 |
 |
| Korean J Intern Med > Volume 33(5); 2018 > Article |
|
Abstract
Background/Aims
Patients with diabetes mellitus (DM) and tuberculosis (TB) have increased morbidity and a high risk of treatment failure or recurrence. It is important to manage both diseases simultaneously. Among anti-diabetic drugs, metformin inhibits intracellular growth of mycobacteria. Therefore, we examined the effects of metformin on TB treatment, especially in patients with DM.
Methods
This retrospective cohort study included patients with culture-positive pulmonary TB diagnosed between 2011 and 2012. The primary study outcome was sputum culture conversion after 2 months of treatment.
Results
Of 499 patients diagnosed with culture-positive pulmonary TB, 105 (21%) had DM at diagnosis. Among them, 62 (59.5%) were treated with metformin. Baseline characteristics, except for the presence of chronic renal disease, were not significantly different between the metformin and non-metformin groups. Metformin treatment had no significant effect on sputum culture conversion (p = 0.60) and recurrence within 1 year after TB treatment completion (p = 0.39). However, metformin improved the sputum culture conversion rate in patients with cavitary pulmonary TB, who have higher bacterial loads (odds ratio, 10.8; 95% confidence interval, 1.22 to 95.63).
The association between diabetes mellitus (DM) and tuberculosis (TB) is well recognized [1]. Various mechanisms on how DM leads to TB are proposed; among them, mechanisms involving indirect effects on phagocytes and lymphocytes, especially T-cells, which are the most important cells for intracellular infection such as TB, are considered significant [2]. It has been shown that blood monocytes, which are responsible for innate immunity through differentiation to macrophages and dendritic cells for antigen presentation and secretion of cytokines, are decreased in number and are less activated in patients with DM and TB [3]. There is controversy according to literature about the T-cell production of cytokines (over expression [4,5] vs. no differences [6]). However, diminished T regulatory cell counts are commonly observed; hence, a relative deficiency in regulatory T-cells may contribute to the increased inflammation in patients with TB and diabetes [7]. Adults with DM have at least a threefold-fold higher risk of developing TB, more frequent treatment failures, relapses, and deaths than non-diabetic patients [3,8,9].
Recently, among the drugs that control diabetes, metformin has attracted attention as a host-directed therapy (HDT). Its effects include augmenting macrophage effectors, reducing inflammation, and preventing lung damage. In in vivo studies, metformin was shown to promote macrophage’s autophagy effect through phagolysosome fusion by activation of the expression of AMP-activated protein kinase (AMPK), production of mitochondrial reactive oxygen species (mROS), and inhibition of Mycobacterium tuberculosis (Mtb) growth [10,11]. Singhal et al. [11] demonstrated that AMPK-activation by metformin inhibits Mtb growth and also showed that mice treated with isoniazid (INH) and metformin had decreased bacillary load in the lungs compared with the INH-treated mice. They assessed the effects of metformin treatment in humans with TB based on their in vivo and in vitro research findings. This study showed that patients treated with metformin had fewer pulmonary cavities and were less likely to die than those not treated with metformin. Thus, we hypothesized that metformin could function as an adjunctive in anti-TB treatment. In this study, we examined the anti-TB treatment effects of metformin on sputum Mtb culture conversion after 2 months of TB treatment.
This retrospective, cohort study recruited patients from among those in the TB Cohort of Seoul Metropolitan Government Seoul National University Boramae Medical Center and Seoul National University Hospital between January 2011 and December 2012. The present study was approved by the Institutional Review Board (IRB no: 201607/26-2016-91/072). The informed consent was waived. The inclusion criteria were as follows: (1) culture-proven pulmonary TB in patients diagnosed with DM; (2) follow-up sputum Mtb cultures after 2 months of treatment; and (3) completion of the World Health Organization (WHO)-recommended TB treatment. The exclusion criteria were as follows: (1) culture-negative pulmonary TB; (2) extrapulmonary TB; (3) no follow-up of sputum Mtb cultures after 2 months of anti-TB treatment; and (4) follow-up loss or death after 2 months of anti-TB treatment.
Among various treatment response indices, we selected sputum culture conversion after 2 months of treatment as the primary outcome, because it was well-known that TB culture conversion at 2 months was a representative indicator of the disease activity, cure, and predictor of relapse [12,13]. The definition of culture-positive pulmonary TB was at least one sputum culture positive for Mtb. We followed the WHO definition of treatment outcomes for TB patients, except for multidrug resistance (MDR) or extensively drug resistant (XDR) TB cases [14]. MDR was defined as TB that did not respond to at least INH and rifampicin, the two most powerful anti-TB drugs. XDR is a form of TB that was resistant to at least four of the core anti-TB drugs, meaning resistance to the two most powerful anti-TB drugs, INH and rifampicin, in addition to resistance to any of the fluoroquinolones and to at least one of the three injectable second-line drugs (amikacin, capreomycin, or kanamycin). MDR and XDR treatments were defined according to the WHO treatment guidelines for drug-resistant TB [15]. The demographic profiles (age, sex, comorbidity, glucose lowering agent, and statin), laboratory findings (hemoglobin, albumin, and glomerular filtration rate according to the Modification of Diet in Renal Disease study equation) [16], random glucose, glycosylated hemoglobin (HbA1c), aspartate transaminase, and alanine transaminase, and chest radiography results (cavitary lesion and infiltration area) were obtained from medical records. Baseline sputum acid-fast bacilli (AFB) smear was the result of a specimen collected before the initiation of anti-TB treatment. Recurrence of TB was defined as proven by isolation of Mtb or clinical and/or radiological evidence of TB after successful completion of treatment.
The primary end point was the achievement of sputum Mtb culture conversion after 2 months of treatment. The secondary end point was the recurrence rate within 1 year after anti-TB treatment completion. The recurrence rate per person-year was calculated. Categorical variables were compared using a chi-square test or Fisher exact test, and continuous variables were compared using an independent unpaired t test. Multivariate analysis was performed to evaluate the factors associated with 2 months sputum culture conversion and recurrence percentages within 1 year. p values ≤ 0.05 were considered significant. All the analyses were performed using the SPSS software version 22.0 (IBM Co., Armonk, NY, USA) and STATA version 13 software (StataCorp, College Station, TX, USA).
A total of 1,286 patients from the TB cohort were evaluated, of which 499 patients had culture-proven pulmonary TB with follow-up sputum Mtb cultures after 2 months of treatment. Of these, a total of 105 patients were pulmonary TB patients diagnosed with DM; 62 of 105 (59.5%) were treated with metformin (Fig. 1).
The baseline characteristics of the enrolled patients according to the use of metformin are presented in TaTable 1. The two groups were comparable regarding age, sex, glycemic control (random glucose, HbA1c), and comorbidity (except chronic kidney disease). Chronic kidney disease was less frequent in metformin users, and the patients with metformin use had a higher use of statins than did patients without metformin (37.1 % vs. 18.6%, p = 0.04). In addition, no statistically significant differences were noted in the percentage of cavitary TB, drug-resistant TB, and sputum AFB smear grades. The comparison of sputum culture conversion rate after 2 months of treatment between metformin and non-metformin group failed to reveal statistical difference (67.7% vs. 62.8%, p = 0.60). All patients were regularly followed during the first 2 months of treatment. Ten patients did not visit the clinic on the expected date more than twice after the intensive treatment period of 2 months. However, they completed the anti-TB treatment in the end. During anti-TB treatment, no one died. However, treatment failure was documented in 11 patients. After completion of anti-TB treatment, 58 of 105 patients were followed more than 1 year. The interval of follow-up after anti-TB treatment varied by the physician.
When we further compared the variables between culture converted group and non-converted group, drug resistance was significantly higher in patients with failure to achieve conversion (10.1% vs. 36.1%, p ≤ 0.01) (Table 2). There were no statistically significant differences in other variables such as age, sex, glycemic control index (random glucose, HbA1c), previous TB history, AFB smear grade, cavity, and statin use.
Multivariate analysis indicated that the odds ratio (OR) of sputum culture conversion at 2 months for patients with metformin use was 1.24 (95% confidence interval [CI], 0.36 to 1.82; p = 0.60), after adjusting for sex, statin use, insulin, cancer, AFB smear grade, and drug resistance (Table 3). The independent risk factors associated with failure of sputum culture conversion at 2 months included drug resistance (OR, 0.17; 95% CI, 0.04 to 0.70; p = 0.03), cavity (OR, 0.36; 95% CI, 0.13 to 0.98; p = 0.04), and AFB smear grade 2+ versus 0 (OR, 0.13; 95% CI, 0.02 to 0.71; p = 0.02).
Table 3 also shows the factor associated with the recurrence of TB episodes within 1 year after anti-TB treatment completion. A total of 58 patients were followed up for more than 1 year, and of these patients, nine had TB recurrence within 1 year. Thus, the rate of recurrence was 0.066 per year in the first year of Mtb infection. We failed to reveal a statistical difference in the recurrence rate with the use of metformin (OR, 1.92; 95% CI, 0.42 to 8.76; p = 0.39) after adjusting for sex, statin, cancer, AFB smear grade, cavity, or drug resistance. The other factors were not associated with the recurrence of TB within 1 year after treatment completion.
Among 105 patients with TB and DM, 39 (37.1%) had cavitary pulmonary TB. We further analyzed patients with cavitary pulmonary TB. Interestingly, in patients with cavitary pulmonary TB, the culture conversion rate after 2 months in the metformin group was much higher than in the non-metformin group (OR, 10.80; 95% CI, 1.22 to 95.63; p = 0.03). This analysis was also conducted in the non-cavitary patients, but we failed to reveal a statistical difference, with no differences were revealed in the culture conversion rate depending on the use of metformin (Supplementary Table 1). Furthermore, a comparison of TB recurrence in the patients with cavitary pulmonary TB between the metformin and non-metformin groups also failed to reveal a statistical difference (OR, 0.73; 95% CI, 0.13 to 4.10; p = 0.72) (Table 4).
In this study, metformin failed to reveal a significant anti-TB effect in TB patients with DM. However, it might be effective in DM patients who have cavitary pulmonary TB, based on the finding that in these patients a higher sputum culture conversion rate was obtained in the metformin group than in the non-metformin group.
As previously mentioned, the association of DM and TB is well established. In this regard, it is presumed that these patients are burdened with the lowered cellular immunity, decreased neutrophil counts, and increased susceptibility to respiratory infections [17]. In addition to drug-susceptible TB, several studies have reported the association between MDR-TB and DM, with a higher prevalence of drug-resistant TB in DM patients [18,19]. MDR-TB treatment is known to be limited and expensive, and some patients experience adverse effects [20]. Therefore, several methods are under development to control both TB and diabetes and to improve the effectiveness and adverse effect of current or future antibiotics in Mtb infection, including drug-susceptible, drug-resistant, and latent TB. Among these methods, HDT is considered a new strategy to augment standard anti-TB treatments. HDT is an adjunctive treatment that modulates host cell immunity to improve pathogen eradication, and it can shorten treatment duration [21]. Furthermore, increased treatment resistance, a problem associated with conventional pathogen-targeted strategies, can be overcome through HDT. Metformin is one of the HDT for treating TB/DM [10,11,22].
To the best of our knowledge, previous studies related to metformin as an HDT were few and were mostly in vitro and in vivo studies. The common and representative effects of metformin on TB were suppression of intracellular Mtb growth in an AMPK-dependent manner, which facilitated phagosome-lysosome fusion in Mtb-infected cells. Based on previous experimental results and retrospective studies using the TB Control Unit of Singapore by Singhal et al. [11], it was hypothesized that metformin could affect Mtb suppression. Thus, we designed this study to prove and expand these findings.
Our study did not show an adjunctive effect on culture conversion after 2 months of treatment or recurrence rate within 1 year except in patients in cavitary pulmonary TB was documented. The discrepancy between the results of a previous study and our study might be explainable by the following. In the previous in vivo study, the dose of metformin was 2,430 mg/day for a 60-kg human. However, the maximum dose of metformin was 1,500 mg, and 500 mg was the usual dose for most patients in our present study. The different doses of metformin did not lead to a positive result. In addition, the heterogeneity of the disease, including cavity or extent of infiltration, and the presentation of the study patients might have affected the various responses to metformin treatment. Chronic hyperglycemia is associated with the dysfunctional immunity to TB in DM patients, and hence, likely to reduce the efficiency of anti-mycobacterial treatment [23] and to also compromise mycobacteria killing by affecting the microvasculature and reducing lung tissue perfusion for optimal immune surveillance [24]. Nevertheless, anti-TB drugs are generally effective in treating drug-sensitive TB patients, even with DM. Therefore, additional HDT effect of metformin may not be essential for successful TB treatment.
However, cavitary pulmonary TB is associated with higher bacterial loads and has been shown to be an important risk factor for treatment failure and relapse [25,26]. In the cavity, the caseous center contains large numbers of extracellular bacilli. Sequestration of bacilli and heterogeneous vascular supply of the cavity make the penetration of anti-TB drugs difficult [27]. Considering this finding, the positive anti-TB effect of metformin in cavitary TB is not due to only intracellular effect. We assumed that it might be due to anti-inflammatory effect by promoting T regulatory and CD8+ T-cells [28]. Thus, the various presentations of cavity or infiltration of the lung among our enrolled patients could have affected our data.
There are limitations to this study. First, this was a retrospective study, and the sample size was relatively small. Second, a large number of patients were excluded due to the absence of follow-up sputum Mtb cultures. Although we used multivariate analysis, these issues can be subject to confounders. Third, the role of metformin in TB patients diagnosed with non-DM was not assessed. Evidence from TB patients without DM might be needed to confirm the anti-inflammatory effect of metformin. Fourth, adherence to the metformin treatment protocol could not be confirmed. Therefore, the preventive effect on the recurrence of metformin cannot be properly interpreted. Given all these issues, further prospective studies with larger numbers of patients are warranted to determine the anti-TB treatment effect of metformin in TB patients.
In conclusion, metformin cannot be recommended as a general adjuvant therapy for TB in patients with DM. However, it may be a candidate HDT for TB in these patients especially in those with a high disease burden, such as in that case of cavitary pulmonary TB. We need further study to find out the role of metformin as an adjunctive anti-TB agent in cavitary pulmonary TB patients with DM, who have a higher burden of bacteria and risk of treatment failure.
1. Concurrent diabetes mellitus (DM) and tuberculosis (TB) is associated with a higher prevalence of TB, more frequent treatment failures and relapses.
2. Macrophage’s autophagy activation mechanism called host-directed therapy by metformin is proposed.
3. Metformin can be an effective adjunctive anti-TB agent in DM patients with a high bacillary burden.
Acknowledgments
This study was supported by grant No. 201607/26-2016- 91/072 from the Seoul Metropolitan Government Seoul National University Boramae Medical Center and Seoul National University Hospital Research Fund, of the Republic of Korea. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Materials
Supplementary Table 1.
Multivariate logistic regression analysis for factors associated with sputum AFB culture conversion at 2 months in patients in whom have not cavity
Figure 1.
Flow chart. BRMH, Seoul Metropolitan Government Seoul National University Boramae Medical Center; SNUH, Seoul National University Hospital; TB, tuberculosis; AFB, acid-fast bacilli. a Misdiagnosed: NTM (n = 20), no TB (n = 10), sarcoidosis (n = 6), b Etc.: cutaneous TB (n =2), renal TB (n = 5), CNS TB (n =3), adrenal TB (n = 1), prostate TB (n = 1), TB arthritis (n = 2), TB pericarditis (n = 2), TB spondylitis (n = 5).
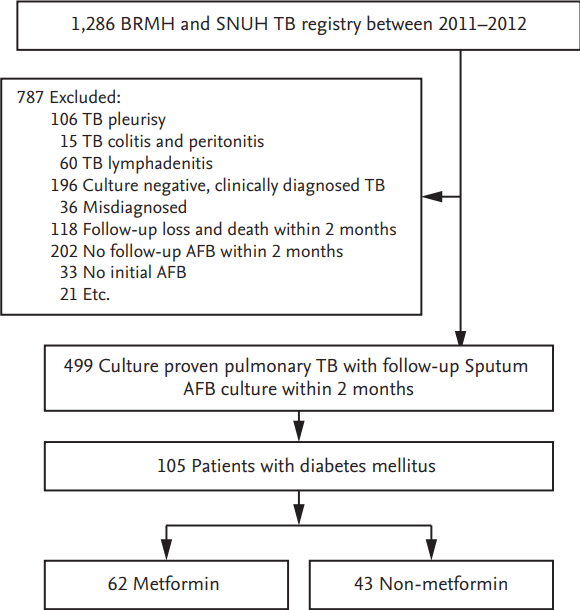
Table 1.
Baseline characteristics according to metformin therapy
Table 2.
Comparison of clinical variables according to the result of sputum AFB culture conversion at 2 months
Table 3.
Multivariate logistic regression model of factors associated with sputum AFB culture conversion at 2 months and recurrence rate within 1 year
Table 4.
Multivariate logistic regression analysis for factors associated with sputum AFB culture conversion at 2 months and recurrence rate within 1 year in patients in whom have cavity
REFERENCES
1. Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 2009;9:737–746.



2. Wang CH, Yu CT, Lin HC, Liu CY, Kuo HP. Hypodense alveolar macrophages in patients with diabetes mellitus and active pulmonary tuberculosis. Tuber Lung Dis 1999;79:235–242.


3. Gomez DI, Twahirwa M, Schlesinger LS, Restrepo BI. Reduced Mycobacterium tuberculosis association with monocytes from diabetes patients that have poor glucose control. Tuberculosis (Edinb) 2013;93:192–197.



4. Restrepo BI, Fisher-Hoch SP, Pino PA, et al. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis 2008;47:634–641.




5. Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, Babu S. Expansion of pathogen-specific T-helper 1 and T-helper 17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. J Infect Dis 2013;208:739–748.




6. Stalenhoef JE, Alisjahbana B, Nelwan EJ, et al. The role of interferon-gamma in the increased tuberculosis risk in type 2 diabetes mellitus. Eur J Clin Microbiol Infect Dis 2008;27:97–103.


7. Kumar Nathella P, Babu S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology 2017;152:13–24.



8. Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 2011;9:81.




9. Alisjahbana B, Sahiratmadja E, Nelwan EJ, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis 2007;45:428–435.



10. Wallis RS, Hafner R. Advancing host-directed therapy for tuberculosis. Nat Rev Immunol 2015;15:255–263.



11. Singhal A, Jie L, Kumar P, et al. Metformin as adjunct antituberculosis therapy. Sci Transl Med 2014;6:263ra159.


12. Zierski M, Bek E, Long MW, Snider DE Jr. Short-course (6 month) cooperative tuberculosis study in Poland: results 18 months after completion of treatment. Am Rev Respir Dis 1980;122:879–889.

13. Mitchison DA. Assessment of new sterilizing drugs for treating pulmonary tuberculosis by culture at 2 months. Am Rev Respir Dis 1993;147:1062–1063.


14. World Health Organization. Treatment of Tuberculosis: Guidelines. 4th ed. Geneva: World Health Organization, 2010.
15. Falzon D, Jaramillo E, Schunemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J 2011;38:516–528.


16. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–254.


17. Niazi AK, Kalra S. Diabetes and tuberculosis: a review of the role of optimal glycemic control. J Diabetes Metab Disord 2012;11:28.



18. Bashar M, Alcabes P, Rom WN, Condos R. Increased incidence of multidrug-resistant tuberculosis in diabetic patients on the Bellevue Chest Service, 1987 to 1997. Chest 2001;120:1514–1519.


19. Fisher-Hoch SP, Whitney E, McCormick JB, et al. Type 2 diabetes and multidrug-resistant tuberculosis. Scand J Infect Dis 2008;40:888–893.



20. World Health Organization. Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. Geneva: World Health Organization, 2014.
21. Zumla A, Rao M, Parida SK, et al. Inflammation and tuberculosis: host-directed therapies. J Intern Med 2015;277:373–387.


22. Zumla A, Rao M, Wallis RS, et al. Host-directed therapies for infectious diseases: current status, recent progress, and future prospects. Lancet Infect Dis 2016;16:e47–e63.


23. Restrepo BI, Schlesinger LS. Impact of diabetes on the natural history of tuberculosis. Diabetes Res Clin Pract 2014;106:191–199.



24. Koziel H, Koziel MJ. Pulmonary complications of diabetes mellitus. Pneumonia. Infect Dis Clin North Am 1995;9:65–96.

25. Palaci M, Dietze R, Hadad DJ, et al. Cavitary disease and quantitative sputum bacillary load in cases of pulmonary tuberculosis. J Clin Microbiol 2007;45:4064–4066.



- TOOLS
-
METRICS

- Related articles
-
Musculoskeletal complications in patients with diabetes mellitus2022 November;37(6)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print


