INTRODUCTION
Aminoglycoside antibiotics, including gentamicin (GM), are widely used in the treatment of gram-negative infections. A major complication of the use of these drugs is nephrotoxicity, accounting for 10% to 15% of all cases of acute renal failure1, 2). Based on comparative clinical nephrotoxicity, GM is the most nephrotoxic drug2). The specificity of GM for renal toxicity is apparently related to its preferential accumulation in the renal proximal convoluted tubules. Although derangement of many cellular alterations has been proposed, the specific mechanism whereby GM induces proximal tubular cell injury remains to be determined.
Apoptosis has recently been recognized as an important mechanism of cell death mediating chemical and hypoxic injury to epithelial cells3, 4). Apoptosis is an active form of cell death whose morphological features consist of both cytosolic and nuclear shrinkage, quite distinct from necrosis. Apoptotic cells and bodies are rapidly phagocytosed and destroyed by macrophages and neighboring cells, so apoptos is is usually inconspicuous in tissue sections even when it is responsible for extensive and rapid cell loss. Recently, apoptosis has been demonstrated to occur in the kidney following ischemic injury or partial ureteral obstruction5). Therefore, the role played by apoptosis in aminoglycoside nephrotoxicity has become an important issue. Whether or not apoptosis of the renal proximal tubular epithelial cells is induced by GM has not been previously studied.
In order to further clarify the mechanisms of renal tubular cell death induced by aminoglycosides, we examined the renal proximal tubule-like cell line, LLCPK1, after chronic treatment with GM to produce apoptosis. The change in the expression of the Fas was also investigated.
MATERIALS AND METHODS
Reagents: Gentamicin sulfate, DulbeccoŌĆÖs modified EagleŌĆÖs medium and fetal calf serum were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The concentration of GM was determined based on the weight of GM sulfate salt in 100 mM Tris/HCl (pH 7.5).
1. Cell culture
The porcine proximal tubule-like cell line LLC- PK1 (ATCC CRL-1392) was obtained from the American Type Culture Collection (Rockville, MD, USA). The cells were cultured on 100 mm culture-grade plastic dishes in nutrient ╬▒-modified EagleŌĆÖs medium supplemented with 3% fetal calf serum (FCS) in an atmosphere of 5% CO2 at 37┬░C. The monolayers were plated at 3├Ś105 cells per well on six-well plates and incubated four to six days until confluent. A fresh medium was provided on days three and five after plating. The experiments were performed on confluent cells between the 20th and 50th passages.
GM treatment: The confluent monolayers were washed three times in HBSS and provided with either a fresh medium (control) or a medium containing GM in various concentrations (0.1, 1, and 10 mM) and incubated for 15 days. Fresh study media were provided every three days until the cells were harvested for the apoptosis analysis.
2. The assessment of apoptosis
Fluorescence-activated cell sorter (FACS) analysis: A total of 950 ╬╝L of cell suspension at a concentration of 106 cells/mL was incubated with 50 ╬╝L propidium iodide (PI, 100 ╬╝g/mL, Calbiochem-Novabiochem Co., La Jolla, CA. USA) in the dark at 4┬░C. The cells were analyzed by FACScan flow cytometry (FACSort; Becton Dickson, USA). The selected gate corresponded to the cells according to the dot-blot histogram (10,000 to 50,000 cells were counted). The inter-and intra-assay variation coefficient was less than 6%.
DNA fragmentation on agarose gels: The DNA fragmentation assay was performed according to the method published by Van de Water et al.6) Adherent cells were scraped into phosphate-buffered saline and pelleted by centrifugation at 200 g for 5 min. The spontaneously detached cells were processed separately and combined with the adherent cells before extracting a single fraction of DNA for electrophoresis. The cell pellets were lysed by overnight incubation in a 300 ╬╝L lysis buffer containing 10 mM Tris-HCl, 10 mM EDTA, 50 mM NaCl, 0.2% SDS, and 200 ╬╝g/mL proteinase K, pH 7.6 at 42┬░C. The lysate was added to the pellet of the pooled washings and placed on ice for 15 min. The lysates were centrifuged at 13,000 g for 20 minutes and the fragmented DNA present in the supernatant was extracted with phenol-chloroformisoamyl(25:24:1) and ethanol precipitation. The pellet was resuspended in 20ŌĆō30 ╬╝L of Tris-EDTA buffer containing RNAse A, incubated for 30 minutes at 37┬░C and then separated by electrophoresis on a 1% agarose gel containing ethidium bromide. The DNA was visualized by ultraviolet examination. The size of the DNA fragments was compared with a 123-bp standard DNA ladder.
3. Fas expression
Each well was washed with ice-cold phosphatebuffered saline and treated with a lysis buffer (150 mM NaCl, 1% NP-40, 0.5% DOC, 50 mM Tris, and 0.1% SDS, pH 8.0) including protease inhibitors for an hour on ice. The lysed cells were centrifuged at 12,000 g for 10 minutes at 4┬░C. Twenty micrograms of the cell extracts were resolved on 10% SDS-PAGE. The separated proteins were then transferred to a nitrocellulose membrane. After blocking with 5% nonfat dry milk, the membrane was incubated for one hour with 1:1000 polyclonal (rabbit) anti-Fas antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted in 5% milk non-fat proteins/Tris-buffered salinetween buffer. The blot was washed again and incubated with a goat anti-rabbit horseradish peroxidase conjugated secondary antibody (Amersham, Pharmacia Biotech UK Ltd., Buckinghamshire, England). The blot was developed using enhanced chemiluminescence.
RESULTS
1. Effects of GM on apoptosis
On the flow cytometric assessment of apoptosis using light scatter measurements, 5.7┬▒3.3% of the control cells appeared in a region of decreased forward light scatter (FSC) and increased side light scatter (SSC), where both indices represent the characteristics of apoptotic cell death. Compared to the control, treatment with GM for more than 12 days significantly increased the proportion of cells in the apoptotic region in a dose dependent manner (Table 1, Figure 1). The maximum apoptosis occurred at 15 days post-treatment (5.7┬▒ 3.3% vs 23.9┬▒8.5% at 10 mM GM, p<0.05). No significant apoptosis was observed in the cells treated with GM for less than 12 days.
These findings were supported by electrophoretic analysis of the DNA extracted from the GM-treated cells. The DNA extracted from the GM-treated cells was visualized as a series of bands corresponding to integer multiples of 200 base pairs that are characteristic of apoptosis (Figure 2).
2. The effect of GM on Fas expression
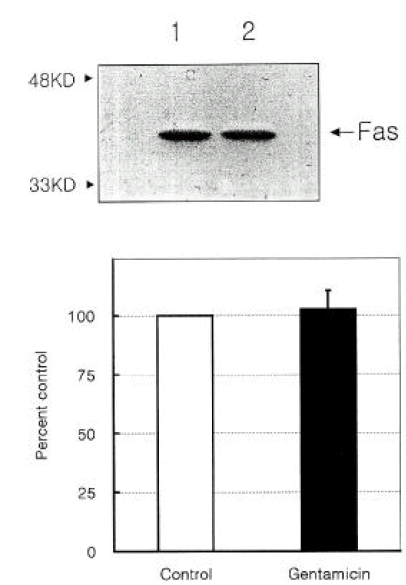
To assess the changes in the Fas expression following GM exposure, Western blots were performed on the cells exposed to 10 mM GM for 7 to 15 days every 24 hours. Although there was a significant increase in the number of apoptotic cells, there as no significant difference in the expression of the 45 kD Fas protein between the GM- treated and control cells (Figure 3).
DISCUSSION
Aminoglycos ides are polycationic compounds that are not metabolized and are minimally protein bound1). After glomerular filtration, aminoglycosides bind to the apical membrane of renal proximal tubular cells and are internalized by adsorptive endocytosis7). After uptake into the cells, a number of intracellular processes are disrupted by the presence of aminoglycosides. Although the mechanism of aminoglycosideelicited renal proximal tubular cell injury has been investigated in many studies, the exact biochemical determinant of tubular cell injury remains unknown.
Apoptosis is a physiologic, programmed cell death that occurs under both physiological and pathological conditions. In particular, apoptosis has been reported to be an important mechanism that mediates ischemic, chemical hypoxic and oxidant injury of renal tubules8). Duncan-Achanzar et al.9) reported that apoptosis in response to 35 ╬╝M HgCl2 was confirmed by observing the morphological features characteristic of apoptotic cells. Lieberthal et al.10) also suggested apoptosis as the mechanism of death induced by cis-platin in proximal tubular epithelial cells. In the present study, we demonstrated that apoptosis occurred in LLC-PK1 cells after treatment with GM for 15 days. Nakagawa et al.11) reported the GM-induced apoptosis of vestibular hair cells of guinea pigs. To our knowledge, our finding is the first evidence of GM-induced apoptosis in renal tubular epithelial cells.
Chronic exposure of renal tubular epithelial cells to high levels of GM leads to the accumulation of GM within the lysosomes accompanied by cell injury. Ford et al.12) reported that LLC-PK1 cells exposed to 2 mM GM for six days displayed a significant decrease in the specific activities of lysosomal and apical membrane enzymes with an increased release into the medium. Hori et al.13) and Schwertz et al.14) also evaluated the alterations in enzyme activities and cellular lipid levels, as well as performing a morphological examination of renal tubular epithelial cell injuries, after exposure to GM for three and seven days. In our study, treatment with GM at a concentration of 10 mM for 15 days induced apoptosis in the LLC-PK1 cells. Based on these findings, we postulate that apoptosis occurs in renal tubular epithelial cells after long-term exposure to GM, and this process may be crucial for cell injury, subsequent, and eventual regeneration. It remains to be determined, however, whether the 10 mM of GM can be achieved in the renal proximal tubular cells in experimental animals and in humans treated with GM.
The process leading to apoptosis is complex and various primary biochemical events that may initiate the apoptosis process are believed to exist including deprivation of growth factors, mitochondrial injury, oxidative stress, F-actin cytoskeletal injury and perturbation of intracellular Ca2+ homeostasis4, 8). The mechanism(s) responsible for apoptosis in GM-induced injury to LLC-PK1 cells is not known. The ability of GM to alter mitochondrial respiration has been well documented in reports of both in vitro and in vivo studies15, 16). Walker and Shah15) observed that the generation of hydrogen peroxide by the mitochondria was enhanced from less than 0.5 nmol in the absence of GM to more than 6 nmol/mg/min in the presence of GM. Recently, GM has also been shown to enhance the generation of superoxide anion and hydroxyl radical in renal cortical mitochondria. Reactive oxygen species play an important role in the activation of the endonuclease and consequent DNA damage4, 10). Based on these findings, we postulated that apoptosis may occur through the enhanced generation of reactive oxygen metabolites following exposure to GM.
We have determined experimentally that the DNA ladder pattern characteristic of apoptosis occurs in the LLC-PK1 cell monolayers 14 days after 10 mM GM exposure. This result demonstrates that the endonuclease-mediated cleavage of the DNA into nucleosome-sized fragments occurs after long-term exposure to GM. Reactive oxygen species have been reported to play an important role in the activation of endonuclease and consequent DNA damage. In addition, Kozek et al.17) reported that the treatment with GM upsets the process of intracellular Ca2+ homeostas is, which may induce apoptos is due to its activation by calcium of the Ca2+/Mg2+-dependent endonucleases that are involved in the cleavage of DNA. Therefore, it can be postulated that the activation of endonuclease following the perturbation of cytosolic calcium caused by GM is another factor for producing apoptosis.
The Fas and its ligand are important mediators of apoptosis and show several structural and functional similarities to the tumor necrosis factor receptor system8, 18). The ability of the Fas to induce death is dependent on the balance between the expression of death factors, such as the interleukin-1-converting enzyme and the expression of protective factors, such as Bcl-2 and Fas-associated phosphatase-18). In the LLC-PK1 cells, a cyclosporine A treatment caused an increase in the express ion of the 45 kD Fas protein. Healy et al.18) suggested that the Fas may be an important mediator of cyclosporine A-induced apoptosis in renal proximal tubular cells. In this study, however, we were unable to observe that the GM exposure was associated with an increase in the expression of the Fas protein.
The use of whole-animal models to study the primary aminoglycoside-induced proximal tubule cell injury is complicated by the structural and functional heterogeneity of the kidney. Furthermore, tubular cell necros is resulting from aminoglycoside exposure is focal in nature, and cellular regeneration coexists temporally19). Many of these complicating factors can be eliminated by the use of a cultured cell model. The LLC-PK1 cell line has renal proximal tubule cell-like properties, including high levels of proximal tubular marker enzymes9). Hori et al.12) concluded from their investigation that the LLC-PK1 cell line could be a useful model for studying aminoglycoside nephrotoxicity in vitro. Thus, we have used the LLC-PK1 cells as a model system for GM nephrotoxicity.
In conclusion, long-term exposure to GM induces apoptosis of the renal tubular epithelial cells and this process may contribute to certain aminoglycoside nephrotoxicities. Further studies are needed on the mechanism(s) of apoptosis induced by GM.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





