A 57-year-old woman presented with worsening chest pain, dysphagia, and vomiting. She denied having prior episodes of heartburn or acid regurgitation. Nine months prior, she had been diagnosed with tubal cancer with peritoneal metastasis. The patient was treated with neoadjuvant chemotherapy using paclitaxel and carboplatin, followed by radical cytoreductive surgery, and subsequent adjuvant chemotherapy using paclitaxel and cisplatin was completed. However, 5 months after surgery, peritoneal recurrence was detected. Thus, palliative chemotherapy with bi-weekly bevacizumab and liposomal doxorubicin was initiated, but after administration of bevacizumab, the patient suffered from severe upper gastrointestinal symptoms.
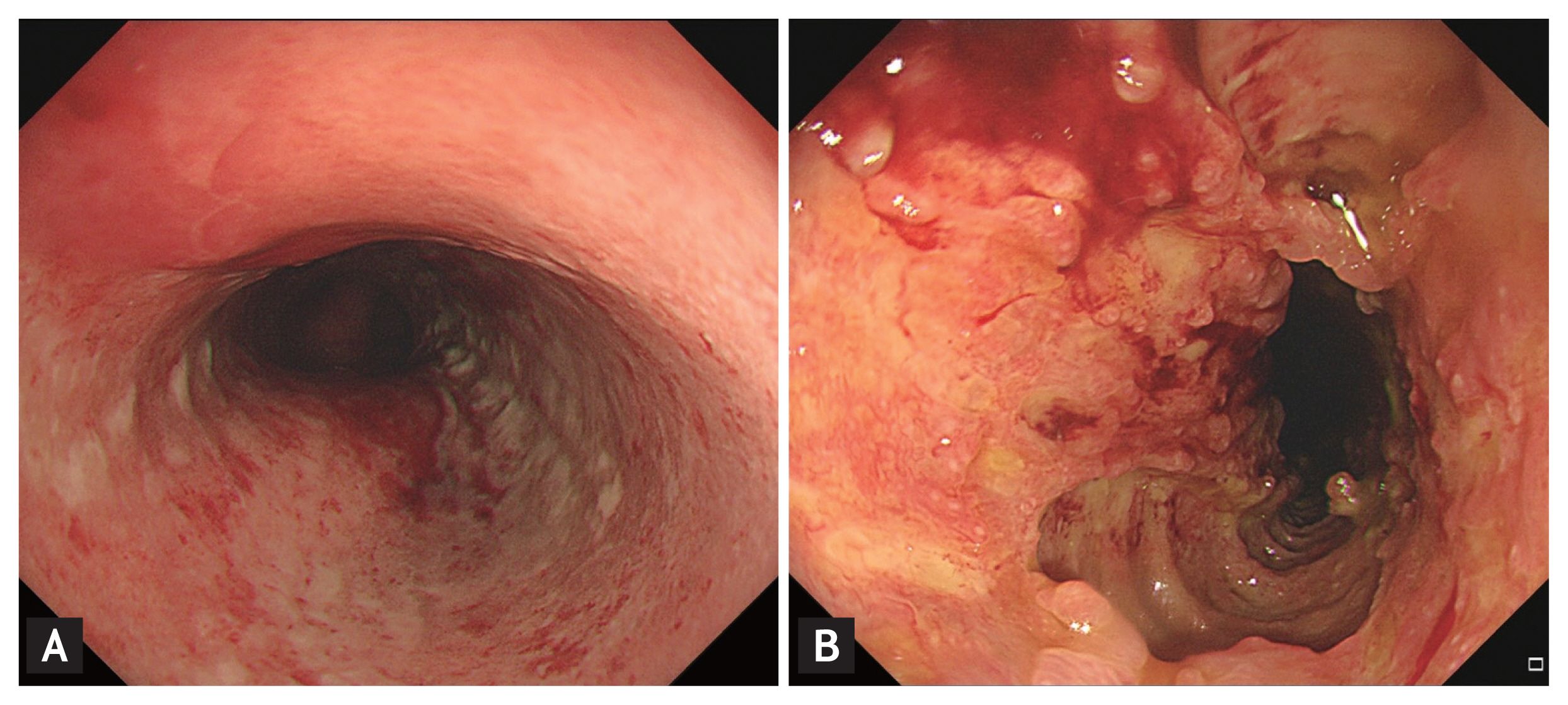
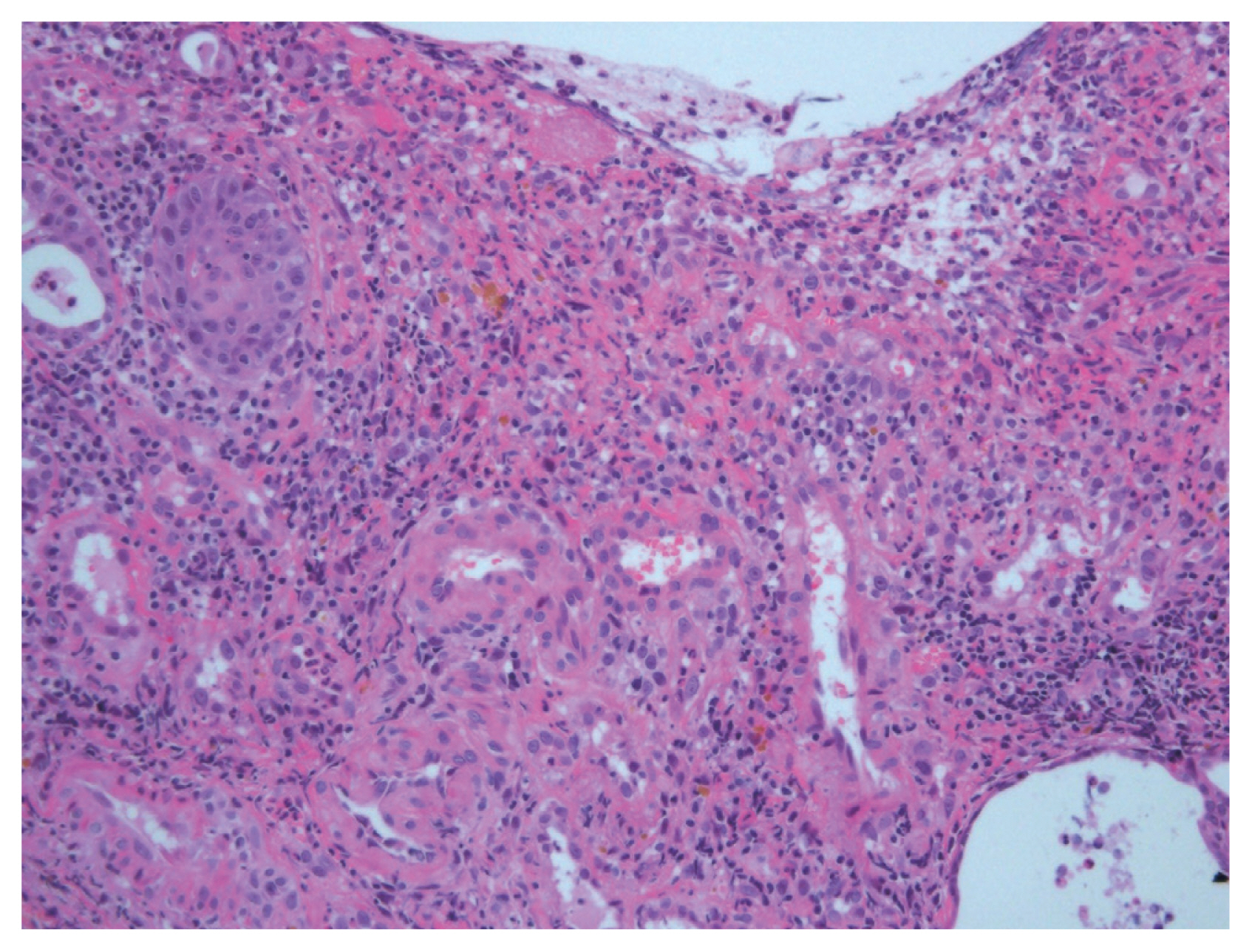
Upper endoscopy revealed moderate to severe esophagitis in the middle esophagus (Fig. 1A). A high-dose proton-pump inhibitor was administered, and the patient continued receiving bevacizumab therapy. After three cycles, 1 month after initiation of bevacizumab, she presented with worsening chest pain and dysphagia. A second endoscopy revealed multiple deep ulcers in the middle esophagus with impending sign of perforation (Fig. 1B). Forceps biopsies were carefully performed; histologic findings showed superficial surface erosion which displays fibrinopurulent exudates and stroma which displays mixed inflammatory cells and increased vasculature. It was negative for cytomegalovirus and malignant cell (Fig. 2). Serum cytomegalovirus antibody was also negative. These results suggested bevacizumab-induced severe esophageal injury. Therefore, bevacizumab therapy was discontinued and proton pump inhibitor was maintained, resulting in gradual improvement in upper gastrointestinal symptoms. Informed consent was obtained from the patient.
Although bevacizumab is generally well tolerated, several serious adverse events including gastrointestinal perforation had been reported. Bevacizumab is an anti-vascular endothelial growth factor monoclonal antibody; thus, vascular injury and ischemia of the gastrointestinal tract is thought to contribute to these complications. When patients taking bevacizumab develop severe upper gastrointestinal symptoms, prompt endoscopic evaluation should be considered to enable early diagnosis and proper treatment.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





