 |
 |
| Korean J Intern Med > Volume 15(2); 2000 > Article |
|
Abstract
Background
Inflammation and activation of immune cells have important roles in the pathogenesis of atherosclerosis. We analyzed the involvement of various immune cells in the pathogenesis of atherosclerosis.
Methods
We investigated the presence of foam cells, lymphocytes and killer cells in 11 atherosclerotic plaque specimens removed from Korean patients who underwent carotid endoarterectomy. Atherosclerotic plaques were analyzed by immunohistochemistry using monoclonal antibody specific to foam cells (anti-CD68), pan-T cells (anti-CD3), helper-T cells (anti-CD4), cytotoxic T cells (anti-CD8), granular component of killer cells (anti-TIA-1) and pan-B cells (anti-CD20).
Results
Analysis revealed a general infiltration of immune cells not only in atherosclerotic plaques but also in the vascular wall adjacent to the plaque. Heavy infiltration of CD68+ macrophage was observed in all cases. In addition, significant infiltration of CD3+ T-lymphocytes was observed in all cases, while CD20+ B-cells were observed in only a few cases. Majority of the CD3+ cells was found to be CD4+ helper-T cells. CD8+ cytotoxic T cells and TIA-1+ cells were less prominent.
Increasing evidence suggests that inflammatory changes and activation of immune cells are involved in the initial and acute phase of atherosclerosis1). The activation of monocytes by agents such as oxidized-low density lipoprotein (oxi-LDL) is believed to initiate atherosclerosis. Subsequent transformation of monocytes to macrophage/foam cells leads to the formation of the hallmark of atherosclerosis: fatty streak. Foam cells produce various growth factors and extracellular matrix proteins that stimulate the proliferation of vascular smooth muscle cells and lead to the formation of atherosclerotic plaques2). Foam cells are involved in not only the initiation but also the acute rupture of plaque and further formation of thrombus through the expression of matrix metalloproteinases, stromelysin and tissue factor3–7).
Activation of T-lymphocytes was also observed in atherosclerotic plaque. Hansson et al. demonstrated the presence of T-cells expressing very late activation antigen-1 (VLA-1) and HLA-DR, which in T cells are synthesized only in an activated state8). Furthermore, at least a subtraction of the activated T-cells in the atherosclerotic plaques appears to be specific to oxi-LDL, which is a known inducer of foam cell transformation of monocytes9). These observations indicate that the presence of activated T-lymphocytes is a result of specific immune response to atherogenic components. Activated T-lymphocytes derived cytokines, such as Interferon(IFN)-γ, are also known to induce instability of the plaque6,10–12). Furthermore, it has been reported that T-lymphocytes isolated from unstable angina patients can activate the pro-coagulating activity of monocytes isolated from normal individuals13). In contrast, monocytes isolated from unstable angina patients did not have pro-coagulant activities. These results suggest that T-lymphocytes may control the response of monocytes to atherogenic stimuli.
Previous analysis of atherosclerotic lesions indicated that B-cells may play some important roles. B-cells perform many functions with potential relevance to atherogenesis, such as formation of immune complexes, complement-mediated cytotoxicity (CMC) and antibody dependent cell-mediated cytotoxicity (ADCMC). Immune complexes and complements have been identified in atherosclerotic lesions. These B-cell-mediated immune responses could contribute to the core of necrotic debris seen in advanced complicated atherosclerotic lesions16,17). Components of activated complements are chemotactic for mononuclear cells, can activate monocyte/macrophages and polymorphonuclear leukocytes, and have profound regulatory effects on both T and B lymphocytes18,19).
Although lots of researchers reported the presence of macrophages, T cells and B cells in atherosclerotic plaque, none of them integrated the results in a single setting. We investigated the distribution of foam cells, helper-T cells, cytotoxic-T cells, killer cells and B cells in the atherosclerotic plaques removed from patients during the carotid endoarterectomy. Results indicated that foam cells and helper-T cells constitute a major population of immune cells in the atherosclerotic plaques.
We selected 11 patients, aged from 63 to 81, who underwent carotid endoarterectomy at Samsung Seoul Hospital. The demographic and clinical features of the study subjects are shown in Table 1. Atherosclerotic plaques were washed with saline and fixed with 4% paraformaldehyde within 1 hr after removal.
Standard 5-μm sections of the tissues were made after the fixation in 4% paraformaldehyde, dehydration and paraffin embedding. The sections were stained with haematoxylin and eosin. Immunohistochemistry was performed using the LSAB kit (DAKO, Copenhagen, Denmark) according to the manual provided by the manufacturer. Monoclonal antibodies to CD68 (KP1), CD3 (UCHT1), CD4 (MT310), CD8 (DK25), CD20 (L26), and TIA-1 were purchased from DAKO.
Heavy infiltration of mononuclear cells was observed in the carotid specimens in all cases. Most of the infiltrated areas included the shoulder regions of the plaques in most of the cases and fibrous cap regions in some cases. Infiltration was also observed in the arterial walls adjacent to the plaque (data not shown).
CD68 is known to be a cellular marker specifically expressed in human monocyte/macrophages20). In all cases, heavy staining with anti-CD68 antibody was observed indicating that most of the infiltrating immune cells are in monocyte/macrophage lineage (Table 2 and Figure 1D). The morphological characteristics of these CD68 positive cells indicate that these are foam cells. Most of the CD68 positive foam cells were seen consistently in the intima but were absent from the media and were either isolated or grouped focally.
Various levels of staining, but positive in all cases, were observed with anti-CD3 antibody suggesting that infiltration of T-lymphocytes is involved in the pathogenesis of atherosclerosis (Table 2 and Figure 1E). To find out whether these cells are helper-T cells or cytotoxic-T cells, sections were stained with either anti-CD4, which recognizes the helper-T cells, or an anti-CD8, which recognizes the cytotoxic-T cells. Results indicated that most of the CD3 positive cells are helper-T cells, while cytotoxic-T cells are less prominent and less frequent (Figure 1F and 1G). Most of the CD3 and CD4 positive cells were found in areas where most of the foam cells were found.
Staining with anti-TIA-1 antibody (Figure 1H), which binds the serine esterase-positive granule membrane components of cytotoxic T-lymphocytes and natural killer cells21,22), also resulted in low incidences (Table 2 and Figure 1H). Staining for a pan-B cell marker, CD20, revealed that positive staining was observed only in 3 out of 11 cases (Table 2).
The most important observation made in this study is that all of the carotid atherosclerotic plaques are heavily infiltrated with foam cells and helper-T cells. This indicates that helper-T cells as well as foam cells may play important roles in the formation of plaques. In contrast, other types of cells, such as B cells and cytotoxic-T cells, are not likely to be involved in atherosclerotic plaque formation. Our study, however, does not provide any evidence that the observed T-lymphocytes activate and contribute to lesion progression. We are currently analyzing the activation status of these cells by determining the expression of activation markers.
Development of atherosclerosis proceeds through a complex process involving the deposition of plasma lipoproteins and the proliferation of cellular elements in the artery wall. This chronic condition advances through a series of stages, beginning with fatty streak lesions composed largely of lipid-engorged macrophage foam cells, and ultimately progresses to complex plaques consisting of the core of a lipid and necrotic cell debris covered by fibrous caps. High prevalence of Helper T-cells and monocytes in all atherosclerotic plaques indicate that they either have major roles in the development of plaques or that the development of plaques activate the infiltration of these cells. Several observations suggest that the former is the case. First, monocytes express many cytokines by stimulation with oxidatively modified-LDL. Second, plaques contain oxi-LDL specific T-lymphocytes9).
The CD4+ helper-T cells observed in this study appear to be associated with monocyte/macrophage lineage cells. Helper-T cells may regulate the attachment and penetration stages of the monocytes. It is also possible that the T-lymphocytes within the subendothelial intimal space play a significant role in establishing and maintaining the presence of monocyte/macrophage cells in atherosclerotic lesions through the expression of chemotactic factors, such as IL-8 and MCP-125,26). Cytokines secreted by activated T cells can activate macrophages and regulate their lipoprotein uptake. Activated macrophages, in turn, can secrete a wide variety of potentially atherogenic agents.
Helper-T cells play an important role in a wide variety of human diseases. It is likely that the helper-T cells within the subendothelial intimal space have the potential ability to directly modify the functions of neighboring endothelial cells, monocyte/macrophages and SMCs, rather than being mere passive onlookers. The possible interaction between these cell types may mediate in many pro-atherogenic events.
Acknowledgments
This work was supported by grants from Samsung (C-98-004) and 1998 National R&D program, MOST, ROK (#98-N1-02-02-A-05).
Figure 1.
Histological analysis of human athereosclerotic plaques. (A) H&E staining of atherosclerotic plaque showing gross morphology. Lipid rich core (triangle) and fibrous cap with focal infiltration of immune cells (arrow) are indicated. (B) H&E staining of a region of heavy infiltration. (C) H&E staining showing a foam cell rich region (arrow). Immunohistochemical staining with anti-CD68 (D), anti-CD3 (E), anti-CD4 (F), anti-CD8 (G) and anti-TIA-1 (H) monoclonal antibodies. Magnification: (A), ×40; (B) and (E), ×100; (C), (D), (F), (G), and (H) ×200.
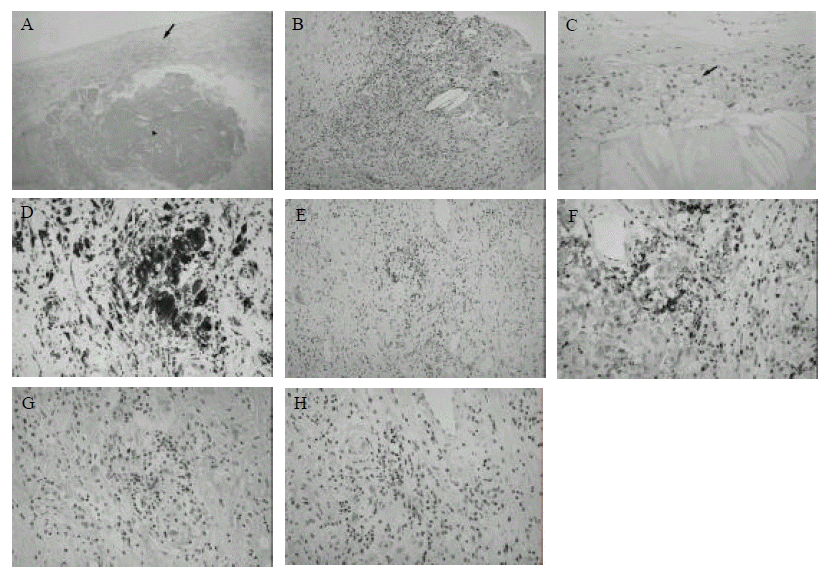
Table 1.
Characteristics of the study subjects.
| Parameter | value |
|---|---|
| n | 11 |
| Age (yrs) | 70.5±9.9 |
| Sex (male/female) | 11/0 |
| BMI (kg/m2) | 23.1±3.0 |
| Total Cholesterol (mg/dL) | 182.0±30.8 |
| Smoking (current/ex/non-smoker) | 3/3/2* |
| Hypertension (n) | 8 |
| Diabetes Mellitus (n) | 5 |
Table 2.
Summary of immunophenotypic analysis of infiltrating inflammatory cells in atherosclerotic plaque
REFERENCES
1. Azar RR, Waters DD. The inflammatory etiology of unstable angina [editorial]. Am Heart J 132:1101–11061996.


2. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–8091993.


3. Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol 7:330–3351996.


4. Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, Fallon JT, Regnstrom J, Fuster V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation 2:1565–15691995.
5. Galis ZS, Sukhova GK, Kranzhofer R, Clark S, Libby P. Macrophage foam cells from experimental atheroma const itutively produce matrix-degrading proteinases. Proc Natl Acad Sci USA 92:402–4061995.



6. Libby P, Sukhova G, Lee RT, Galis ZS. Cytokines regulate vascular functions related to stability of the atherosclerotic plaque. J Cardiovasc Pharmacol 25:S9–121995.

7. Mach F, Schonbeck U, Bonnefoy JY, Pober JS, Libby P. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40: induction of collagenase, stromelysin and tissue factor. Circulation 96:396–3991997.


8. Hansson GK, Holm J, Jonasson L. Detection of activated T-lymphocytes in the human atherosclerotic plaque. Am J Pathol 135:169–1751989.


9. Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T-lymphocytes from human atherosclerotic plagues recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA 92:3893–38971995.



10. Geng YJ, Hansson GK. Interferon-gamma inhibits scavenger receptor expression and foam cell formation in human monocyte-derived macrophages. J Clin Invest 89:1322–13301992.



11. Jonasson L, Hansson GK, Bondjers G, Noe L, Etienne J. Interferon-gamma inhibits lipoprotein lipase in human monocyte-derived macrophages. Biochim Biophys Acta 1053:43–481990.


12. Hansson GK, Jonasson L, Seifert PS, Stemme S. Immune mechanisms in atherosclerosis. Arteriosclerosis 9:567–5781989.


13. Serneri GG, Abbate R, Gori AM, Attanasio M, Martini F, Giusti B, Dabizzi P, Poggesi L, Modesti PA, Trotta F, et al. Transient intermittent lymphocyte activation is responsible for the instability of angina. Circulation 86:790–7971992.


14. Hollander W, Colombo MA, Kirkpatrick B, Paddock J. Soluble proteins in the human atherosclerotic plaque. With spectral reference to immunoglobulins, C3-complement component, alpha 1-antitrypsin and alpha 2-macroglobulin. Atherosclerosis 34:391–4051979.


15. Vlaicu R, Rus HG, Niculescu F, Cristea A. Immunoglobulins and complement components in human aortic atherosclerotic intima. Atherosclerosis 55:35–501985.


17. Cerottini JC, Brunner KT. Cell-mediated cytotoxicity, allograft rejection and tumor immunity. Adv Immunol 18:67–1321974.


18. Weigle WO, Goodman MG, Morgan EL, Hugli TE. Regulation of immune response by components of the complement cascade and their activated fragments. Springer Semin Immunopathol 6:173–1941983.


20. Pulford KA, Sipos A, Cordell JL, Stross WP, Mason DY. Distribution of the CD68 macrophage/myeloid associated antigen. Int Immunol 2:973–9801990.


21. Tenner-Racz K, Racz P, Thome C, Meyer CG, Anderson PJ, Schlossman SF, Letvin NL. Cytotoxic effector ceil granules recognized by the monoclonal antibody TIA-1 are present in CD8+ lymphocytes in lymph nodes of human immunodeficiency virus-1-infected patients. Am J Pathol 142:1750–17581993.


22. Sale GE, Anderson P, Browne M, Myerson D. Evidence of cytotoxic T-cell destruction of epidermal cells in human graft-vs-host disease. Immunohistology with monoclonal antibody TIA-1. Arch Pathol Lab Med 116:622–6251992.

23. Golay JT, Clark EA, Beverley PC. The CD20 (Bp35) antigen is involved in activation of B cells from the G0 to the G1 phase of the cell cycle. J Immunol 135:3795–38011985.



24. Barath P, Fishbein MC, Cao J, Berenson J, Helfant RH, Forrester JS. Detection and localization of tumor necrosis factor in human atheroma. Am J Cardiol 65:297–3021990.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



