killip class | 0.941 | < 0.001 | |||||
| I | 3,652 (78.1) | 2,057 (85.6) | 1,167 (83.4) | 724 (83.5) | 443 (83.3) | ||
| II–IV | 1,022 (21.9) | 346 (14.4) | 232 (16.6) | 143 (16.5) | 89 (16.7) | ||
| Final diagnosis | 0.013 | < 0.001 | |||||
| Non-ST segment elevation MI | 2,462 (52.7) | 969 (40.3) | 704 (50.3) | 459 (52.9) | 245 (46.1) | ||
| ST segment elevation MI | 2,212 (47.3) | 1,434 (59.7) | 695 (49.7) | 408 (47.1) | 287 (53.9) | ||
| Left ventricular ejection fraction, % | 51.77 ± 0.17 | 52.87 ± 0.21 | 52.13 ± 0.29 | 52.45 ± 0.37 | 51.62 ± 0.47 | 0.163 | < 0.001 |
| Creatinine clearance, mL/min/1.73 m2 | 72.74 ± 0.53 | 87.78 ± 0.89 | 83.82 ± 1.01 | 86.75 ± 1.28 | 79.02 ± 1.63 | < 0.001 | < 0.001 |
| Vascular access | 0.956 | < 0.001 | |||||
| Trans-radial approach | 1,729 (37.0) | 1,095 (45.6) | 701 (50.1) | 435 (50.2) | 266 (50.0) | ||
| Trans-femoral approach | 2,945 (63.0) | 1,308 (54.4) | 698 (49.9) | 432 (49.8) | 266 (50.0) | ||
| Infarct-related artery | 0.424 | 0.263 | |||||
| Left anterior descending | 2,169 (46.4) | 1,133 (47.2) | 664 (47.5) | 410 (47.3) | 254 (47.7) | ||
| Left circumflex | 838 (17.9) | 431 (17.9) | 216 (15.4) | 135 (15.6) | 81 (15.2) | ||
| Right coronary | 1,559 (33.4) | 795 (33.1) | 483 (34.5) | 295 (34.0) | 188 (35.3) | ||
| Left main | 108 (2.3) | 44 (1.8) | 36 (2.6) | 27 (3.1) | 9 (1.7) | ||
| Involved vessel number | 0.047 | 0.001 | |||||
| Single vessel | 2,200 (47.1) | 1,236 (51.4) | 712 (50.9) | 423 (48.8) | 289 (54.3) | ||
| Left main or multi-vessel disease | 2,474 (52.9) | 1,167 (48.6) | 687 (49.1) | 444 (51.2) | 243 (45.7) | ||
| ACC/AHA lesion classification | 0.931 | < 0.001 | |||||
| Type A/B1 | 755 (16.2) | 227 (9.4) | 157 (11.2) | 98 (11.3) | 59 (11.1) | ||
| Type B2/C | 3,919 (83.8) | 2,176 (90.6) | 1,242 (88.8) | 769 (88.7) | 473 (88.9) | ||
| Procedure for target lesion | 0.805 | 0.001 | |||||
| Only balloon angioplasty | 276 (5.9) | 108 (4.5) | 72 (5.1) | 42 (4.8) | 30 (5.6) | ||
| Bare metal stent | 150 (3.2) | 44 (1.8) | 39 (2.8) | 24 (2.8) | 15 (2.8) | ||
| Drug-eluting stent | 4,248 (90.9) | 2,251 (93.7) | 1,288 (92.1) | 801 (92.4) | 487 (91.6) | ||
| Use of glycoprotein IIb/IIIa inhibitor | 514 (11.0) | 457 (19.0) | 238 (17.0) | 157 (18.1) | 81 (15.2) | 0.187 | < 0.001 |
| In-hospital medications | |||||||
| Aspirin | 4,668 (99.9) | 2,402 (100.0) | 1,396 (99.8) | 864 (99.7) | 532 (100.0) | 0.292 | 0.311 |
| Clopidogrel | 4,674 (100.0) | - | 1,399 (100.0) | 867 (100.0) | 532 (100.0) | - | - |
| Ticagrelor | - | 1,748 (72.7) | 894 (63.9) | 503 (58.0) | 391 (73.5) | < 0.001 | - |
| Prasugrel | - | 655 (27.3) | 505 (36.1) | 364 (42.0) | 141 (26.5) | < 0.001 | - |
| Cilostazol | 585 (12.5) | 12 (0.5) | 42 (3.0) | 17 (2.0) | 25 (4.7) | 0.005 | < 0.001 |
| Beta-blocker | 3,869 (82.8) | 2,048 (85.2) | 1,167 (83.4) | 750 (86.5) | 417 (78.4) | < 0.001 | 0.031 |
| Calcium channel blockers | 322 (6.9) | 103 (4.3) | 77 (5.5) | 47 (5.4) | 30 (5.6) | 0.904 | < 0.001 |
| ACEi or ARB | 3,725 (79.7) | 1,908 (79.4) | 1,107 (79.1) | 706 (81.4) | 401 (75.4) | 0.008 | 0.886 |
| Statin | 4,307 (92.1) | 2,284 (95.0) | 1,323 (94.6) | 824 (95.0) | 499 (93.8) | 0.333 | < 0.001 |
| Oral anticoagulants | 156 (3.3) | 29 (1.2) | 46 (3.3) | 8 (0.6) | 38 (7.1) | < 0.001 | < 0.001 |
RI, receptor inhibitor; C-P, from clopidogrel to potent P2Y12RI; P-C, from potent P2Y12RI to clopidogrel; MI, myocardial infarction; CVA, cerebrovascular accident; TIA, transient ischemic attack; ST, stent thrombosis; ACC, American College of Cardiology; AHA, American Heart Association; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Table 2
| Variable | Unchanged clopidogrel (n = 4,674) | Unchanged potent P2Y12RIs (n = 2,403) | In-hospital P2Y12RI switching | X2a | p valuea | X2b | p valueb | ||
|---|---|---|---|---|---|---|---|---|---|
| All patients (n = 1,399) | The C-P (n = 867) | The P-C (n = 532) | |||||||
| All-cause death | 162 (3.5) | 60 (2.5) | 24 (1.7) | 9 (1.0) | 15 (2.8) | 13.664 | 0.001 | 17.377 | 0.001 |
| Cardiac death | 138 (3.0) | 49 (2.0) | 16 (1.1) | 4 (0.5) | 12 (2.3) | 16.887 | < 0.001 | 21.428 | < 0.001 |
| Non-fatal MI | 9 (0.2) | 9 (0.4) | 11 (0.8) | 11 (1.3) | 0 | 11.235 | 0.004 | 26.799 | < 0.001 |
| Definite stent thrombosis | 7 (0.1) | 7 (0.3) | 10 (0.7) | 10 (1.2) | 0 | 12.183 | 0.002 | 27.717 | < 0.001 |
| Stroke | 35 (0.7) | 3 (0.1) | 8 (0.6) | 4 (0.5) | 4 (0.8) | 11.474 | 0.003 | 11.990 | 0.007 |
| MACEs | 174 (3.7) | 61 (2.5) | 35 (2.5) | 19 (2.2) | 16 (3.0) | 9.756 | 0.008 | 10.468 | 0.015 |
| TIMI major bleeding | 63 (1.3) | 65 (2.7) | 28 (2.0) | 11 (1.3) | 17 (3.2) | 16.418 | < 0.001 | 23.193 | < 0.001 |
| TIMI minor bleeding | 108 (2.3) | 92 (3.8) | 51 (3.6) | 22 (2.5) | 29 (5.5) | 15.454 | < 0.001 | 25.194 | < 0.001 |
| TIMI major or minor bleeding | 151 (3.2) | 132 (5.5) | 66 (4.7) | 28 (3.2) | 38 (7.1) | 22.106 | < 0.001 | 34.895 | < 0.001 |
| NACEs | 222 (4.7) | 117 (4.9) | 58 (4.1) | 28 (3.2) | 30 (5.6) | 1.137 | 0.566 | 5.425 | 0.143 |
RI, receptor inhibitor; C-P, from clopidogrel to potent P2Y12RI; P-C, from potent P2Y12RI to clopidogrel; MI, myocardial infarction; MACE, major adverse cardiovascular event; TIMI, thrombolysis in myocardial infarction; NACE, net adverse cardiovascular event.
Table 3
| Variable | No. (%) | Unadjusted | Multivariate-adjusted | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR (95% CI) | p valuea | OR (95% CI) | p value | |||
| Unchanged clopidogrel vs. The C-P | 4,674 | 867 | ||||
|
|
||||||
| All-cause death | 162 (3.5) | 9 (1.0) | 0.292 (0.149–0.574) | < 0.001 | 0.778 (0.242–2.507) | 0.674 |
|
|
||||||
| Cardiac death | 138 (3.0) | 4 (0.5) | 0.152 (0.056–0.413) | < 0.001 | 0.169 (0.030–0.970) | 0.046 |
|
|
||||||
| Non-fatal MI | 9 (0.2) | 11 (1.3) | 6.661 (2.752–16.122) | < 0.001 | 7.075 (2.858–17.517) | < 0.001 |
|
|
||||||
| Definite stent thrombosis | 7 (0.1) | 10 (1.2) | 7.780 (2.953–20.494) | < 0.001 | 5.977 (2.195–16.278) | < 0.001 |
|
|
||||||
| Stroke | 35 (0.7) | 4 (0.5) | 0.614 (0.218–1.733) | 0.357 | 0.828 (0.286–2.396) | 0.728 |
|
|
||||||
| MACEs | 174 (3.7) | 19 (2.2) | 0.579 (0.359–0.936) | 0.026 | 1.246 (0.669–2.318) | 0.488 |
|
|
||||||
| TIMI major bleeding | 63 (1.3) | 11 (1.3) | 0.941 (0.494–1.792) | 0.852 | 1.085 (0.542–2.170) | 0.818 |
|
|
||||||
| TIMI minor bleeding | 108 (2.3) | 22 (2.5) | 1.101 (0.692–1.751) | 0.685 | 1.386 (0.843–2.281) | 0.198 |
|
|
||||||
| TIMI major or minor bleeding | 151 (3.2) | 28 (3.2) | 1.000 (0.663–1.506) | 0.999 | 1.276 (0.818–1.990) | 0.282 |
|
|
||||||
| NACEs | 222 (4.7) | 28 (3.2) | 0.669 (0.449–0.998) | 0.049 | 1.222 (0.750–1.993) | 0.421 |
|
|
||||||
| Unchanged potent P2Y12RIs vs. The C-P | 2,403 | 867 | ||||
|
|
||||||
| All-cause death | 60 (2.5) | 9 (1.0) | 0.410 (0.202–0.829) | 0.013 | 0.168 (0.026–1.085) | 0.061 |
|
|
||||||
| Cardiac death | 49 (2.0) | 4 (0.5) | 0.223 (0.080–0.619) | 0.004 | 0.015 (0.001–0.355) | 0.009 |
|
|
||||||
| Non-fatal MI | 9 (0.4) | 11 (1.3) | 3.418 (1.412–8.277) | 0.006 | 3.944 (1.619–9.606) | 0.003 |
|
|
||||||
| Definite stent thrombosis | 7 (0.3) | 10 (1.2) | 3.994 (1.516–10.526) | 0.005 | 4.661 (1.756–12.371) | 0.002 |
|
|
||||||
| Stroke | 3 (0.1) | 4 (0.5) | 3.708 (0.828–16.601) | 0.087 | 3.595 (0.801–16.133) | 0.095 |
|
|
||||||
| MACEs | 61 (2.5) | 19 (2.2) | 0.860 (0.511–1.448) | 0.571 | 1.373 (0.683–2.757) | 0.374 |
|
|
||||||
| TIMI major bleeding | 65 (2.7) | 11 (1.3) | 0.462 (0.243–0.880) | 0.019 | 0.454 (0.230–0.895) | 0.023 |
|
|
||||||
| TIMI minor bleeding | 92 (3.8) | 22 (2.5) | 0.654 (0.408–1.048) | 0.078 | 0.639 (0.389–1.049) | 0.076 |
|
|
||||||
| TIMI major or minor bleeding | 132 (5.5) | 28 (3.2) | 0.574 (0.379–0.870) | 0.009 | 0.570 (0.366–0.889) | 0.013 |
|
|
||||||
| NACEs | 117 (4.9) | 28 (3.2) | 0.652 (0.428–0.992) | 0.046 | 0.707 (0.431–1.159) | 0.170 |
|
|
||||||
| Unchanged clopidogrel vs. The P-C | 4,674 | 532 | ||||
|
|
||||||
| All-cause death | 162 (3.5) | 15 (2.8) | 0.808 (0.472–1.382) | 0.436 | 1.238 (0.477–3.213) | 0.661 |
|
|
||||||
| Cardiac death | 138 (3.0) | 12 (2.3) | 0.759 (0.418–1.378) | 0.364 | 1.163 (0.391–3.459) | 0.785 |
|
|
||||||
| Non-fatal MI | 9 (0.2) | 0 | - | - | - | - |
|
|
||||||
| Definite stent thrombosis | 7 (0.1) | 0 | - | - | - | - |
|
|
||||||
| Stroke | 35 (0.7) | 4 (0.8) | 1.004 (0.355–2.836) | 0.994 | 0.904 (–0.315–2.598) | 0.852 |
|
|
||||||
| MACEs | 174 (3.7) | 16 (3.0) | 0.802 (0.477–1.349) | 0.405 | 0.922 (0.455–1.867) | 0.821 |
|
|
||||||
| TIMI major bleeding | 63 (1.3) | 17 (3.2) | 2.416 (1.403–4.160) | 0.001 | 2.379 (1.310–4.321) | 0.004 |
|
|
||||||
| TIMI minor bleeding | 108 (2.3) | 29 (5.5) | 2.437 (1.601–3.710) | < 0.001 | 2.683 (1.714–4.202) | < 0.001 |
|
|
||||||
| TIMI major or minor bleeding | 151 (3.2) | 38 (7.1) | 2.304 (1.595–3.328) | < 0.001 | 2.575 (1.722–3.851) | < 0.001 |
|
|
||||||
| NACEs | 222 (4.7) | 30 (5.6) | 1.198 (0.810–1.774) | 0.366 | 1.534 (0.935–2.518) | 0.090 |
|
|
||||||
| Unchanged potent P2Y12RIs vs. The P-C | 2,403 | 532 | ||||
|
|
||||||
| All-cause death | 60 (2.5) | 15 (2.8) | 1.133 (0.638–2.011) | 0.670 | 0.259 (0.049–1.378) | 0.113 |
|
|
||||||
| Cardiac death | 49 (2.0) | 12 (2.3) | 1.109 (0.586–2.099) | 0.752 | 0.374 (0.071–1.961) | 0.245 |
|
|
||||||
| Non-fatal MI | 9 (0.4) | 0 | - | - | - | - |
|
|
||||||
| Definite stent thrombosis | 7 (0.3) | 0 | - | - | - | - |
|
|
||||||
| Stroke | 3 (0.1) | 4 (0.8) | 6.061 (1.352–27.159) | 0.019 | 3.892 (0.799–18.969) | 0.093 |
|
|
||||||
| MACEs | 61 (2.5) | 16 (3.0) | 1.190 (0.681–2.081) | 0.541 | 0.912 (0.389–2.135) | 0.832 |
|
|
||||||
| TIMI major bleeding | 65 (2.7) | 17 (3.2) | 1.187 (0.690–2.042) | 0.535 | 0.990 (0.547–1.794) | 0.974 |
|
|
||||||
| TIMI minor bleeding | 92 (3.8) | 29 (5.5) | 1.448 (0.944–2.223) | 0.090 | 1.301 (0.821–2.061) | 0.262 |
|
|
||||||
| TIMI major or minor bleeding | 132 (5.5) | 38 (7.1) | 1.323 (0.911–1.923) | 0.142 | 1.147 (0.761–1.727) | 0.512 |
|
|
||||||
| NACEs | 117 (4.9) | 30 (5.6) | 1.168 (0.773–1.764) | 0.462 | 0.957 (0.573–1.597) | 0.866 |
OR, odds ratio; CI, confidence interval; C-P, from clopidogrel to potent P2Y12RI; MI, myocardial infarction; MACE, major adverse cardiovascular event; TIMI, thrombolysis in myocardial infarction; NACE, net adverse cardiovascular event; RI, receptor inhibitor; P-C, from potent P2Y12RI to clopidogrel.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



