 |
 |
| Korean J Intern Med > Volume 31(4); 2016 > Article |
|
Abstract
Background/Aims:
Overt hypothyroidism is frequently found in patients with type 2 diabetes mellitus (T2DM), but it remains unknown the relationship between subclinical hypothyroidism (SCH) and T2DM. We attempt to evaluate the difference in prevalence of SCH between patients with T2DM and general population, and the association between SCH and glycemic control status ofdiabetic patients.
Methods:
This was cross-sectional study. Total 8,528 subjects who visited for health check-up were recruited. SCH was defined as an elevated level of serum thyroid stimulating hormone (> 4.0 mIU/L) with a normal level of free thyroxine. T2DM group was categorized into three groups by glycosylated hemoglobin (HbA1c): < 7% (reference), Ōēź 7% and < 9%, Ōēź 9%.
Results:
Finally, 7,966 subjects were included. The prevalence of SCH was not different between healthy controls and subjects with T2DM (8.1% vs. 7.4%, p = 0.533; in men, 5.7% vs. 5.1%, p = 0.573; in women, 10.9% vs. 11.7%, p = 0.712), but it was increased with highest HbA1c group more than well controlled group, especially in women. The risk of SCH was increased by group with poorer glycemic control; the odds ratio for HbA1c Ōēź 9% compared to < 7% was 2.52 (95% confidence interval [CI], 1.09 to 5.86; p = 0.031), and 4.58 (95% CI, 1.41 to 14.87; p = 0.011) in women, but not significant in men.
Subclinical hypothyroidism (SCH) is defined as an elevated level of serum thyroid stimulating hormone (TSH) with a normal level of serum free thyroxine (FT4) [1]. A number of studies have provided that SCH is associated with dyslipidemia, impaired vascular function, atherosclerosis, myocardial dysfunction, and progression to overt hypothyroidism [2-7]. Because advanced assays enable to measure thyroid hormones more accurately, the incidence of SCH is tend to increase in the last few decades [8]. The overall prevalence of SCH is reported to range from 4% to 10% in large general population screening surveys [9], although it varies with age, sex, and race [7,10,11].
It is assumed that type 2 diabetes mellitus (T2DM) is associated with SCH [12,13]. Previous studies suggested SCH was much more likely in patients with T2DM than general population, and the prevalence was reported to be 2.2% to 17% [14-18]. However, some investigators reported no differences between groups [16] and more studies are needed to clarify the relationship between T2DM and SCH. With regard to controversy, few studies compared the SCH of patients with T2DM to that of general population in Korea. Moreover, little is known about the prevalence of SCH according to glycemic control status in diabetic patients.
The aims of this study are to compare the prevalence of SCH in patients with T2DM and general population, andto evaluate the association between SCH and glycemic control in patients with T2DM.
This was a cross-sectional study. Between January 2008 and December 2009, 8,528 subjects were enrolled who underwent a health examination at the Yeungnam University Health Promotion Center, Daegu, South Korea. Their ages ranged from 20 to 85 years. We excluded individuals who had followings: overt hypothyroidism or overt hyperthyroidism (FT4 > 1.9, < 0.9 ng/dL), severe anemia (hemoglobin < 8 g/dL), chronic kidney disease (serum creatinine > 1.5 mg/dL), abnormal liver function test (total or direct bilirubin, aspartate transaminase or alanine transaminase > 2-fold of upper normal limit), and abnormal tumor markers (╬▒-fetoprotein > 15 mg/mL, carbohydrate antigen 19-9 > 37 U/mL, carcinoembryonic antigen > 10 mg/mL, cancer antigen 125 > 35 U/mL in women, prostate-specific antigen > 4 mg/mL in men), individuals with chronic infection, acute illness, pregnant women, postpartum women, and self-reported use of thionamides, L-thyroxine, amiodarone, or lithium, were excluded. The study protocol was approved by the Institutional Review Board of Yeungnam University Medical Center (YUMC 2014-11-002). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
SCH was defined as an elevated level of serum TSH (>4.0 mIU/L) with a normal level of serum FT4 (0.9 to 1.9 ng/dL). The criteria for diagnosis of diabetes were glycosylated hemoglobin (HbA1c) Ōēź 6.5% or fasting plasma glucose (FPG) Ōēź 126 mg/dL according to the guidelines of the American Diabetes Association [17]. Self-reported diabetes medication use and/or self-reported medical diagnosis of diabetes (other thangestational diabetes) are also included. Subjects with T2DM were divided into three groups by glycemic control: HbA1c < 7%; HbA1c Ōēź 7% and < 9%; and HbA1c Ōēź 9%.
Body mass index (BMI) was calculated as weight (kg) divided by the square of height in meters (m2). Blood pressure was measured with a standard sphygmomanometer after at least 10 minutes of rest in the sitting position. Hypertension was defined by a systolic blood pressure Ōēź 140 mmHg and/or a diastolic blood pressure Ōēź 90 mmHg, or self-reported anti-hypertensive medication use. Venous blood samples were obtained from each individual after a 12-hour overnight fast. FPG was measured using the hexokinase method (AU 5400 Autoanalyzer, Olympus, Tokyo, Japan). HbA1c was measured by high performance liquid chromatography (HLC-723G7, Tosoh, Tokyo, Japan). Total cholesterol was measured by enzyme colorimetry (Kyowa Medex Co. Ltd., Tokyo, Japan), while triglyceride was measured using the glycerol elimination method, and high density lipoprotein cholesterol (HDL-C) was measured using direct enzymatic assays (Kyowa Medex Co. Ltd.). Low density lipoprotein cholesterol (LDL-C) was calculated using FriedewaldŌĆÖs formula. The levels of TSH and FT4 were measured by an electrochemiluminescence immunoassay (Modular Analytics E170, Hitachi, Tokyo, Japan; and Immunoassay Kit, Roche Diagnostics GmbH, Mannheim, Germany).
All numerical variables are expressed as the mean ┬▒ standard deviation, and categorical data were expressed as percentages. Comparisons of continuous variables between groups were conducted using Student t tests, while one-way analysis of variance was conducted to compare continuous variables among glycemic control groups. Chi-square test was used to estimate differences of the prevalence of hypertension and SCH between subgroups. Multivariate logistic regression analyses were performed to estimate the odds ratio (OR) for the presence of SCH in poorly glycemic controlled diabetic patients. All statistical analyses were performed using SPSS version 20.0 (IBM Co., Armonk, NY, USA). A value of p < 0.05 was considered to indicate statistical significance.
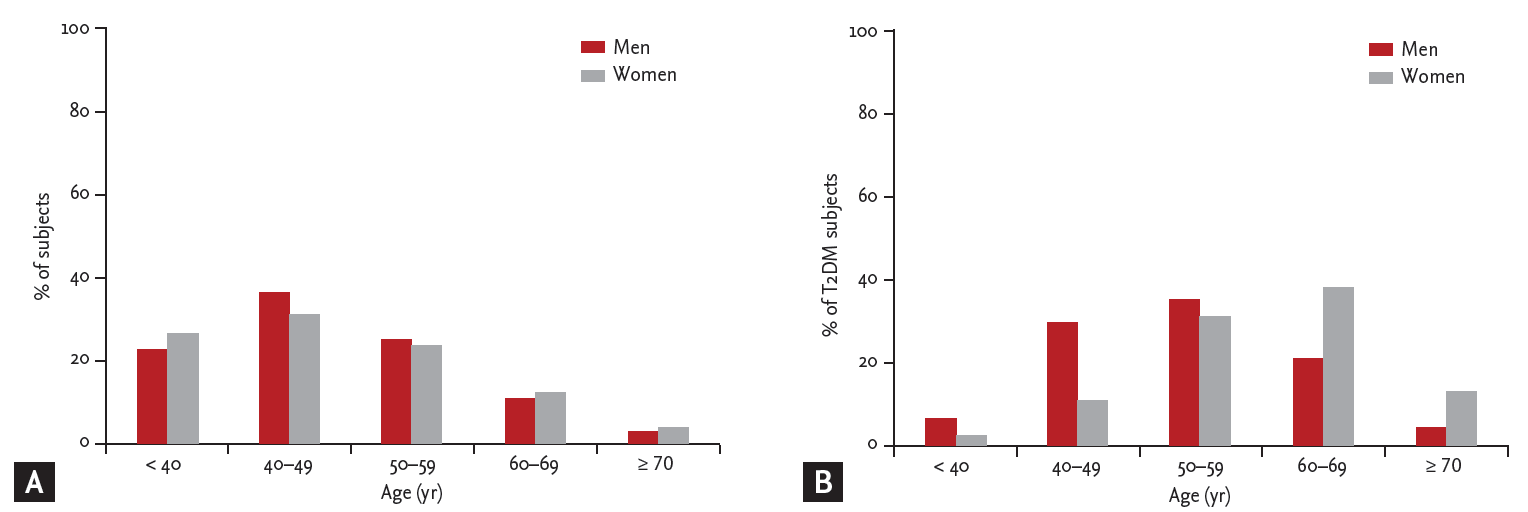
After exclusion, 7,966 subjects (4,483 men; mean age, 47.6 years) were finally eligible for this study. Of them, 678 were patients with T2DM. The age distribution of subjects is shown in Fig. 1. The clinical characteristics of the subjects are summarized in Table 1. Subjects with T2DM were older, more often men and had a higher prevalence of hypertension than subjects without T2DM. Moreover, BMI, systolic and diastolic blood pressure, FPG, HbA1c, blood urea nitrogen (BUN), total cholesterol, and triglyceride values were higher, and HDL-C was lower in subjects with T2DM than in those without T2DM regardless of gender. However, TSH and FT4 levels did not differ significantly between subjects with and without T2DM.
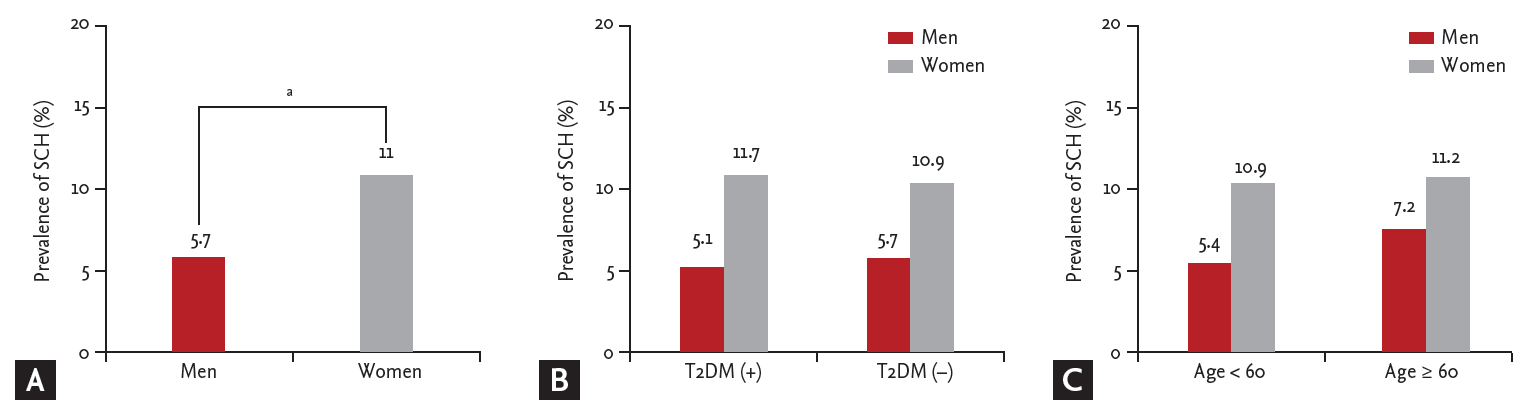
SCH was more prevalent in women than in men (11.0% [382/3,483] vs. 5.7% [255/4,483], p = 0.000) (Fig. 2A). The prevalence of SCH was 5.1% (23/447) in male subjects with T2DM, while it was 5.7% (232/4,036) in those without T2DM (p = 0.573). In women, the prevalence of SCH was 11.7% (27/231) in the T2DM subjects compared with 10.9% in those without T2DM (p = 0.712). There was no significant differencein both men and women (Fig. 2B). Additionally, the prevalence of SCH was not significantly higher in older age (> 60 years; in men, 5.4% [208/3,831] vs. 7.2% [47/652], p = 0.070; in women, 10.9% [317/2,901] vs. 11.2% [65/582], p = 0.865) (Fig. 2C).
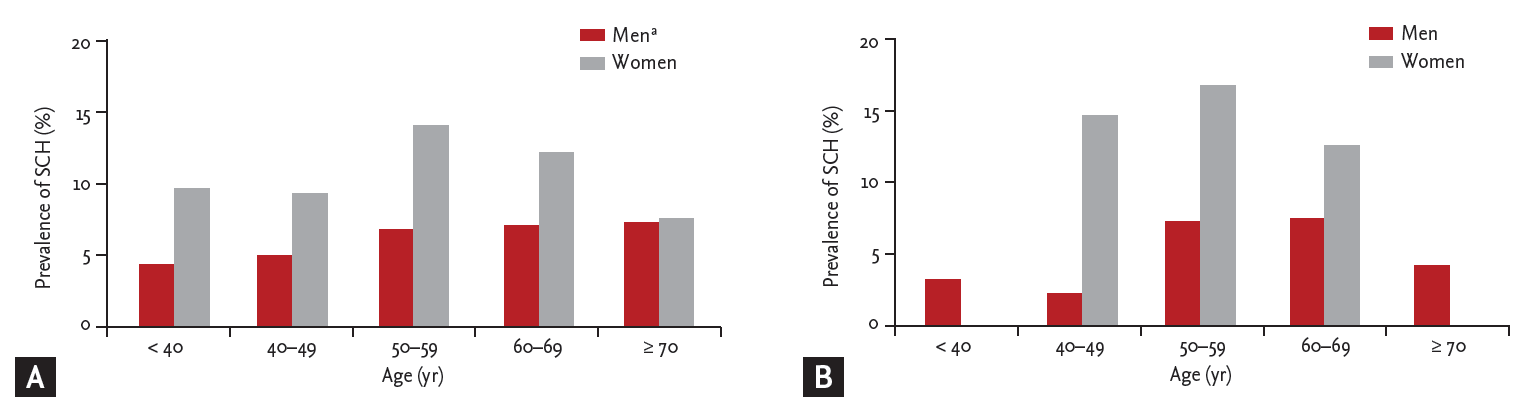
Subjects were analyzed by age group. TSH level increased with age in men but not in women (Fig. 3A). In T2DM subjects, age was not associated with prevalence of SCH in both men and women (Fig. 3B)
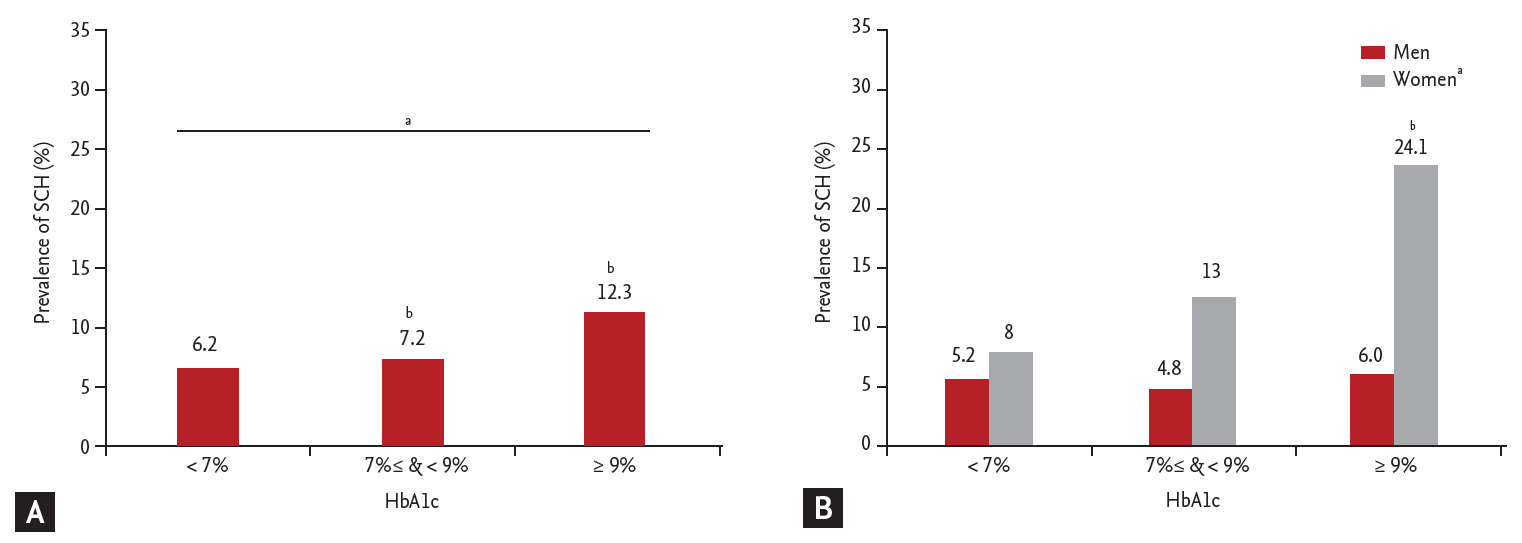
Subjects with T2DM were divided into two groups according to thyroid function, those with SCH or those with euthyroidism (Table 2). Diabetic subjects with SCH were characterized by higher TSH. BMI, hypertension, systolic and diastolic blood pressure, FPG, BUN, creatinine, total cholesterol, HDL-C, triglyceride, LDL-C, and FT4 levels did not differ between groups. Moreover, no significant between group difference was observed in HbA1c, although there was a tendency toward higher HbA1c in diabetic subjects with SCH in both men and women (in men, 7.67% ┬▒ 1.30% vs. 7.53% ┬▒ 1.33%, p = 0.62; in women, 8.34% ┬▒ 2.38% vs. 7.42% ┬▒ 1.29%, p = 0.05). When subjects with T2DM were categorized into three groups according to HbA1c, the prevalence of SCH was higher in the poorly glycemic control group (Fig. 4A). Also, the prevalence of SCH was higher in the poorly glycemic control group in women significantly (Fig. 4B).
We attempt to investigate the relationship between SCH and glycemic control in subjects with T2DM (Table 3). After adjustment for age, sex, BMI, hypertension, total cholesterol, triglyceride, HDL-C, and LDL-C, multivariate logistic regression analysis identified a positive association between SCH and glycemic control. The OR for HbA1c Ōēź 9% compared to < 7% was 2.52 (95% CI, 1.09 to 5.86; p = 0.031). It tended to more increase in older age (age over 60) and women (OR for HbA1c Ōēź 9% compared to < 7%, 4.77; 95% CI, 1.18 to 19.29; p = 0.028, and OR, 4.58; 95% CI, 1.41 to 14.87; p = 0.011, respectively). Moreover, the relationship was more obvious, especially in older women (OR for HbA1c Ōēź 9% compared to < 7%, 12.76; 95% CI, 1.41 to 115.68; p = 0.024) but not in men.
In the present study, the prevalence of SCH was not different between patients with T2DM and healthy control. However, in group with T2DM, poor glycemic control was related with a higher prevalence of SCH, and that was prominent in older women.
We found that the prevalence of SCH was similar regardless of diabetes (in men, 5.7% control vs. 5.1% T2DM, p = 0.573; in women, 10.9% vs. 11.7%, p = 0.712). Controversy still exists with regard to relationship between SCH and T2DM. Several reports documented thyroid dysfunction was more often in the diabetic population [14,18-21]. However, most of these studies included diabetic subjects with type 1 as well as type 2, and lacked control subjects. Selection bias also cannot be rule out because chances of thyroid test are higher in diabetic patients rather than healthy individuals. Our result correspond with the report of Ishay et al. [16] which showed that there was no significant difference between a T2DM group and a non-diabetic control. Recent cohort study showed SCH risk for T2DM was not prominent by comparison with overt hypothyroidism [22], SCH doesnŌĆÖt seem to be more frequent in diabetes but confirmatory evidences are needed. SCH was also shown to be more prevalent in women than in men in the present study, which is in accordance with previous studies [6,11,23].
An important finding in our study is that the risk of SCH is increased with poor glycemic control, especially HbA1c > 9%. It was more obvious to elderly women. To our knowledge, only few studies about the association between SCH and degree of glycemic control in T2DM. Insulin resistance may be involved in the association of SCH with poor glycemic control. Fasting hyperinsulinemia was reported in patients with SCH [24]. Moreover, Maratou et al. [25] demonstrated that patients with SCH have insulin resistance comparable to that of the patients with hypothyroidism. Previous investigation showed impairment of insulin-stimulated glucose disposal was found in SCH, and it caused by impaired translocation of glucose transporter type 4 (GLUT4) on the cell surface [25]. Although our study did not include insulin resistance indexes such as homeostasis model assessment of insulin resistance (HOMA-IR), it can be speculated that insulin resistance would be elevated in the poor glycemic control group. Previous work indicated that HOMA-IR was higher in diabetic subjects with SCH than in those with normal thyroid function [26]. El-Eshmawy et al. [27] also revealed positive correlations between TSH levels and HOMA-IR. Interestingly, there are the opposite results were reported. Some investigators showed mild elevated TSH protected rats with diabetes from oxidative stress in comparison with isolated diabetes group. They suggested the increased incidence of SCH could be a reflection of a physiological adaptation against damage wrought by diabetes [28]. Taken together, these findings indicate that an elevated level of serum TSH may be associated with fasting hyperglycemia and insulin resistance in patients with HbA1c levels above 9%. The relationship between SCH and T2DM seems to have a complex interdependent interaction, and more studies clarifying the exact mechanisms are still needed.
Overt hypothyroidism is clearly involved with abnormal lipid profiles regardless of diabetes [29,30] but there is disagreement between SCH and dyslipidemia. A recent meta-analysis showed that SCH is not associated with dyslipidemia [31]. In the present study, diabetic patients with SCH had better triglyceride and HDL-C profiles than those with normal thyroid function. We could not determine the effect of SCH on lipid profile in this study for some limitationsŌĆöcross-sectional study and history of medications, such as statins, was not taken. But our result may be a clue to answer the question and well-designed studies are needed in the future.
A study in Korean reported the prevalence of SCH in patients with T2DM was 12.4% [26], whereas only 7.4% in the present study. This discrepancy is appeared to depend on characteristics of enrolled subjects. In their study [26], all participants had microvascular complications and glycemic control was generally poorer than that of our study.
Our study have a strength for large sample size, butit should be noted that this study has some limitations. Firstly, blood test for thyroid function was done for a single time. Secondly, this was a cross-sectional study; therefore, it could not determine causal relationship between SCH and glycemic control. Well-designed prospective studies are warranted to confirm the association between SCH and glycemic control in patients with T2DM. Furthermore, future studies will be required to determine whether thyroid hormone replacement therapy can improve glycemic control in diabetic patients with SCH. Lastly, medications being taken by the patients were not checked, which may have an effect on thyroid functionŌĆömultivitamins and/or health supplements.
Although annual thyroid screening is currently only recommended for children and adolescents with type 1 diabetes, there is no consensus as to whether screening for thyroid disorders should be mandatory in T2DM [17]. With regard to clinical application of this study, wepropose the possibility of SCH as a comorbid condition should be considered in poorly controlled diabetes. It would be needed screening for thyroid function when T2DM patient is not controlled adequately, especially in elderly women.
In conclusion, the prevalence of SCH was similar between T2DM and healthy population. However, the risk of SCH was increased with poor glycemic control in patients with T2DM, and obviously in elder women. These results suggest SCH as comorbidity may be considered in elderly women with poor glycemic control.
Acknowledgments
This study was supported by a grant of the Korea Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A111345).
Figure┬Ā1.
Age distributions of subjects. (A) The percentage of all subjects by sex and age. (B) The percentage of diabetic subjects by sex and age. T2DM, type 2 diabetes mellitus.
Figure┬Ā2.
Comparison of the prevalence of subclinical hypothyroidism (SCH) in the different groups. (A) The prevalence of SCH was higher in women than men. (B) There was no significant difference in the prevalence of SCH between subjects with and without type 2 diabetes mellitus (T2DM), in both men and women. (C) The prevalence of SCH was not significantly higher in older individuals (age over 60). Differences were analyzed by Student t test. ap < 0.05.
Figure┬Ā3.
Comparison of the prevalence of subclinical hypothyroidism (SCH) according to age groups. (A) In male subjects, the prevalence of SCH was increased with advanced age (p for trend = 0.003). However, the prevalence of SCH was not associated with age in women. (B) In type 2 diabetes mellitus subjects, age was not associated with prevalence of SCH in both men and women. Differences were analyzed by analysis of variance. ap < 0.05 for trends.
Figure┬Ā4.
The prevalence of subclinical hypothyroidism (SCH) within subjects with type 2 diabetes mellitus (T2DM). (A) Among tertiles, SCH was significantly increased in the highest glycosylated hemoglobin (HbA1c) group (HbA1c Ōēź 9%) compared with well controlled group (HbA1c < 7%). (B) In female subjects, SCH was significantly increased in the highest HbA1c group compared with well controlled group (p for trend = 0.037) but not in men. Differences were analyzed by analysis of variance followed by Tukey test. ap < 0.05 for trends, bp < 0.05 for post hoc analysis vs. HbA1c < 7%.
Table┬Ā1.
Clinical characteristics of the study subjects
| Characteristic |
Subjects without T2DM (n = 7,288) |
Subjects with T2DM (n = 678) |
||||
|---|---|---|---|---|---|---|
| Total | Men (n = 4,036) | Women (n = 3,252) | Total | Men (n = 447) | Women (n = 231) | |
| Age, yra | 46.8 ┬▒ 10.9 | 46.9 ┬▒ 10.5 | 46.7 ┬▒ 11.4 | 55.4 ┬▒ 9.9 | 53.5 ┬▒ 9.6 | 59.2 ┬▒ 9.5 |
| BMI, kg/m2,a | 23.6 ┬▒ 2.9 | 24.3 ┬▒ 2.7 | 22.8 ┬▒ 2.9 | 25.1 ┬▒ 3.0 | 25.2 ┬▒ 2.6 | 25.0 ┬▒ 3.8 |
| Hypertensionb | 865 (11.9) | 591 (14.6) | 274 (8.4) | 170 (25.1) | 109 (24.4) | 61 (26.4 ) |
| Systolic BP, mmHga | 114.6 ┬▒ 12.9 | 118.1 ┬▒ 11.1 | 110.3 ┬▒ 13.6 | 122.9 ┬▒ 13.1 | 122.9 ┬▒ 11.7 | 123.0 ┬▒ 15.6 |
| Diastolic BP, mmHga | 73.2 ┬▒ 10.5 | 76.0 ┬▒ 9.2 | 69.8 ┬▒ 10.9 | 79.0 ┬▒ 9.8 | 79.2 ┬▒ 9.0 | 78.8 ┬▒ 11.4 |
| FPG, mg/dLa | 89.7 ┬▒ 9.3 | 91.1 ┬▒ 9.4 | 87.9 ┬▒ 8.8 | 136.5 ┬▒ 39.4 | 139.6 ┬▒ 38.4 | 130.6 ┬▒ 40.9 |
| HbA1c, %a | 5.5 ┬▒ 0.4 | 5.5 ┬▒ 0.3 | 5.5 ┬▒ 0.3 | 7.5 ┬▒ 1.4 | 7.5 ┬▒ 1.3 | 7.5 ┬▒ 1.4 |
| BUN, mg/dLa | 13.5 ┬▒ 3.6 | 14.2 ┬▒ 3.6 | 12.6 ┬▒ 3.5 | 14.8 ┬▒ 4.0 | 15.1 ┬▒ 3.8 | 14.4 ┬▒ 4.3 |
| Creatinine, mg/dL | 0.9 ┬▒ 0.1 | 1.0 ┬▒ 0.1 | 0.7 ┬▒ 0.1 | 0.9 ┬▒ 0.1 | 0.9 ┬▒ 0.1 | 0.7 ┬▒ 0.1 |
| Total cholesterol, mg/dLa | 200.3 ┬▒ 34.6 | 202.0 ┬▒ 34.1 | 198.2 ┬▒ 35.2 | 210.56 ┬▒ 43.1 | 206.3 ┬▒ 40.5 | 218.7 ┬▒ 46.7 |
| Triglyceride, mg/dLa | 131.0 ┬▒ 85.8 | 153.1 ┬▒ 95.7 | 103.6 ┬▒ 61.4 | 186.5 ┬▒ 140.5 | 195.6 ┬▒ 149.5 | 169.0 ┬▒119.4 |
| HDL-C, mg/dLa | 59.5 ┬▒ 15.1 | 55.5 ┬▒ 13.8 | 64.4 ┬▒ 15.2 | 53.9 ┬▒ 14.6 | 52.1 ┬▒ 13.1 | 57.5 ┬▒ 16.6 |
| LDL-C, mg/dL | 114.6 ┬▒ 31.6 | 115.8 ┬▒ 32.0 | 113.0 ┬▒ 31.1 | 119.2 ┬▒ 40.5 | 115.0 ┬▒ 39.4 | 127.4 ┬▒ 41.4 |
| TSH, mlU/L | 2.1 ┬▒ 1.6 | 1.9 ┬▒ 1.5 | 113.0 ┬▒ 31.1 | 1.9 ┬▒ 1.5 | 1.8 ┬▒ 1.4 | 2.2 ┬▒ 1.6 |
| Free T4, ng/dL | 1.2 ┬▒ 0.4 | 1.3 ┬▒ 0.2 | 1.2 ┬▒ 0.2 | 1.2 ┬▒ 0.4 | 1.3 ┬▒ 0.2 | 1.3 ┬▒ 0.1 |
| SCH | 587 (8.1) | 232 (5.7) | 355 (10.9) | 50 (74) | 23 (5.1) | 27 (11.7) |
T2DM, type 2 diabetes mellitus; BMI, body mass index; BP, blood pressure; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; BUN, blood urea nitrogen; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TSH, thyroid stimulating hormone; T4, thyroxine; SCH, subclinical hypothyroidism.
Table┬Ā2.
Clinical characteristics of type 2 diabetes with and without subclinical hypothyroidism
| Characteristic |
Euthyroidism (n = 628) |
Subclinical hypothyroidism (n = 50) |
||||
|---|---|---|---|---|---|---|
| Total | Men (n = 424) | Women (n = 204) | Total | Men (n = 23) | Women (n = 27) | |
| Age, yr | 55.3 ┬▒ 10.1 | 53.3 ┬▒ 9.6 | 59.4 ┬▒ 9.3 | 56.9 ┬▒ 8.1 | 56.6 ┬▒ 9.4 | 57.2 ┬▒ 6.9 |
| BMI, kg/m2 | 25.1 ┬▒ 2.9 | 25.2 ┬▒ 2.6 | 24.8 ┬▒ 3.5 | 25.7 ┬▒ 4.3 | 25.0 ┬▒ 2.7 | 26.3 ┬▒ 5.3 |
| Hypertension | 152 (24.2) | 99 (23.3) | 53 (26.0) | 18 (36.0) | 10 (43.5) | 8 (29.6) |
| Systolic BP, mmHg | 122.7 ┬▒ 12.7 | 122.7 ┬▒ 11.5 | 122.9 ┬▒ 14.9 | 125.0 ┬▒ 17.7 | 126.5 ┬▒ 14.6 | 123.7 ┬▒ 20.2 |
| Diastolic BP, mmHg | 79.0 ┬▒ 9.7 | 79.0 ┬▒ 8.9 | 78.9 ┬▒ 11.2 | 80.2 ┬▒ 12.0 | 82.1 ┬▒ 10.4 | 78.5 ┬▒ 13.2 |
| FPG, mg/dL | 135.9 ┬▒ 38.3 | 139.6 ┬▒ 38.3 | 128.3 ┬▒ 37.2 | 143.7 ┬▒ 51.6 | 139.1 ┬▒ 39.6 | 147.6 ┬▒ 60.5 |
| HbA1c, % | 7.5 ┬▒ 14 | 7.5 ┬▒ 1.3 | 7.4 ┬▒ 13 | 8.0 ┬▒ 2.0 | 7.6 ┬▒ 1.3 | 8.3 ┬▒ 2.3 |
| BUN, mg/dL | 14.8 ┬▒ 4.1 | 15.0 ┬▒ 3.9 | 14.4 ┬▒ 4.4 | 14.7 ┬▒ 3.6 | 15.0 ┬▒ 3.4 | 14.6 ┬▒ 3.8 |
| Creatinine, mg/dL | 0.9 ┬▒ 0.1 | 0.9 ┬▒ 0.1 | 0.7 ┬▒ 0.1 | 0.9 ┬▒ 0.1 | 1.0 ┬▒ 0.1 | 0.7 ┬▒ 0.1 |
| Total cholesterol, mg/dL | 210.8 ┬▒ 43.1 | 206.6 ┬▒ 40.4 | 219.6 ┬▒ 47.2 | 207.1 ┬▒ 42.7 | 200.7 ┬▒ 42.3 | 212.5 ┬▒ 43.1 |
| Triglyceride, mg/dL | 189.2 ┬▒ 144.3 | 198.2 ┬▒ 152.6 | 170.5 ┬▒ 123.6 | 153.4 ┬▒ 70.4 | 147.9 ┬▒ 56.7 | 158.2 ┬▒ 81.1 |
| HDL-C, mg/dL | 53.5 ┬▒ 14.2 | 51.8 ┬▒ 12.85 | 57.08 ┬▒ 16.1 | 58.9 ┬▒ 18.5 | 57.0 ┬▒ 16.9 | 60.7 ┬▒ 19.9 |
| LDL-C, mg/dL | 119.4 ┬▒ 40.5 | 115.1 ┬▒ 39.3 | 128.4 ┬▒ 41.6 | 117.4 ┬▒ 40.4 | 114.0 ┬▒ 42.3 | 120.2 ┬▒ 39.4 |
| TSH, mIU/La | 1.6 ┬▒ 0.8 | 1.6 ┬▒ 0.8 | 1.8 ┬▒ 0.8 | 6.0 ┬▒ 2.4 | 6.1 ┬▒ 2.8 | 5.8 ┬▒ 2.1 |
| Free T4, ng/dL | 1.2 ┬▒ 0.4 | 1.3 ┬▒ 0.2 | 1.3 ┬▒ 0.2 | 1.2 ┬▒ 0.4 | 1.3 ┬▒ 0.2 | 1.2 ┬▒ 0.1 |
Table┬Ā3.
Multivariate logistic regression analysis of association between subclinical hypothyroidism and glycemic control in subjects with type 2 diabetes mellitus
| Variable |
Total |
Men |
Women |
||||||
|---|---|---|---|---|---|---|---|---|---|
| ╬▓ | OR (95% CI) | p value | ╬▓ | OR (95% CI) | p value | ╬▓ | OR (95% CI) | p value | |
| All age | |||||||||
| ŌĆā< 7% | Reference | Reference | Reference | ||||||
| ŌĆā7% Ōēż & < 9% | 0.25 | 1.28 (0.67ŌĆō2.49) | 0.46 | 0.00 | 1.00 (0.40ŌĆō2.52) | 0.10 | 0.67 | 1.96 (0.74ŌĆō5.20) | 0.18 |
| ŌĆāŌēź 9% | 0.92 | 2.52 (1.09ŌĆō5.86) | 0.03a | 0.39 | 1.48 (0.37ŌĆō5.91) | 0.93 | 2.27 | 4.58 (1.41ŌĆō14.87) | 0.01a |
| Age Ōēź 60 yr | |||||||||
| ŌĆā< 7% | Reference | Reference | Reference | ||||||
| ŌĆā7% Ōēż & < 9% | 0.25 | 1.28 (0.40ŌĆō4.13) | 0.68 | 0.08 | 1.08 (0.21ŌĆō5.51) | 0.93 | 0.46 | 1.58 (0.25ŌĆō9.82) | 0.63 |
| ŌĆāŌēź 9% | 1.56 | 4.77 (1.18ŌĆō19.29) | 0.03a | 0.86 | 2.36 (0.17ŌĆō32.77) | 0.52 | 3.91 | 12.76 (1.41ŌĆō115.68) | 0.02a |
REFERENCES
1. Helfand M, Redfern CC. Clinical guideline, part 2. Screening for thyroid disease: an update. American College of Physicians. Ann Intern Med 1998;129:144ŌĆō158.


2. Bindels AJ, Westendorp RG, Frolich M, Seidell JC, Blokstra A, Smelt AH. The prevalence of subclinical hypothyroidism at different total plasma cholesterol levels in middle aged men and women: a need for case-finding? Clin Endocrinol (Oxf ) 1999;50:217ŌĆō220.


3. Tsimihodimos V, Bairaktari E, Tzallas C, Miltiadus G, Liberopoulos E, Elisaf M. The incidence of thyroid function abnormalities in patients attending an outpatient lipid clinic. Thyroid 1999;9:365ŌĆō368.


4. Pirich C, Mullner M, Sinzinger H. Prevalence and relevance of thyroid dysfunction in 1922 cholesterol screening participants. J Clin Epidemiol 2000;53:623ŌĆō629.


5. Tzotzas T, Krassas GE, Konstantinidis T, Bougoulia M. Changes in lipoprotein(a) levels in overt and subclinical hypothyroidism before and during treatment. Thyroid 2000;10:803ŌĆō808.


6. Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med 2000;132:270ŌĆō278.


7. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000;160:526ŌĆō534.


8. Badman MK, Chowdhury TA. Should thyroid function tests be done annually in all patients with diabetes? Diabet Med 2002;19 Suppl 3:7ŌĆō9.


9. McDermott MT, Ridgway EC. Subclinical hypothyroidism is mild thyroid failure and should be treated. J Clin Endocrinol Metab 2001;86:4585ŌĆō4590.


11. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489ŌĆō499.


13. Gray RS, Irvine WJ, Clarke BF. Screening for thyroid dysfunction in diabetics. Br Med J 1979;2:1439.



14. Perros P, McCrimmon RJ, Shaw G, Frier BM. Frequency of thyroid dysfunction in diabetic patients: value of annual screening. Diabet Med 1995;12:622ŌĆō627.


15. Smithson MJ. Screening for thyroid dysfunction in a community population of diabetic patients. Diabet Med 1998;15:148ŌĆō150.


16. Ishay A, Chertok-Shaham I, Lavi I, Luboshitzky R. Prevalence of subclinical hypothyroidism in women with type 2 diabetes. Med Sci Monit 2009;15:CR151ŌĆōCR155.

17. American Diabetes Association. Standards of medical care in diabetes: 2014. Diabetes Care 2014;37 Suppl 1:S14ŌĆōS80.



18. Palma CC, Pavesi M, Nogueira VG, et al. Prevalence of thyroid dysfunction in patients with diabetes mellitus. Diabetol Metab Syndr 2013;5:58.



19. Papazafiropoulou A, Sotiropoulos A, Kokolaki A, Kardara M, Stamataki P, Pappas S. Prevalence of thyroid dysfunction among Greek type 2 diabetic patients attending an outpatient clinic. J Clin Med Res 2010;2:75ŌĆō78.



20. Celani MF, Bonati ME, Stucci N. Prevalence of abnormal thyrotropin concentrations measured by a sensitive assay in patients with type 2 diabetes mellitus. Diabetes Res 1994;27:15ŌĆō25.

21. Hage M, Zantout MS, Azar ST. Thyroid disorders and diabetes mellitus. J Thyroid Res 2011;2011:439463.




22. Gronich N, Deftereos SN, Lavi I, Persidis AS, Abernethy DR, Rennert G. Hypothyroidism is a risk factor for new-onset diabetes: a cohort study. Diabetes Care 2015;38:1657ŌĆō1664.


23. Sawin CT, Castelli WP, Hershman JM, McNamara P, Bacharach P. The aging thyroid: thyroid deficiency in the Framingham Study. Arch Intern Med 1985;145:1386ŌĆō1388.


24. Tuzcu A, Bahceci M, Gokalp D, Tuzun Y, Gunes K. Subclinical hypothyroidism may be associated with elevated high-sensitive c-reactive protein (low grade inflammation) and fasting hyperinsulinemia. Endocr J 2005;52:89ŌĆō94.


25. Maratou E, Hadjidakis DJ, Kollias A, et al. Studies of insulin resistance in patients with clinical and subclinical hypothyroidism. Eur J Endocrinol 2009;160:785ŌĆō790.


26. Kim BY, Kim CH, Jung CH, Mok JO, Suh KI, Kang SK. Association between subclinical hypothyroidism and severe diabetic retinopathy in Korean patients with type 2 diabetes. Endocr J 2011;58:1065ŌĆō1070.


27. El-Eshmawy MM, Abd El-Hafez HA, El Shabrawy WO, Abdel Aal IA. Response: subclinical hypothyroidism is independently associated with microalbuminuria in a cohort of prediabetic egyptian adults (Diabetes Metab J 2013;37:450-7). Diabetes Metab J 2014;38:85ŌĆō86.



28. Ashwini S, Bobby Z, Joseph M. Mild hypothyroidism improves glucose tolerance in experimental type 2 diabetes. Chem Biol Interact 2015;235:47ŌĆō55.


29. Staub JJ, Althaus BU, Engler H, et al. Spectrum of subclinical and overt hypothyroidism: effect on thyrotropin, prolactin, and thyroid reserve, and metabolic impact on peripheral target tissues. Am J Med 1992;92:631ŌĆō642.


-
METRICS

- Related articles
-
Musculoskeletal complications in patients with diabetes mellitus2022 November;37(6)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


