 |
 |
| Korean J Intern Med > Volume 40(3); 2025 > Article |
|
Abstract
Follicular lymphoma (FL) is the most common type of indolent lymphoma, and the prognosis is favorable for most patients. However, FL remains generally incurable, and relapse is common. Patients are at risk of developing treatment-resistant lymphoma, particularly when early disease progression occurs or transformation to aggressive lymphoma takes place. Furthermore, lymphoma is the leading cause of death among patients with FL, emphasizing the need for more effective treatment strategies. This review summarizes therapeutic approaches for FL, with a focus on therapies currently in development. Recent biological insights have driven the emergence of highly effective treatments, including novel immune and targeted therapies. Clinical trials are assessing the efficacy of these novel approaches, which are increasingly used in earlier line settings. In the future, FL therapy is expected to rely less on chemotherapeutic methods, extend remission, and potentially enable cures for a growing number of patients.
Follicular lymphoma (FL) is a common type of B-cell non-Hodgkin lymphoma, with an annual incidence of 2.2 per 100,000 individuals in the United States [1]. The median age at diagnosis is 64 years [1]. The incidence varies according to geography and ethnicity for largely unknown reasons [2], implying that FL incidence is influenced by multiple factors, including population demographics, genetic predis-position, early detection, and potentially lifestyle. FL is an indolent disease with typically slow progression. Although 50–60% of patients present with advanced-stage disease, outcomes are generally favorable; the 5-year survival rate is 90% [1]. Despite high response rates and often durable responses to frontline therapy, relapse is frequent, and FL is considered incurable; lymphoma is the leading cause of death in patients with FL [3]. Early progression after systemic therapy is associated with poor survival and often results from transformation to aggressive lymphoma, which carries an increased risk of mortality [4–8]. This review examines the impacts of recent therapeutic advances on patient care.
Histologically, FL consists of smaller centrocyte-like and larger centroblast-like cells organized in a microanatomical structure, forming follicles that, unlike normal germinal centers, lack polarization into dark and light zones. Malignant cells are also found outside the follicular areas. The relative proportions of these cell types are reflected in grading; grades 1, 2, and 3A represent indolent disease. In contrast, grade 3B is typically associated with poorer outcomes and has been classified as an aggressive form of lymphoma. Since the most recent update of the World Health Organization Classification of Haematolymphoid Tumours [9], grading has become optional, and most FL cases are classified as classic FL. Rarer cases with a predominantly diffuse pattern are classified as diffuse FL; even rarer cases with unusual cytological features are designated FL with unusual cytological features. Grade 3B FL has been renamed follicular large B-cell lymphoma to reflect its distinction from indolent disease. Malignant cells express B-cell-associated antigens, including CD20, CD22, and CD79a; they typically exhibit germinal center markers such as CD10 and BCL6. A translocation between chromosomes 14 and 18 is observed in 85% of cases, leading to aberrant overexpression of the anti-apoptotic factor BCL2 [10]. Although the t(14;18) translocation is a hallmark of FL, it is not a defining feature because it can also occur in other lymphoid malignancies and is absent in a small subset of FL cases, particularly in early-stage cases (~50%) [11]. Certain immunohistochemical markers, such as high expression of Ki-67, IRF4, and FOXP1, are associated with worse outcomes but are not used in clinical practice for treatment decision-making [12,13]. Several FL variants are recognized, including in situ follicular neoplasms, pediatric-type FL, duodenal-type FL, and primary cutaneous follicle center lymphoma [14–17]; these variants are associated with favorable outcomes.
Advances in genomic methods have substantially improved the overall understanding of the molecular basis of FL, enabling studies of FL at both bulk and single-cell levels [18,19]. Furthermore, the development of sophisticated animal models has illuminated the effects of genetic alterations. FL frequently harbors mutations in genes encoding histone modifiers or chromatin remodelers (e.g., KMT2D, CREBBP, EZH2, ARID1A, EP300) and histone linker genes [20–22]. Epigenetic reprogramming of normal transcriptional profiles is a distinguishing feature of FL and other B-cell malignancies. Other genes, including TNFRSF14, FOXO1, STAT6, IRF8, MEF2B, and genes related to the mTOR pathway (RRAGC, ATP6V1B2, and ATP6AP1), are also mutated in FL [23–25]. The most common gene mutations are not currently actionable, with the exception of mutant EZH2, which can be targeted by small-molecule inhibitors. Patterns of gene mutations may clarify molecular subtypes that influence therapeutic responses and outcomes [26,27]. Similar to other lymphoid malignancies, TP53 mutations have been consistently associated with a worse prognosis, although they have not been adopted as biomarkers [28–30] even though treatment principles, including chemotherapy resistance due to mutant TP53, do not substantially differ from those in other indolent lymphoid malignancies. Clonal heterogeneity exists in FL, which may cause a tissue-based biomarker to yield different results depending on the anatomical site of biopsy [31,32].
Single-cell studies have provided insights into the transcriptional footprints of FL tumor cells, challenging the simplistic view that FL cells are universally germinal center-like [33–36]. The transcriptional profiles in FL are desynchronized, possibly reflecting the loss of polarization in FL nodules [33]. FL transcriptional phenotypes are complex, representing a continuum of gene expression states where some cells express programs similar to normal germinal-center cells and others resemble normal memory B cells [34]. Importantly, memory-like FLs are associated with a higher risk of transformation and shorter progression-free survival (PFS), indicating that molecular-level interpatient heterogeneity translates into distinct clinical trajectories [34,37].
The tumor microenvironment (TME) plays a key role in FL, comprising a significant portion of the tumor mass, and its composition is associated with patient outcomes [38]. The TME consists of interacting immune cells, including CD4+ and CD8+ T cells and macrophages, as well as stromal cells and non-cellular components such as the extracellular matrix. Collectively, these elements support tumor growth and immune evasion [39]. Distinct FL subtypes have been identified based on TME composition, and reduced T-cell counts have been linked to unfavorable outcomes [40,41]. Moreover, the pathogenetic effects of certain gene mutations extend beyond cell-intrinsic changes and contribute to TME remodeling. For example, CREBBP and EZH2 mutations downregulate antigen-presentation genes, such as major histocompatibility complex class I or II [42,43], emphasizing the complexity of interactions between tumor cells and the TME.
Several clinical factors are associated with an increased likelihood of disease progression after treatment. These factors have been incorporated into clinical-risk assessment tools such as the Follicular Lymphoma International Prognostic Index (FLIPI) [44], FLIPI-2 [45], PRIMA-PI [46], and Follicular Lymphoma Evaluation Index (FLEX) [47]. These prognostic scores, which are calculated using routinely available clinical and laboratory data, enable risk stratification and evaluation of the likelihood of disease progression. However, their discriminative accuracy remains insufficient for risk-adjusted treatment strategies and does not provide insight into potential therapeutic targets in high-risk patients. In addition to clinical indices, functional imaging with positron emission tomography-computed tomography (PET-CT) offers valuable prognostic information. For example, baseline tumor metabolic volume is associated with shorter PFS [48]. Similarly, positive PET-CT findings (i.e., a Deauville score of 4 or 5) upon completion of immunochemotherapy are associated with inferior PFS [49,50]. Efforts have been made to incorporate molecular characteristics into prognostic scores. For example, the m7-FLIPI combines the mutation statuses of seven genes with the FLIPI and performance status [29], whereas the 23-gene predictor assesses risk based on expression levels of 23 genes [51–53]. Additionally, TME-derived signatures show potential for identifying high-risk patients [40,41,54,55]. Notably, the prognostic values of certain biomarkers depend on the treatment received. For instance, EZH2 mutations are favorable prognostic indicators in patients treated with rituximab-cyclophosphamide-vincristine-prednisone (R-CVP) or rituximab-cyclophosphamidedoxorubicin-vincristine-prednisone (R-CHOP) [27,29,56,57]. However, no such association has been detected in patients treated with bendamustine-based immunochemotherapy [53,58]. Despite ongoing efforts to enhance prognostic methods, predictions of early progression and transformation remain challenging [59], posing an obstacle to personalized treatment for at-risk patients.
The fundamental aspects of treatment are determined by disease stage, tumor burden, presence or absence of symptoms, and potential occurrence of laboratory abnormalities (e.g., cytopenias). In addition to clinical examination, cross-sectional imaging is required for staging and is ideally performed with PET-CT [60], which is more sensitive than conventional CT [61,62]. PET-CT can also guide additional biopsies when transformation is clinically suspected. The treatment approach for FL is shown in Figure 1.
Patients with stage I or contiguous stage II disease are typically considered candidates for radiation therapy. This strategy has historically been associated with favorable outcomes; half of the patients with stage I disease do not experience relapse after extended follow-up, raising the question of whether radiation in this context is curative [63–65]. It remains unclear, however, whether these favorable outcomes are due to the intervention or possibly reflect favorable disease biology. Two retrospective series reported no survival advantage of radiation over observation or other systemic treatment modalities [66,67], whereas other studies suggested improved overall survival when radiation is used upfront for early-stage FL [61,68]. Patients with presumed early-stage disease should be fully staged with PET-CT and bone marrow biopsy. Thoroughly staged patients with stage I–II disease receiving ≥ 24 Gy had a 5-year freedom-from-progression rate of 69% (74% for stage I disease vs. 49% for stage II disease) in a multicenter retrospective study by the International Lymphoma Radiation Oncology Group (ILROG) [69]. In-field relapses were uncommon (2%), indicating excellent local disease control. A radiation dose of 24 Gy, divided into 12 fractions, is typically used because it is as effective as higher doses [70]. Doses lower than 24 Gy may result in inferior outcomes. A randomized controlled trial showed a 2-year local progression-free rate of 94% vs. 80% in patients treated with 24 Gy vs. a very low dose of 4 Gy [71]. In a retrospective series, very low-dose radiation achieved an overall response rate (ORR) and complete response (CR) rate of 90% and 66%, respectively [72].
Numerous out-of-field relapses and frequent circulation of the t(14;18) translocation in peripheral blood [73] suggest that systemic disease is often undetected at diagnosis, supporting the rationale for adding systemic therapy to radiation. Rituximab has been proposed as an adjunct treatment based on retrospective and prospective data [74,75]. The strongest evidence supporting systemic therapy comes from the TROG 99.03 trial, in which patients were randomized to receive involved-field radiotherapy with 30–36 Gy, with or without (R)-CVP [76]. Combined therapy significantly improved PFS but not OS. Although efforts to achieve long-term remission after radiotherapy are essential, research concerning minimally toxic strategies that avoid the genotoxicity of chemotherapy is also needed.
The question of whether asymptomatic patients with a low tumor burden and advanced-stage disease require treatment or can be safely observed has been investigated in several clinical trials. Two studies from the pre-rituximab era showed no survival advantage from upfront systemic therapy with oral chlorambucil, prednimustine, or interferon alfa [77,78]. Subsequently, a randomized phase III trial compared rituximab induction, with or without maintenance, to a watch-and-wait approach [79]. As expected, rituximab extended the time before subsequent therapy was needed. At 10 years, 49% and 65% of patients in the rituximab induction and maintenance arms, respectively, had not started a new treatment, compared to 29% in the watch-and-wait arm. Moreover, cause-specific survival exceeded 80% at 10 years, indicating that a low tumor burden is associated with a favorable prognosis. In the RESORT trial, patients received rituximab induction, followed by either maintenance or retreatment as needed [80]. The retreatment strategy, requiring fewer rituximab doses, was preferred, with only a minor difference in the 3-year freedom-from-cytotoxic-therapy rate (84% vs. 95%). Patients assigned to the maintenance arm in the RESORT trial received rituximab every 3 months until treatment failure. A different approach was adopted in the phase III FLIRT trial, where four maintenance doses were administered at 2-month intervals; maintenance was associated with a higher 4-year PFS rate (58% vs. 41%) [81]. New therapies may further improve outcomes for patients with a low tumor burden. However, these patients have a favorable prognosis, and no treatment has yet demonstrated a survival advantage. Therefore, it is essential to minimize treatment toxicity.
Patients requiring systemic therapy typically meet the criteria of the Groupe d’Etude des Lymphomes Folliculaires (GELF) or the British National Lymphoma Investigation (BNLI), as outlined in Table 1. These criteria are not intended as risk stratification tools; their primary purpose is to aid in identifying patients with a high tumor burden. Clinicians have some discretion in applying these criteria. Since the introduction of rituximab and the discovery that its combination with chemotherapy substantially prolongs both PFS and OS [82–84], immunochemotherapy has become the standard approach for patients with high-tumor-burden FL. Various chemotherapy backbones are available for use. The phase III StiL trial showed that bendamustine and rituximab (BR) achieved better PFS than R-CHOP, with superior tolerability [85]. The confirmatory BRIGHT phase III trial demonstrated the non-inferiority of BR compared to R-CHOP or R-CVP; BR showed higher incidences of vomiting and drug hypersensitivity but lower rates of peripheral neuropathy and alopecia [86]. Longer follow-up from the BRIGHT study revealed superior PFS with BR, but OS did not differ between groups [87]. The PRIMA study showed that rituximab maintenance, administered after R-CHOP, R-CVP, or R-FCM, significantly prolonged PFS [88]. Even with extended follow-up, an OS improvement has not been observed, although rituximab maintenance shows excellent long-term disease control, with a median PFS of 11 years compared to 4 years in the observation arm [89]. Similarly, in the FOLL05 study, the 8-year PFS rate was 48% [90]. Rituximab maintenance after BR induction has not been extensively studied. In a retrospective series of 640 patients, those with a partial response after BR had an improved duration of response when receiving rituximab maintenance, whereas those who achieved complete remission after BR did not [91]. The FOLL12 trial evaluated whether patients with a complete metabolic and minimal residual disease-negative response could forgo rituximab maintenance [92]. Despite an excellent response to induction treatment, the experimental arm showed inferior PFS. In the FOLL12 study, rituximab maintenance resulted in longer PFS in patients treated with R-CHOP and in those treated with BR [93]. To further enhance the efficacy of CD20-directed therapy, the GALLIUM trial randomized patients to receive immunochemotherapy with either rituximab or obinutuzumab. PFS was improved in the obinutuzumab arm [94]. Additionally, obinutuzumab was associated with a lower risk of early progression [95]. A subsequent analysis of the GALLIUM trial highlighted the risk of grade 5 toxicity, particularly in patients treated with bendamustine, due to substantial reductions in CD4+ and CD8+ T-cell counts [96]. The coronavirus disease 2019 pandemic has prompted a re-evaluation of the risk-benefit balance of frontline therapy in FL, with BR associated with increased risks of adverse outcomes [97–99].
Due to the acute and long-term side effects of chemotherapy, there is considerable interest in developing non-chemotherapeutic approaches. After several phase II trials demonstrated promising activity of rituximab combined with lenalidomide (R2) [100–102], the RELEVANCE study compared R2 to R-chemotherapy [103]. At 6 years of follow-up, PFS was nearly identical in the two arms (59% and 60%) [104]. Although neutropenia and febrile neutropenia were more frequent in the R-chemotherapy arm, other side effects, such as cutaneous reactions, were more common in the R2 arm. Obinutuzumab was combined with lenalidomide in the phase II GALEN study, yielding an ORR of 92% and a 2-year PFS rate of 82% [105]. Overall, R2 is a therapeutic option with comparable efficacy but a distinct toxicity profile relative to immunochemotherapy.
Because FL is incurable, recurrence is common. However, outside of clinical trials, the utility of regular imaging-based monitoring is limited [106,107]. Considering the unfavorable prognosis regarding POD24, it is tempting to hypothesize that routine imaging can identify early recurrence. However, POD24 detected by incidental routine imaging was not associated with reduced survival [108]. Consequently, most patients should undergo clinical monitoring without systematic, serial imaging. Principles similar to those applied in initial treatment should guide the management of recurrent disease. In relapsed/refractory disease, biopsies are recommended (particularly to exclude transformation) because this scenario requires a different treatment approach and is associated with a worse prognosis [8].
If relapse is limited to a single site or adjacent sites, radiation (even at very low doses) can achieve reasonable disease control [72]. Patients who are asymptomatic and exhibit a low tumor burden can be managed with a watch-and-wait strategy [109]. Conversely, systemic therapy is generally indicated for patients with a high tumor burden or lymphoma-related symptoms. Second- and third-line treatment options differ to some extent because many recently approved novel therapies are based on studies conducted after two or more lines of therapy. With successive lines of treatment, the duration of response tends to diminish [110–112]. As the number of treatment options rapidly increases, it is important to balance potential toxicity, intended therapeutic goals, and patient preferences.
There is no universally accepted treatment approach after recurrence [113–115], and treatment practices vary among jurisdictions [113]. In the second-line setting, re-treatment with chemoimmunotherapy is often considered, particularly if the response to initial therapy was prolonged (e.g., > 5 yr). Typically, a different chemotherapy backbone is chosen to minimize the risk of treatment resistance. In a randomized non-inferiority trial, BR demonstrated superior PFS and OS compared to fludarabine and rituximab in 219 patients, half of whom had FL [116]. In the GADOLIN trial, patients with rituximab-refractory disease were randomized to receive either bendamustine alone or bendamustine combined with obinutuzumab, followed by obinutuzumab maintenance [117]. Patients in the experimental arm experienced improved PFS, and an OS improvement was later demonstrated [117,118]. Notably, the trial did not include rituximab in the control arm, precluding a definitive comparison of obinutuzumab vs. rituximab efficacy in this setting. Because BR is frequently used in the first-line setting, obinutuzumab is often combined with CHOP or CVP for rituximab-refractory FL. However, the optimal sequence of chemotherapy backbones remains unclear. Additionally, the benefit of rituximab maintenance therapy for rituximab-sensitive disease in the second-line setting is uncertain [119], considering that anti-CD20 therapy is routinely administered with upfront treatment.
The immunomodulator lenalidomide induces the recruitment and subsequent degradation of the Ikaros and Aiolos transcription factors via the CRL4CRBN E3 ubiquitin ligase [120–122]. Lenalidomide also enhances NK-cell function, repairs T-cell immunologic synapses, and acts synergistically with rituximab [123–126]. Accordingly, several phase II trials reported encouraging response rates of 65–77% in the relapsed setting [127–129], setting the stage for exploration of this regimen’s efficacy in larger clinical trials. The phase III AUGMENT trial randomized patients with relapsed/refractory FL or marginal zone lymphoma to R2 or rituximab alone [130]. Patients had received a median of one prior therapy and were not rituximab-refractory; the majority (82%) had FL. At the 5-year follow-up, the median PFS was 28 months with R2 vs. 14 months with rituximab alone; an improvement in OS was noted [131]. The R2 regimen showed comparable efficacy in patients with and without POD24 [130]. In the MAGNIFY trial, patients with relapsed/refractory FL, marginal zone lymphoma, or mantle cell lymphoma received induction with R2, followed by randomization to R2 vs. rituximab maintenance. Patients had received a median of two prior lines of therapy. The induction phase showed an ORR of 72% and a CR of 42% in patients with FL, with a median PFS of 41 months [132]. Lenalidomide combined with obinutuzumab was evaluated in the GALEN trial, revealing an ORR of 84% and a 2-year PFS of 65% [133]. Cereblon E3 ligase modulators (CELMoDs) other than lenalidomide are also under investigation. The combination of golcadomide (CC-99282) with rituximab demonstrated encouraging activity in a phase I/II study [134]. Considering these promising results, the combination of lenalidomide with anti-CD20 therapy is a frequently used second-line treatment, especially for patients who did not experience durable responses to immunochemotherapy in the frontline setting.
Hematopoietic stem cell transplantation (SCT) has long been a commonly used treatment approach in FL. In the pre-rituximab era, long-term follow-up data from the Dana-Farber Cancer Institute showed an 8-year disease-free survival rate of 42% and an OS rate of 66% in patients with relapsed FL who underwent autologous SCT (auto-SCT) [135]. Negative PCR for the t(14;18) rearrangement during follow-up was associated with sustained complete remission. Later follow-up indicated a remission plateau at 48% at 12 years, suggesting the curative potential of chemotherapy intensification and auto-SCT [136]. In a randomized controlled trial of 140 European patients, high-dose chemotherapy was associated with improved PFS and OS [137]. A retrospective re-analysis of the GLSG1996 and GLSG2000 trials showed improved PFS and OS in patients with POD24, suggesting that treatment intensification can overcome the poor prognosis in these patients [138]. In the GELA/GOELAMS FL2000 study, auto-SCT was associated with improved PFS and OS [139]. Encouraging long-term outcomes were reported in a cohort of patients with relapsed FL, 64% of whom had received prior anti-CD20 therapy, with a plateau of 59% in after 9 years [140]. In a retrospective analysis by the LymphoCare Study and the Center for International Blood and Marrow Transplant Research (CIBMTR), auto-SCT was associated with improved OS in patients receiving auto-SCT within 1 year of treatment failure [141]. However, in the follow-up of patients from the PRIMA trial, only those with biopsy-proven transformation benefited from auto-SCT; patients with persistent FL on biopsy did not [8].
Other findings favor allogeneic SCT (allo-SCT). For patients with early treatment failure, matched sibling and matched unrelated donor allo-SCT were associated with better disease control but higher non-relapse mortality relative to auto-SCT [142]. A combined analysis by the European Society for Blood and Marrow Transplantation and CIBMTR reported 5-year OS and PFS rates of 61% and 52%, with worse outcomes in patients who had received multiple lines of therapy and those undergoing myeloablative conditioning [143]. The use of SCT is expected to decrease, particularly where novel treatment options are available. If auto-SCT is considered, the available data suggest it is most effective during the first or second remission, especially for POD24.
In FL, tumor cells reside within a complex cellular ecosystem, interacting closely with the TME. FL cells depend on signals from the tumor microenvironment and evade immune recognition. Disrupting the interaction between FL cells and the TME is intuitively thought to have therapeutic value. Amplification of programmed death ligands is uncommon in FL tumor cells [144]. The PD-1 checkpoint is expressed in subsets of tumor-infiltrating T cells [145]. CD4+ PD-1high expressing cells are found in the follicles and exhibit a follicular T helper phenotype, whereas PD-1low CD4+ T cells reside outside of follicles, show reduced cytokine production, and transduce fewer signals [146]. Although immune checkpoint inhibitors have significantly impacted multiple cancers, trials of single-agent immune checkpoint inhibitors in FL have yielded disappointing results, with minimal response rates [147–149] (Table 2). However, combining checkpoint inhibitors with anti-CD20-directed therapy has shown promise. For example, in a single-center phase II trial, 30 patients with rituximab-sensitive relapse after one or more lines of treatment received pembrolizumab and rituximab, achieving an ORR of 67%, a CR rate of 50%, and a median PFS of 13 months [150]. In a phase Ib study of 26 patients with FL who had a median of two prior lines of therapy, the combination of atezolizumab and obinutuzumab produced an ORR of 54%, a CR rate of 23%, and a median PFS of 9 months [151]. Triplet therapies have been tested to enhance therapeutic efficacy. In a phase Ib/II trial of patients treated with atezolizumab, obinutuzumab, and lenalidomide, the CR rate was 72%, and the 3-year PFS rate was 68% [152]. The combination of atezolizumab, obinutuzumab, and polatuzumab vedotin was less effective and more toxic [153]. Further studies are warranted to identify the most effective combination strategies and to understand the effects of checkpoint inhibitors when combined with other targeted agents.
The adoptive transfer of autologous T cells engineered to recognize CD19 can result in high response rates and durable responses [154–157]. Three phase II studies have confirmed these promising early results. The ZUMA-5 study evaluated 86 patients with FL who had received two or more prior lines of therapy and were infused with axicabtagene ciloleucel; the ORR and CR rates were 94% and 79%, respectively [158]. The rates of grade 3 or higher cytokine release syndrome (CRS) and neurotoxicity were 6% and 15%. The 3-year follow-up of 127 FL patients from ZUMA-5 showed a median PFS of 40 months [159]. Similar efficacy was observed in the phase II ELARA trial of tisagenlecleucel, where the OR and CR rates were 86% and 69%, respectively, in patients with relapsed/refractory FL after two or more lines of therapy or relapse following auto-SCT [160]. The rates of grade 3 or higher CRS and immune effector cell-associated neurotoxicity syndrome (ICANS) were 0% and 1%, respectively. The most recent update from the ELARA trial showed a 24-month PFS of 57% [161]. The phase II TRANSCEND FL study evaluated lisocabtagene maraleucel in patients with relapsed/refractory FL, 18% of whom had received only one prior line of therapy [162]. Remarkably, in second-line patients, the ORR and CR rates were 96%, with similar results in third- or later-line patients (ORR 97% and CR rate 94%). Grade 3 or higher CRS occurred in 1% of patients, whereas grade 3 or higher neurological toxicity was noted in 2%. Without direct comparative data, it is not possible to conclude that one CAR T-cell product is superior to another. However, the real-world efficacy of CAR T-cell therapy aligns with clinical trial results [163,164], and indirect comparisons suggest that CAR T-cell therapy has greater efficacy than other standard-of-care therapies [165,166].
Another promising advance is the development of CD20 × CD3 bispecific antibodies, which target a B-cell epitope while simultaneously activating T cells near neoplastic cells. In a phase I dose-escalation study, mosunetuzumab achieved OR and CR rates of 66% and 49%, respectively, with a median duration of response of 17 months in patients with relapsed/ refractory indolent lymphomas after two or more prior lines of therapy [167]. The corresponding phase II study reported OR and CR rates of 80% and 60%, respectively [168]. Grade 3 or higher CRS occurred in only two patients (2%), and no grade 3 or 4 neurological toxicity was noted. Extended follow-up confirmed the previous findings, with a high response rate of 88% in eight patients exhibiting indolent lymphomas who relapsed after achieving a CR and were re-treated [169]. A phase I study examined the safety of glofitamab, a bispecific antibody with a 2:1 configuration for bivalent binding to CD20, showing that 4% of patients experienced grade 3 or higher CRS and 1% experienced grade 3 ICANS [170]. Among patients with FL treated with the recommended phase II dose, the OR and CR rates were 62% and 52%, respectively. In a dose-escalation study of subcutaneously administered epcoritamab, the OR and CR rates were 90% and 50% in patients with FL treated at full doses, with no grade 3 or higher CRS events [171]. Similar results were observed in the phase II EPCORE NHL-1 study, where OR and CR rates were 82% and 63%, respectively, with grade 3 CRS in only 2% of patients and no high-grade ICANS [172]. Finally, odronextamab was evaluated in the phase I ELM-1 study, where the OR and CR rates were 78% and 63%, respectively, in patients with relapsed/refractory FL; grade 3 or higher CRS was noted in 7% and grade 3 or higher ICANS was observed in 3% of the overall cohort [173]. The phase II ELM-2 study reported OR and CR rates of 80% and 73%, respectively, in relapsed/refractory FL; only one patient experienced grade 3 CRS and no patients exhibited grade 3 or higher ICANS, after optimized stepup dosing [174]. Across these studies, observed toxicity was generally mild, with frequent pyrexia, mainly during stepup dosing in the first treatment cycle, and cases of neutropenia. However, the risk of infection may be underestimated, as a recent meta-analysis of patients treated with bispecific antibodies showed high frequencies of all-grade (44%), grade 3 or higher (20%), and fatal (3%) infections [175]. Viral infections were overrepresented in fatal cases, indicating a need for preventative measures. Despite these risks, bispecific antibodies are expected to play an increasing role in managing FL, as they achieve high response rates with a comparatively low risk of high-grade toxicity. Bispecific antibodies have not been directly compared with CAR T-cell therapy or other approved therapies, representing an unmet need. A matching-adjusted indirect comparison by the LEO consortium suggested that mosunetuzumab had a higher response rate than real-world data but similar PFS at 12 months [176], with similar results reported by others [177]. The treatment schedules and routes of administration vary among bispecific antibodies, which may influence decision-making in real-world settings.
In addition to immune therapies that rely on T cells for anti-tumor effects, the inhibition of “do-not-eat-me” signals emitted by tumor cells can enhance macrophage phagocytic function and may synergize with rituximab, as demonstrated in a preclinical study [178]. In a phase Ib study of seven patients with relapsed/refractory FL treated with the CD47-blocking antibody Hu5F9-G4 (magrolimab) and rituximab, 71% and 43% of patients achieved an objective and complete response, respectively [179]. In a subsequent extended phase Ib and preliminary phase II report, the OR and CR rates were 66% and 24%, respectively, in patients with indolent lymphomas [180]. Anemia, an on-target toxicity, and infusion reactions were common. TTI-622 (maplirpacept), a fusion protein that blocks the signal-regulatory protein α (SIRPα)–CD47 axis, was evaluated in a phase Ia/Ib dose-escalation and expansion trial involving four patients with FL [181]. Combination therapy studies are underway to better define the role of CD47-directed therapies.
Epigenetic disruption plays a key role in the development of FL. EZH2 gain-of-function mutations increase trimethylation of lysine 27 on histone 3, suppressing gene expression programs that typically drive terminal differentiation [182]. Accordingly, specific inhibitors of EZH2 have been developed. Proof-of-concept was demonstrated in a phase I study of tazemetostat involving 21 patients with B-cell non-Hodgkin lymphoma, including 7 who had FL [183]. Considering its favorable safety profile, a phase II study was conducted in which tazemetostat was administered orally twice daily [184]. The ORR was 69% in EZH2-mutant cases and 35% in wild-type cases. However, the median PFS was similar between the two cohorts (14 and 11 mo), suggesting that the therapeutic efficacy of EZH2 inhibition is not restricted to EZH2-mutant FL. Intriguingly, an EZH2 gene expression signature is present in certain wild-type cases [185], possibly due to alternative causes of EZH2 hyperactivity, such as chromosomal gains [57]. Importantly, EZH2 mutations are frequently subclonal [31,186], indicating that tissue-based tumor sampling could miss this targetable alteration. The favorable safety profile of tazemetostat supports ongoing efforts to evaluate combination therapies that may yield more durable responses. For instance, the SYMPHONY-1 study is assigning patients to R2 with or without tazemetostat, after a phase Ib lead-in that showed good tolerability and promising responses [187]. In preclinical studies, tazemetostat increased the efficacy of CAR T-cell therapy [188,189]. Other epigenetic therapies, including histone deacetylase inhibitors, have been investigated in FL, with variable response rates and less favorable toxicity profiles relative to other emerging therapies [190–195]. Mutations of the KMT2D histone methyltransferase are among the most common genetic alterations in FL. In a preclinical study, inhibition of the histone demethylase KDM5 reversed the activation of both KMT2D-dependent and independent genes, with efficacy demonstrated in an animal model [196].
The preservation of surface immunoglobulin expression and selection against mutations causing structural changes [197] suggest that a functional B-cell receptor is positively selected during FL development. Altered B-cell receptor signaling patterns have been reported in FL [198–200] and may be critical in transmitting tumor-promoting signals from the TME [201,202]. Spleen tyrosine kinase (Syk) docks to the B-cell receptor upon engagement and activates Bruton’s tyrosine kinase (BTK), which then triggers intracellular signaling pathways, such as the phosphatidylinositol 3-kinase (PI3K) pathway [203]. The therapeutic potential of Syk inhibition has been explored in several clinical studies, yielding mixed results. Fostamatinib, the first clinical-grade, orally available Syk inhibitor, showed limited activity in FL (ORR 10%) [204]. Response rates were also low with entospletinib (17% in FL) [205] and the dual Syk/JAK inhibitor cerdulatinib (31%) [206]. In contrast, response rates were higher with the dual Syk/FLT3 inhibitor TAK-659 [207] and sovleplenib (61%) [208].
BTK inhibition, which has led to high response rates in chronic lymphocytic leukemia and mantle cell lymphoma, has also been evaluated in FL. Ibrutinib alone demonstrated a low ORR of 21% in the phase II DAWN study [209]. In a separate phase II study, ibrutinib also showed a modest ORR of 38%; rates were higher in patients with rituximab-sensitive disease than in those with rituximab-refractory disease [210]. The phase III SELENE study randomized patients with relapsed/refractory FL or marginal zone lymphoma after one or more prior therapies to receive immunochemotherapy (BR or R-CHOP) with or without ibrutinib [211]. The experimental group showed a non-significant trend toward improved PFS. Acalabrutinib has been evaluated in combination with rituximab in patients with relapsed/refractory FL and one or more prior lines of therapy, achieving an ORR of 33–39% [212,213]. Acalabrutinib combined with R2 demonstrated an ORR of 76% [213]. In a phase I study, zanubrutinib combined with obinutuzumab achieved an ORR of 72% in relapsed/refractory FL [214]. The phase II ROSEWOOD study randomized patients to receive zanubrutinib with obinutuzumab or obinutuzumab alone [215], revealing improvements in ORR (69% vs. 46%), CR rate (39% vs. 19%), and median PFS (28 vs. 10 mo). Grade 3 or higher toxicities were primarily cytopenias and pneumonia. Therefore, BTK inhibitors combined with other targeted agents may be effective against relapsed/refractory FL. Intriguingly, BTK mutations have been detected in samples from patients with FL who had not been previously exposed to BTK inhibitors; these mutations differ from mutations that confer resistance in chronic lymphocytic leukemia [216,217]. Several BTK mutations identified in FL also induce resistance to BTK inhibition [216]. Therefore, further research is required to develop precision medicine strategies related to BTK inhibition.
PI3K is involved in transducing several signaling pathways, including those from the B-cell receptor, and its inhibition has been evaluated for efficacy in relapsed/refractory indolent lymphomas. In a phase II study, inhibition of the delta isoform (PI3Kδ) with idelalisib led to a response rate of 57% [218]. The ORR was 59% for copanlisib, a pan-class I PI3K inhibitor [219], and 47% (42% in patients with FL) for duvelisib, an oral dual inhibitor of PI3Kδ and γ [220]. In the phase III CHRONOS-3 trial, copanlisib combined with rituximab improved PFS compared to rituximab alone (22 vs. 14 mo). Additional PI3K inhibitors include umbralisib [119], buparlisib [120], KA2237 [121], zandelisib [221], and TQB3525 [222]. Although effective, PI3K inhibitors can cause severe side effects, such as pneumonitis, colitis, transaminitis, and rash. Inhibitors targeting the δ isoform, as well as the ubiquitously expressed α isoform, are associated with hyperglycemia and hypertension [223]. Due to increased toxicity and fatalities in several phase III trials, the development of PI3K inhibitors has largely ceased, and previously approved agents have been withdrawn from the market [224]. The future of this therapeutic class remains uncertain.
BCL2 is typically overexpressed in FL due to the t(14;18) translocation and plays a key role in its development. Despite the strong rationale for investigating BCL2 inhibitors in FL, venetoclax alone showed a response rate of only 38% in a phase I trial involving 29 patients with relapsed/refractory FL [225]. In the phase II CONTRALTO study, venetoclax and rituximab, with or without bendamustine, were evaluated in 163 patients with relapsed/refractory FL [226]. The ORR for venetoclax and rituximab was 35%, increasing to 84% when bendamustine was added, although with increased toxicity. This finding suggests that while t(14;18) is necessary in the early stages of FL pathogenesis, BCL2 overexpression may not be essential in later stages.
Regarding mTOR inhibitors, temsirolimus achieved OR and CR rates of 54% and 26%, respectively [227]. In a subsequent study, the mTORC1 inhibitor everolimus demonstrated an ORR of 61% in relapsed/refractory FL [228]. It remains unclear whether mTOR inhibitors are more effective in patients with FL harboring mTORC1-activating mutations [24,25,229]. A small study of 21 patients revealed that CREBBP mutations, specifically those affecting the histone acetyltransferase domain, were associated with responses to everolimus or temsirolimus [230]. No correlation between response and mTORC1 pathway mutations was observed, but the number of samples with such mutations was small. Further studies in this subset of patients are warranted, particularly given recent findings suggesting that mTOR pathway mutations define a subset of FLs with distinct pathobiological characteristics [26,230].
Naked antibodies and antibody-drug conjugates are under investigation. Among 34 patients with relapsed/refractory FL, tafasitamab (MOR208) demonstrated an ORR of 29% [231]. The ROMULUS trial included 20 FL patients and evaluated the CD79b-targeting antibody-drug conjugate polatuzumab vedotin, with or without rituximab; the OR and CR rates were 70% and 45%, respectively [232]. In 56 patients with relapsed/refractory FL, polatuzumab vedotin combined with obinutuzumab and lenalidomide showed OR and CR rates of 76% and 63%, respectively [233]. However, in a randomized phase II trial, the addition of polatuzumab vedotin to BR did not improve the response rate [234]. In a phase I trial, the antibody-drug conjugate loncastuximab tesirine achieved an ORR of 79% among 14 evaluable patients with FL [235]. Further research is needed to define the roles of these therapies in FL treatment.
Treatment options for patients with FL have substantially advanced due to the emergence of immune therapies, improved understanding of immunomodulatory treatments, and introduction of targeted therapies. Several novel therapies are currently in phase III evaluation (Table 3). Therefore, the treatment landscape for FL is expected to evolve to include therapies that are more effective and less toxic than those available at present.
In addition to clinical efficacy, there is increasing recognition of the importance of assessing patient-reported outcomes (PROs) and quality of life (QoL) in patients with FL. Given the favorable outcomes for most patients with FL, balancing disease control with QoL is essential. In a population- based registry, patients with FL receiving immunochemotherapy reported worse QoL scores than a normative population [236]. Declines in QoL measures have also been observed with later lines of therapy [237,238]. Additionally, although PROs are typically collected during clinical trials, they are often underreported, highlighting the need to prioritize patient-centered care [239].
Looking ahead, we expect that the biological basis of treatment response will continue to be investigated, and novel therapies will be developed. These efforts will likely lead to the discovery of predictive biomarkers that enable personalized therapeutic approaches. If novel therapies can reduce early progression and prevent the development of aggressive lymphoma, outcomes for FL are expected to improve.
Notes
Acknowledgments
Dr. Kridel is a Lymphoma Research Foundation Grantee (Jaime Peykoff Follicular Lymphoma Research Initiative).
Figure 1
Treatment algorithm for follicular lymphoma in the frontline and first- and later-relapse settings. This is intended as a general guide. Interpretations of evidence from published studies may vary among physicians, and treatment choices can be influenced by funding, drug access, and local guidelines. ASCT, autologous stem cell transplantation; chemo, chemotherapy; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CVP, cyclophosphamide, vincristine, and prednisone; O, obinutuzumab; POD24, progression of disease within 24 months; R, rituximab; R2, rituximab and lenalidomide. Figure created using BioRender.com.
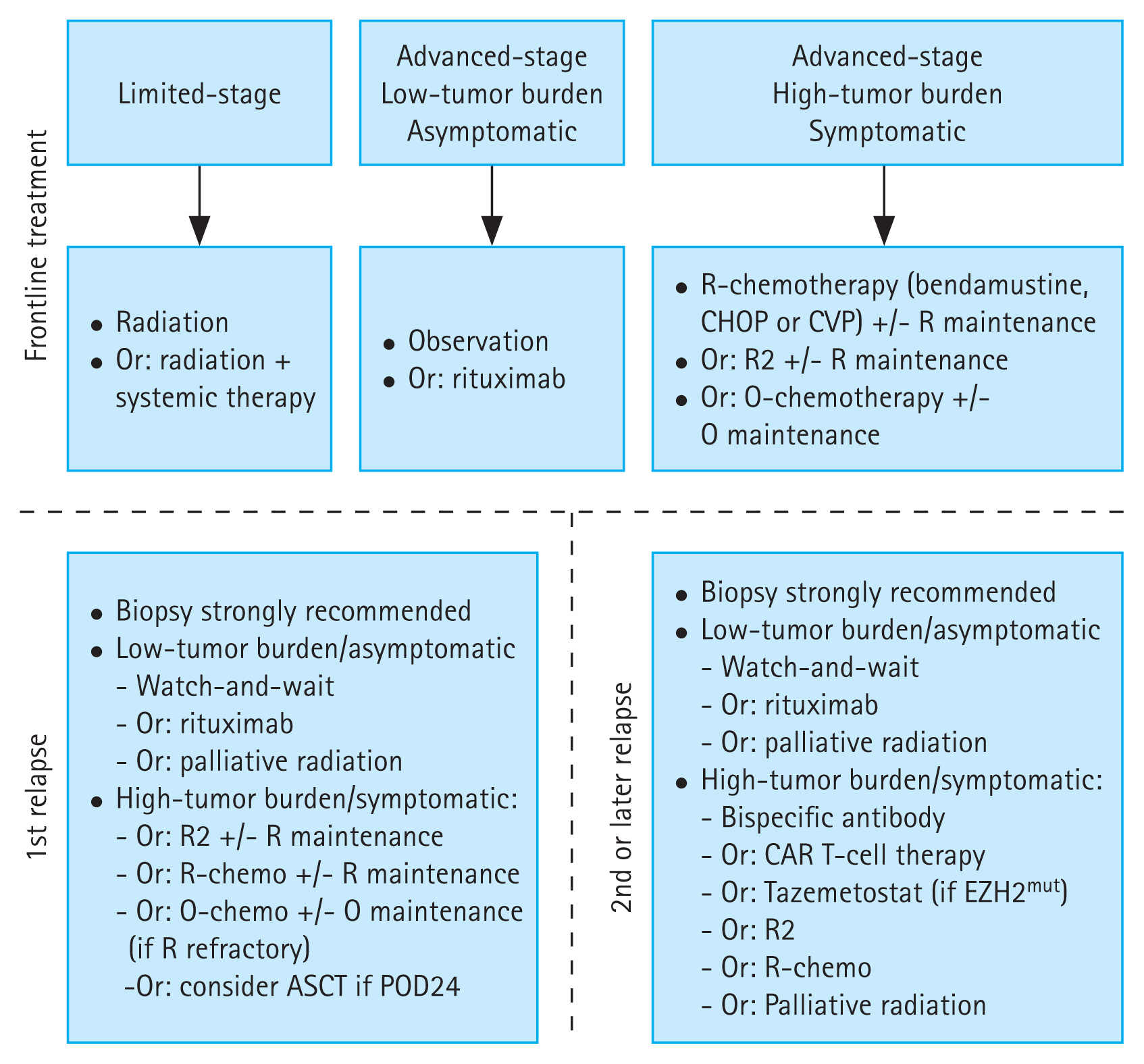
Table 1
Criteria for defining high-tumor-burden follicular lymphoma
| Groupe d’Etude des Lymphomes Folliculaires (GELF) criteria [77]: |
| Any nodal or extranodal tumor mass > 7 cm in diameter; |
| Involvement of at least 3 nodal sites, each with a diameter > 3 cm; |
| Absence of systemic symptoms; |
| Spleen below umbilical line; |
| Local compression (e.g., epidural, ureteral, gastrointestinal); |
| Pleural or peritoneal effusions; |
| Leukemic phase with > 5 × 109/L malignant cells; |
| Cytopenias (absolute neutrophil count < 1 × 109/L or platelet count < 100 × 109/L). |
| British National Lymphoma Investigation (BNLI) criteria [76]: |
| Pruritus or B symptoms; |
| Rapid generalized disease progression in the preceding 3 months; |
| Life-endangering organ involvement; |
| Cytopenias with hemoglobin < 10 g/dL or white blood cell count < 1.5 × 109/L or platelet count < 100 × 109/L, related to bone marrow involvement; |
| Localized bone lesions detected on radiography or isotope scan; |
| Renal infiltration; |
| Macroscopic as opposed to microscopic liver involvement. |
Table 2
Results from selected trials of immune therapies in FL
| Therapeutic agent | Phase | No.a) | ORR | CRR | Median PFS (mo) | CRS (grades 3–4) | Neurological events (grades 3–4) |
|---|---|---|---|---|---|---|---|
| Checkpoint inhibitor | |||||||
| Nivolumab [148] | II | 92 | 4% | 1% | 2 | - | - |
| Pembrolizumab [149] | II | 18 | 11% | 0% | Not reported | - | - |
| Pembrolizumab + rituximab [150] | II | 30 | 67% | 50% | 13 | - | - |
| Atezolizumab + obinutuzumab [151] | Ib | 26 | 54% | 23% | 9 | - | - |
| Atezolizumab + obinutuzumab + lenalidomide [152] | Ib/II | 38 | 78% | 72% | Not reported | - | - |
| CAR T-cell therapy | |||||||
| Axicabtagene ciloleucel [158,159] | II | 127 | 94% | 79% | 40 | 6% | 15% |
| Tisagenlecleucel [160,161] | II | 98 | 86% | 68% | Not reached | 0% | 1% |
| Lisocabtagene maraleucel [162] | II | 139 | 97% | 94% | Not reached | 1% | 2% |
| Bispecific antibodies | |||||||
| Mosunetuzumab [168] | II | 90 | 80% | 60% | 18 | 2% | 0% |
| Epcoritamab [172] | II | 128 | 82% | 63% | Not reported | 2% | 0% |
| Odronextamab [174] | II | 128 | 80% | 73% | 21 | 2% | 0% |
| CD47 blockade | |||||||
| Magrolimab [180] | Ib/II | 28 | 66%b) | 24%b) | Not reported | - | - |
Table 3
Selected ongoing phase III trials in FL
CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone; BR, bendamustine and rituximab; R2, rituximab and lenalidomide.
Trials were identified from ClinicalTrials.gov using the following search strategy: Condition/disease is “Follicular Lymphoma” and Study Phase is “Phase 3” and Study Status is (looking for participants is “Not yet recruiting” or “Recruiting”, or No longer looking for participants is “Active, not recruiting”). Trials are grouped by Setting, and ordered by Study number.
REFERENCES
1. Surveillance, Epidemiology, and End Results Program (SEER). Cancer stat facts: NHL - follicular lymphoma [Internet] Bethesda (MD): National Cancer Institute, c2024. [cited 2024 Jul 28]. Available from: https://seer.cancer.gov/statfacts/html/follicular.html.
2. Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin’s lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol 1998;9:717–720.

3. Sarkozy C, Maurer MJ, Link BK, et al. Cause of death in follicular lymphoma in the first decade of the rituximab era: a pooled analysis of French and US cohorts. J Clin Oncol 2019;37:144–152.



4. Mozessohn L, Cheung MC, Crump M, et al. Chemoimmunotherapy resistant follicular lymphoma: predictors of resistance, association with transformation and prognosis. Leuk Lymphoma 2014;55:2502–2507.


5. Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National Lympho-Care Study. J Clin Oncol 2015;33:2516–2522.



6. Freeman CL, Kridel R, Moccia AA, et al. Early progression after bendamustine-rituximab is associated with high risk of transformation in advanced stage follicular lymphoma. Blood 2019;134:761–764.


7. Casulo C, Dixon JG, Le-Rademacher J, et al. Validation of POD24 as a robust early clinical end point of poor survival in FL from 5225 patients on 13 clinical trials. Blood 2022;139:1684–1693.




8. Sarkozy C, Trneny M, Xerri L, et al. Risk factors and outcomes for patients with follicular lymphoma who had histologic transformation after response to first-line immunochemotherapy in the PRIMA trial. J Clin Oncol 2016;34:2575–2582.


9. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022;36:1720–1748.


10. Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 1984;226:1097–1099.


11. Leich E, Hoster E, Wartenberg M, et al. Similar clinical features in follicular lymphomas with and without breaks in the BCL2 locus. Leukemia 2016;30:854–860.



12. Xerri L, Bachy E, Fabiani B, et al. Identification of MUM1 as a prognostic immunohistochemical marker in follicular lymphoma using computerized image analysis. Hum Pathol 2014;45:2085–2093.


13. Mottok A, Jurinovic V, Farinha P, et al. FOXP1 expression is a prognostic biomarker in follicular lymphoma treated with rituximab and chemotherapy. Blood 2018;131:226–235.




14. Cong P, Raffeld M, Teruya-Feldstein J, Sorbara L, Pittaluga S, Jaffe ES. In situ localization of follicular lymphoma: description and analysis by laser capture microdissection. Blood 2002;99:3376–3382.



15. Liu Q, Salaverria I, Pittaluga S, et al. Follicular lymphomas in children and young adults: a comparison of the pediatric variant with usual follicular lymphoma. Am J Surg Pathol 2013;37:333–343.


16. Louissaint A Jr, Ackerman AM, Dias-Santagata D, et al. Pediatric-type nodal follicular lymphoma: an indolent clonal proliferation in children and adults with high proliferation index and no BCL2 rearrangement. Blood 2012;120:2395–2404.



17. Takata K, Okada H, Ohmiya N, et al. Primary gastrointestinal follicular lymphoma involving the duodenal second portion is a distinct entity: a multicenter, retrospective analysis in Japan. Cancer Sci 2011;102:1532–1536.


18. Lackraj T, Goswami R, Kridel R. Pathogenesis of follicular lymphoma. Best Pract Res Clin Haematol 2018;31:2–14.


19. Kumar E, Pickard L, Okosun J. Pathogenesis of follicular lymphoma: genetics to the microenvironment to clinical translation. Br J Haematol 2021;194:810–821.



20. Morin RD, Johnson NA, Severson TM, et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet 2010;42:181–185.




21. Pasqualucci L, Dominguez-Sola D, Chiarenza A, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature 2011;471:189–195.




22. Li H, Kaminski MS, Li Y, et al. Mutations in linker histone genes HIST1H1 B, C, D, and E; OCT2 (POU2F2); IRF8; and ARID1A underlying the pathogenesis of follicular lymphoma. Blood 2014;123:1487–1498.




23. Cheung KJ, Johnson NA, Affleck JG, et al. Acquired TNFRSF14 mutations in follicular lymphoma are associated with worse prognosis. Cancer Res 2010;70:9166–9174.



24. Okosun J, Wolfson RL, Wang J, et al. Recurrent mTORC1-activating RRAGC mutations in follicular lymphoma. Nat Genet 2016;48:183–188.

25. Wang F, Gatica D, Ying ZX, et al. Follicular lymphoma-associated mutations in vacuolar ATPase ATP6V1B2 activate autophagic flux and mTOR. J Clin Invest 2019;129:1626–1640.



26. Shelton V, Detroja R, Liu T, et al. Identification of genetic subtypes in follicular lymphoma. Blood Cancer J 2024;14:128.




27. Russler-Germain DA, Krysiak K, Ramirez C, et al. Mutations associated with progression in follicular lymphoma predict inferior outcomes at diagnosis: Alliance A151303. Blood Adv 2023;7:5524–5539.




28. O’Shea D, O’Riain C, Taylor C, et al. The presence of TP53 mutation at diagnosis of follicular lymphoma identifies a high-risk group of patients with shortened time to disease progression and poorer overall survival. Blood 2008;112:3126–3129.




29. Pastore A, Jurinovic V, Kridel R, et al. Integration of gene mutations in risk prognostication for patients receiving firstline immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol 2015;16:1111–1122.


30. Burack WR, Li H, Adlowitz D, et al. Subclonal TP53 mutations are frequent and predict resistance to radioimmunotherapy in follicular lymphoma. Blood Adv 2023;7:5082–5090.




31. Araf S, Wang J, Korfi K, et al. Genomic profiling reveals spatial intra-tumor heterogeneity in follicular lymphoma. Leukemia 2018;32:1261–1265.




32. Haebe S, Shree T, Sathe A, et al. Single-cell analysis can define distinct evolution of tumor sites in follicular lymphoma. Blood 2021;137:2869–2880.




33. Milpied P, Cervera-Marzal I, Mollichella ML, et al. Human germinal center transcriptional programs are de-synchronized in B cell lymphoma. Nat Immunol 2018;19:1013–1024.



34. Wang X, Nissen M, Gracias D, et al. Single-cell profiling reveals a memory B cell-like subtype of follicular lymphoma with increased transformation risk. Nat Commun 2022;13:6772.




35. Andor N, Simonds EF, Czerwinski DK, et al. Single-cell RNA-Seq of follicular lymphoma reveals malignant B-cell types and coexpression of T-cell immune checkpoints. Blood 2019;133:1119–1129.




36. Sarkozy C, Wu S, Takata K, et al. Integrated single cell analysis reveals co-evolution of malignant B cells and tumor micro-environment in transformed follicular lymphoma. Cancer Cell 2024;42:1003–1017e6.


37. Laurent C, Trisal P, Tesson B, et al. Follicular lymphoma comprises germinal center-like and memory-like molecular subtypes with prognostic significance. Blood 2024;Oct. 7. [Epub]. 10.1182/blood.2024024496.



38. Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med 2004;351:2159–2169.

39. Kridel R, Sehn LH, Gascoyne RD. Pathogenesis of follicular lymphoma. J Clin Invest 2012;122:3424–3431.



40. Han G, Deng Q, Marques-Piubelli ML, et al. Follicular lymphoma microenvironment characteristics associated with tumor cell mutations and MHC class II expression. Blood Cancer Discov 2022;3:428–443.




41. Tobin JWD, Keane C, Gunawardana J, et al. Progression of disease within 24 months in follicular lymphoma is associated with reduced intratumoral immune infiltration. J Clin Oncol 2019;37:3300–3309.



42. Ennishi D, Takata K, Béguelin W, et al. Molecular and genetic characterization of MHC deficiency identifies EZH2 as therapeutic target for enhancing immune recognition. Cancer Discov 2019;9:546–563.



43. Green MR, Kihira S, Liu CL, et al. Mutations in early follicular lymphoma progenitors are associated with suppressed antigen presentation. Proc Natl Acad Sci U S A 2015;112:E1116–E1125.



44. Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood 2004;104:1258–1265.


45. Federico M, Bellei M, Marcheselli L, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol 2009;27:4555–4562.


46. Bachy E, Maurer MJ, Habermann TM, et al. A simplified scoring system in de novo follicular lymphoma treated initially with immunochemotherapy. Blood 2018;132:49–58.




47. Mir F, Mattiello F, Grigg A, et al. Follicular Lymphoma Evaluation Index (FLEX): a new clinical prognostic model that is superior to existing risk scores for predicting progression-free survival and early treatment failure after frontline immunochemotherapy. Am J Hematol 2020;95:1503–1510.


48. Meignan M, Cottereau AS, Versari A, et al. Baseline metabolic tumor volume predicts outcome in high-tumor-burden follicular lymphoma: a pooled analysis of three multicenter studies. J Clin Oncol 2016;34:3618–3626.


49. Trotman J, Luminari S, Boussetta S, et al. Prognostic value of PET-CT after first-line therapy in patients with follicular lymphoma: a pooled analysis of central scan review in three multicentre studies. Lancet Haematol 2014;1:e17–e27.


50. Trotman J, Barrington SF, Belada D, et al. Prognostic value of end-of-induction PET response after first-line immunochemotherapy for follicular lymphoma (GALLIUM): secondary analysis of a randomised, phase 3 trial. Lancet Oncol 2018;19:1530–1542.

51. Huet S, Tesson B, Jais JP, et al. A gene-expression profiling score for prediction of outcome in patients with follicular lymphoma: a retrospective training and validation analysis in three international cohorts. Lancet Oncol 2018;19:549–561.



52. Silva A, Bassim S, Sarkozy C, et al. Convergence of risk prediction models in follicular lymphoma. Haematologica 2019;104:e252–e255.



53. Bolen CR, Mattiello F, Herold M, et al. Treatment dependence of prognostic gene expression signatures in de novo follicular lymphoma. Blood 2021;137:2704–2707.




54. Bolen CR, McCord R, Huet S, et al. Mutation load and an effector T-cell gene signature may distinguish immunologically distinct and clinically relevant lymphoma subsets. Blood Adv 2017;1:1884–1890.




55. Blaker YN, Spetalen S, Brodtkorb M, et al. The tumour microenvironment influences survival and time to transformation in follicular lymphoma in the rituximab era. Br J Haematol 2016;175:102–114.



56. Stevens WBC, Mendeville M, Redd R, et al. Prognostic relevance of CD163 and CD8 combined with EZH2 and gain of chromosome 18 in follicular lymphoma: a study by the Lunenburg Lymphoma Biomarker Consortium. Haematologica 2017;102:1413–1423.



57. Huet S, Xerri L, Tesson B, et al. EZH2 alterations in follicular lymphoma: biological and clinical correlations. Blood Cancer J 2017;7:e555.




58. Jurinovic V, Passerini V, Oestergaard M, et al. Evaluation of the m7-FLIPI in patients with follicular lymphoma treated within the gallium trial: EZH2 mutation status may be a predictive marker for differential efficacy of chemotherapy. Blood 2019;134(Supplement 1):122.


59. Kridel R, Sehn LH, Gascoyne RD. Can histologic transformation of follicular lymphoma be predicted and prevented? Blood 2017;130:258–266.



60. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059–3068.



61. Pugh TJ, Ballonoff A, Newman F, Rabinovitch R. Improved survival in patients with early stage low-grade follicular lymphoma treated with radiation: a Surveillance, Epidemiology, and End Results database analysis. Cancer 2010;116:3843–3851.


62. Wirth A, Foo M, Seymour JF, Macmanus MP, Hicks RJ. Impact of [18F] fluorodeoxyglucose positron emission tomography on staging and management of early-stage follicular non-hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2008;71:213–219.


63. Mac Manus MP, Hoppe RT. Is radiotherapy curative for stage I and II low-grade follicular lymphoma? Results of a long-term follow-up study of patients treated at Stanford University. J Clin Oncol 1996;14:1282–1290.


64. Wilder RB, Jones D, Tucker SL, et al. Long-term results with radiotherapy for Stage I–II follicular lymphomas. Int J Radiat Oncol Biol Phys 2001;51:1219–1227.


65. Guadagnolo BA, Li S, Neuberg D, et al. Long-term outcome and mortality trends in early-stage, Grade 1–2 follicular lymphoma treated with radiation therapy. Int J Radiat Oncol Biol Phys 2006;64:928–934.


66. Friedberg JW, Byrtek M, Link BK, et al. Effectiveness of first-line management strategies for stage I follicular lymphoma: analysis of the National LymphoCare Study. J Clin Oncol 2012;30:3368–3375.



67. Barzenje DA, Cvancarova Småstuen M, Liestøl K, et al. Radiotherapy compared to other strategies in the treatment of stage I/II follicular lymphoma: a study of 404 patients with a median follow-up of 15 years. PLoS One 2015;10:e0131158.



68. Vargo JA, Gill BS, Balasubramani GK, Beriwal S. What is the optimal management of early-stage low-grade follicular lymphoma in the modern era? Cancer 2015;121:3325–3334.


69. Brady JL, Binkley MS, Hajj C, et al. Definitive radiotherapy for localized follicular lymphoma staged by 18F-FDG PET-CT: a collaborative study by ILROG. Blood 2019;133:237–245.



70. Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol 2011;100:86–92.


71. Hoskin P, Popova B, Schofield O, et al. 4 Gy versus 24 Gy radiotherapy for follicular and marginal zone lymphoma (FoRT): long-term follow-up of a multicentre, randomised, phase 3, non-inferiority trial. Lancet Oncol 2021;22:332–340.


72. Imber BS, Chau KW, Lee J, et al. Excellent response to very-low-dose radiation (4 Gy) for indolent B-cell lymphomas: is 4 Gy suitable for curable patients? Blood Adv 2021;5:4185–4197.




73. Pulsoni A, Starza ID, Frattarelli N, et al. Stage I/II follicular lymphoma: spread of bcl-2/IgH+ cells in blood and bone marrow from primary site of disease and possibility of clearance after involved field radiotherapy. Br J Haematol 2007;137:216–220.


74. Herfarth K, Borchmann P, Schnaidt S, et al. Rituximab with involved field irradiation for early-stage nodal follicular lymphoma: results of the MIR study. Hemasphere 2018;2:e160.


75. Janikova A, Bortlicek Z, Campr V, et al. Radiotherapy with rituximab may be better than radiotherapy alone in first-line treatment of early-stage follicular lymphoma: is it time to change the standard strategy? Leuk Lymphoma 2015;56:2350–2356.


76. MacManus M, Fisher R, Roos D, et al. Randomized trial of systemic therapy after involved-field radiotherapy in patients with early-stage follicular lymphoma: TROG 99.03. J Clin Oncol 2018;36:2918–2925.


77. Ardeshna KM, Smith P, Norton A, et al. Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: a randomised controlled trial. Lancet 2003;362:516–522.


78. Brice P, Bastion Y, Lepage E, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 1997;15:1110–1107.


79. Ardeshna KM, Qian W, Smith P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: an open-label randomised phase 3 trial. Lancet Oncol 2014;15:424–435.


80. Kahl BS, Hong F, Williams ME, et al. Rituximab extended schedule or re-treatment trial for low-tumor burden follicular lymphoma: eastern cooperative oncology group protocol e4402. J Clin Oncol 2014;32:3096–3102.



81. Cartron G, Bachy E, Tilly H, et al. Randomized phase III trial evaluating subcutaneous rituximab for the first-line treatment of low-tumor burden follicular lymphoma: results of a LYSA study. J Clin Oncol 2023;41:3523–3533.


82. Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol 2008;26:4579–4586.


83. Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005;106:3725–3732.


84. Bachy E, Houot R, Morschhauser F, et al. Long-term follow up of the FL2000 study comparing CHVP-interferon to CHVP-interferon plus rituximab in follicular lymphoma. Haematologica 2013;98:1107–1114.



85. Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381:1203–1210.


86. Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 2014;123:2944–2952.




87. Flinn IW, van der Jagt R, Kahl B, et al. First-line treatment of patients with indolent non-Hodgkin lymphoma or mantle-cell lymphoma with bendamustine plus rituximab versus R-CHOP or R-CVP: results of the BRIGHT 5-year follow-up study. J Clin Oncol 2019;37:984–991.



88. Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 2011;377:42–51.


89. Bachy E, Seymour JF, Feugier P, et al. Sustained progression-free survival benefit of rituximab maintenance in patients with follicular lymphoma: long-term results of the PRIMA study. J Clin Oncol 2019;37:2815–2824.



90. Luminari S, Ferrari A, Manni M, et al. Long-term results of the FOLL05 trial comparing R-CVP versus R-CHOP versus R-FM for the initial treatment of patients with advanced-stage symptomatic follicular lymphoma. J Clin Oncol 2018;36:689–696.


91. Hill BT, Nastoupil L, Winter AM, et al. Maintenance rituximab or observation after frontline treatment with bendamustine-rituximab for follicular lymphoma. Br J Haematol 2019;184:524–535.




92. Luminari S, Manni M, Galimberti S, et al. Response-adapted postinduction strategy in patients with advanced-stage follicular lymphoma: the FOLL12 study. J Clin Oncol 2022;40:729–739.


93. Nizzoli ME, Manni M, Ghiggi C, et al. Impact of immunochemotherapy with R-bendamustine or R-CHOP for treatment naïve advanced-stage follicular lymphoma: a subset analysis of the FOLL12 trial by Fondazione Italiana Linfomi. Hematol Oncol 2023;41:655–662.

94. Marcus R, Davies A, Ando K, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med 2017;377:1331–1344.


95. Seymour JF, Marcus R, Davies A, et al. Association of early disease progression and very poor survival in the GALLIUM study in follicular lymphoma: benefit of obinutuzumab in reducing the rate of early progression. Haematologica 2019;104:1202–1208.



96. Hiddemann W, Barbui AM, Canales MA, et al. Immunochemotherapy with obinutuzumab or rituximab for previously untreated follicular lymphoma in the GALLIUM study: influence of chemotherapy on efficacy and safety. J Clin Oncol 2018;36:2395–2404.


97. Gong IY, Prica A, Ante Z, et al. Indolent lymphoma care delivery and outcomes during the COVID-19 pandemic in Ontario, Canada. Br J Haematol 2024;204:805–814.


98. Lamure S, Duléry R, Di Blasi R, et al. Determinants of outcome in Covid-19 hospitalized patients with lymphoma: a retrospective multicentric cohort study. EClinicalMedicine 2020;27:100549.



99. Serna Á, Navarro V, Iacoboni G, et al. Rituximab maintenance after bendamustine-based treatment for follicular lymphoma and mantle cell lymphoma may exert a negative influence on SARS-CoV-2 infection outcomes. Haematologica 2024;Jul. 11. [Epub]. 10.3324/haematol.2024.285219.



100. Martin P, Jung SH, Pitcher B, et al. A phase II trial of lenalidomide plus rituximab in previously untreated follicular non-Hodgkin’s lymphoma (NHL): CALGB 50803 (Alliance). Ann Oncol 2017;28:2806–2812.



101. Zucca E, Rondeau S, Vanazzi A, et al. Short regimen of rituximab plus lenalidomide in follicular lymphoma patients in need of first-line therapy. Blood 2019;134:353–362.



102. Fowler NH, Davis RE, Rawal S, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol 2014;15:1311–1318.



103. Morschhauser F, Fowler NH, Feugier P, et al. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N Engl J Med 2018;379:934–947.



104. Morschhauser F, Nastoupil L, Feugier P, et al. Six-year results from RELEVANCE: lenalidomide plus rituximab (R2) versus rituximab-chemotherapy followed by rituximab maintenance in untreated advanced follicular lymphoma. J Clin Oncol 2022;40:3239–3245.



105. Bachy E, Houot R, Feugier P, et al. Obinutuzumab plus lenalidomide in advanced, previously untreated follicular lymphoma in need of systemic therapy: a LYSA study. Blood 2022;139:2338–2346.



106. Goldman ML, Mao JJ, Strouse CS, et al. Surveillance imaging during first remission in follicular lymphoma does not impact overall survival. Cancer 2021;127:3390–3402.




107. El-Galaly TC, Øvlisen AK, Cheah CY. Routine imaging for disease surveillance in follicular lymphoma-To comfort the patients or their doctors? Cancer 2021;127:3298–3301.



108. Bitansky G, Avigdor A, Vasilev E, et al. Progression of disease within 24 months of initial therapy (POD24) detected incidentally in imaging does not necessarily indicate worse outcome. Leuk Lymphoma 2020;61:2645–2651.


109. Fujino T, Maruyama D, Maeshima AM, et al. The outcome of watchful waiting in patients with previously treated follicular lymphoma. Cancer Med 2022;11:2106–2116.




110. Link BK, Day BM, Zhou X, et al. Second-line and subsequent therapy and outcomes for follicular lymphoma in the United States: data from the observational National LymphoCare Study. Br J Haematol 2019;184:660–663.



111. Rivas-Delgado A, Magnano L, Moreno-Velázquez M, et al. Response duration and survival shorten after each relapse in patients with follicular lymphoma treated in the rituximab era. Br J Haematol 2019;184:753–759.



112. Batlevi CL, Sha F, Alperovich A, et al. Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups. Blood Cancer J 2020;10:74.




113. Ghione P, Palomba ML, Ghesquieres H, et al. Treatment patterns and outcomes in relapsed/refractory follicular lymphoma: results from the international SCHOLAR-5 study. Haematologica 2023;108:822–832.




114. Casulo C, Larson MC, Lunde JJ, et al. Treatment patterns and outcomes of patients with relapsed or refractory follicular lymphoma receiving three or more lines of systemic therapy (LEO CReWE): a multicentre cohort study. Lancet Haematol 2022;9:e289–e300.



115. Salles G, Schuster SJ, Fischer L, et al. A retrospective cohort study of treatment outcomes of adult patients with relapsed or refractory follicular lymphoma (ReCORD-FL). Hemasphere 2022;6:e745.



116. Rummel M, Kaiser U, Balser C, et al. Bendamustine plus rituximab versus fludarabine plus rituximab for patients with relapsed indolent and mantle-cell lymphomas: a multicentre, randomised, open-label, non-inferiority phase 3 trial. Lancet Oncol 2016;17:57–66.


117. Sehn LH, Chua N, Mayer J, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol 2016;17:1081–1093.


118. Cheson BD, Chua N, Mayer J, et al. Overall survival benefit in patients with rituximab-refractory indolent non-hodgkin lymphoma who received obinutuzumab plus bendamustine induction and obinutuzumab maintenance in the GADOLIN study. J Clin Oncol 2018;36:2259–2266.


119. Vidal L, Gafter-Gvili A, Salles G, et al. Rituximab maintenance improves overall survival of patients with follicular lymphoma-Individual patient data meta-analysis. Eur J Cancer 2017;76:216–225.


120. Krönke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014;343:301–305.

121. Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 2014;343:305–309.



122. Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science 2010;327:1345–1350.


123. Hernandez-Ilizaliturri FJ, Reddy N, Holkova B, Ottman E, Czuczman MS. Immunomodulatory drug CC-5013 or CC-4047 and rituximab enhance antitumor activity in a severe combined immunodeficient mouse lymphoma model. Clin Cancer Res 2005;11:5984–5992.



124. Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol 2008;140:36–45.


125. Ramsay AG, Clear AJ, Kelly G, et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood 2009;114:4713–4720.




126. Zhang L, Qian Z, Cai Z, et al. Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol 2009;84:553–559.


127. Leonard JP, Jung SH, Johnson J, et al. Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (Alliance). J Clin Oncol 2015;33:3635–3640.



128. Chong EA, Ahmadi T, Aqui NA, et al. Combination of Lenalidomide and rituximab overcomes rituximab resistance in patients with indolent B-cell and mantle cell lymphomas. Clin Cancer Res 2015;21:1835–1842.



129. Tuscano JM, Dutia M, Chee K, et al. Lenalidomide plus rituximab can produce durable clinical responses in patients with relapsed or refractory, indolent non-Hodgkin lymphoma. Br J Haematol 2014;165:375–381.


130. Leonard JP, Trneny M, Izutsu K, et al. AUGMENT: a phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol 2019;37:1188–1199.



131. Leonard JP, Trneny M, Offner F, et al. Five-year results and overall survival update from the phase 3 randomized study augment: lenalidomide plus rituximab (R2) vs rituximab plus placebo in patients with relapsed/refractory indolent non-hodgkin lymphoma. Blood 2022;140(Supplement 1):561–563.


132. Lansigan F, Andorsky DJ, Coleman M, et al. P1156: magnify phase 3B STUdy of lenalidomide + rituximab (R2) followed by maintenance in relapsed/refractory indolent non-hodgkin lymphoma: complete induction phase analysis. Hemasphere 2022;6(Suppl):1043–1044.


133. Morschhauser F, Le Gouill S, Feugier P, et al. Obinutuzumab combined with lenalidomide for relapsed or refractory follicular B-cell lymphoma (GALEN): a multicentre, single-arm, phase 2 study. Lancet Haematol 2019;6:e429–e437.


134. Chavez JC, Nastoupil L, Jørgensen J, et al. Safety and efficacy of golcadomide, a potential first-in-class celmod agent ± rituximab in a phase 1/2 open-label study of patients with relapsed/refractory (R/R) follicular lymphoma (FL). Eur Hematol Assoc 2024;419219:P1132.
135. Freedman AS, Neuberg D, Mauch P, et al. Long-term follow-up of autologous bone marrow transplantation in patients with relapsed follicular lymphoma. Blood 1999;94:3325–3333.



136. Rohatiner AZ, Nadler L, Davies AJ, et al. Myeloablative therapy with autologous bone marrow transplantation for follicular lymphoma at the time of second or subsequent remission: long-term follow-up. J Clin Oncol 2007;25:2554–2259.


137. Schouten HC, Qian W, Kvaloy S, et al. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin’s lymphoma: results from the randomized European CUP trial. J Clin Oncol 2003;21:3918–3927.


138. Jurinovic V, Metzner B, Pfreundschuh M, et al. Autologous stem cell transplantation for patients with early progression of follicular lymphoma: a follow-up study of 2 randomized trials from the German low grade lymphoma study group. Biol Blood Marrow Transplant 2018;24:1172–1179.


139. Le Gouill S, De Guibert S, Planche L, et al. Impact of the use of autologous stem cell transplantation at first relapse both in naive and previously rituximab exposed follicular lymphoma patients treated in the GELA/GOELAMS FL2000 study. Haematologica 2011;96:1128–1135.



140. Puckrin R, Chua N, Chin K, et al. Long-term follow-up demonstrates curative potential of autologous stem cell transplantation for relapsed follicular lymphoma. Br J Haematol 2023;201:319–325.



141. Casulo C, Friedberg JW, Ahn KW, et al. Autologous transplantation in follicular lymphoma with early therapy failure: a national lymphocare study and center for international blood and marrow transplant research analysis. Biol Blood Marrow Transplant 2018;24:1163–1171.



142. Smith SM, Godfrey J, Ahn KW, et al. Autologous transplantation versus allogeneic transplantation in patients with follicular lymphoma experiencing early treatment failure. Cancer 2018;124:2541–2551.




143. Sureda A, Zhang MJ, Dreger P, et al. Allogeneic hematopoietic stem cell transplantation for relapsed follicular lymphoma: a combined analysis on behalf of the Lymphoma Working Party of the EBMT and the Lymphoma Committee of the CIBMTR. Cancer 2018;124:1733–1742.




144. Twa DD, Chan FC, Ben-Neriah S, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood 2014;123:2062–2065.



145. Myklebust JH, Irish JM, Brody J, et al. High PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumors from peripheral T cells. Blood 2013;121:1367–1376.




146. Yang ZZ, Grote DM, Ziesmer SC, Xiu B, Novak AJ, Ansell SM. PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood Cancer J 2015;5:e281.




147. Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol 2016;34:2698–2704.



148. Armand P, Janssens A, Gritti G, et al. Efficacy and safety results from CheckMate 140, a phase 2 study of nivolumab for relapsed/refractory follicular lymphoma. Blood 2021;137:637–645.




149. Ding W, Laplant B, Witzig TE, et al. PD-1 blockade with pembrolizumab in relapsed low grade non-hodgkin lymphoma. Blood 2017;130(Supplement 1):4055.
150. Nastoupil LJ, Chin CK, Westin JR, et al. Safety and activity of pembrolizumab in combination with rituximab in relapsed or refractory follicular lymphoma. Blood Adv 2022;6:1143–1151.




151. Palomba ML, Till BG, Park SI, et al. Combination of atezolizumab and obinutuzumab in patients with relapsed/refractory follicular lymphoma and diffuse large B-cell lymphoma: results from a phase 1b study. Clin Lymphoma Myeloma Leuk 2022;22:e443–e451.


152. Morschhauser F, Ghosh N, Lossos IS, et al. Obinutuzumab-atezolizumab-lenalidomide for the treatment of patients with relapsed/refractory follicular lymphoma: final analysis of a Phase Ib/II trial. Blood Cancer J 2021;11:147.




153. Topp MS, Eradat H, Florschütz A, et al. Anti-CD20-atezolizumab-polatuzumab vedotin in relapsed/refractory follicular and diffuse large B-cell lymphoma. J Cancer Res Clin Oncol 2023;149:811–817.




154. Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010;116:4099–4102.




155. Cappell KM, Sherry RM, Yang JC, et al. Long-term follow-up of anti-cd19 chimeric antigen receptor T-cell therapy. J Clin Oncol 2020;38:3805–3815.



156. Hirayama AV, Gauthier J, Hay KA, et al. High rate of durable complete remission in follicular lymphoma after CD19 CAR-T cell immunotherapy. Blood 2019;134:636–640.




157. Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017;377:2545–2554.



158. Jacobson CA, Chavez JC, Sehgal AR, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol 2022;23:91–103.


159. Neelapu SS, Chavez JC, Sehgal AR, et al. Three-year follow-up analysis of axicabtagene ciloleucel in relapsed/refractory indolent non-Hodgkin lymphoma (ZUMA-5). Blood 2024;143:496–506.




160. Fowler NH, Dickinson M, Dreyling M, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med 2022;28:325–332.

161. Dreyling M, Fowler NH, Dickinson M, et al. Durable response after tisagenlecleucel in adults with relapsed/refractory follicular lymphoma: ELARA trial update. Blood 2024;143:1713–1725.




162. Morschhauser F, Dahiya S, Palomba ML, et al. Lisocabtagene maraleucel in follicular lymphoma: the phase 2 TRANSCEND FL study. Nat Med 2024;30:2199–2207.




163. Jacobson C, Hemmer MT, Hu ZH, et al. S223: real-world early outcomes of axicabtagene ciloleucel for relapsed or refractory follicular lymphoma. HemaSphere 2023;7(S3):e31464ce.


164. Ysebaert L, Houot R, Casasnovas O, et al. Real-word experience of CAR T-cells in patients with relapsed/refractory follicular lymphoma: a Descart registry analysis from the lysa. Blood 2023;142(Supplement 1):296.


165. Salles G, Schuster SJ, Dreyling M, et al. Efficacy comparison of tisagenlecleucel vs usual care in patients with relapsed or refractory follicular lymphoma. Blood Adv 2022;6:5835–5843.




166. Kambhampati S, Wang HL, Yan J, et al. Comparative effectiveness of axicabtagene ciloleucel vs historical standardof-care in patients with relapsed or refractory follicular lymphoma: an analysis of CIBMTR and SCHOLAR-5 data. Blood 2023;142(Supplement 1):2121.


167. Budde LE, Assouline S, Sehn LH, et al. Single-agent mosunetuzumab shows durable complete responses in patients with relapsed or refractory B-cell lymphomas: phase I dose-escalation study. J Clin Oncol 2022;40:481–491.



168. Budde LE, Sehn LH, Matasar M, et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol 2022;23:1055–1065.


169. Budde LE, Assouline S, Sehn LH, et al. Durable responses with mosunetuzumab in relapsed/refractory indolent and aggressive B-cell non-Hodgkin lymphomas: extended follow-up of a phase I/II study. J Clin Oncol 2024;42:2250–2256.



170. Hutchings M, Morschhauser F, Iacoboni G, et al. Glofitamab, a novel, bivalent CD20-targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase I trial. J Clin Oncol 2021;39:1959–1970.



171. Hutchings M, Mous R, Clausen MRJ, et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet 2021;398:1157–1169.


172. Linton KM, Vitolo U, Jurczak W, et al. Epcoritamab monotherapy in patients with relapsed or refractory follicular lymphoma (EPCORE NHL-1): a phase 2 cohort of a single-arm, multicentre study. Lancet Haematol 2024;11:e593–e605.


173. Bannerji R, Arnason JE, Advani RH, et al. Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol 2022;9:e327–e339.



174. Kim TM, Taszner M, Novelli S, et al. Safety and efficacy of odronextamab in patients with relapsed or refractory follicular lymphoma. Ann Oncol 2024;35:1039–1047.


175. Reynolds GK, Maclean M, Cliff ERS, et al. Infections in patients with lymphoma treated with bispecific antibodies: a systematic review and meta-analysis. Blood Adv 2024;8:3555–3559.




176. Maurer MJ, Casulo C, Larson MC, et al. Matching-adjusted indirect comparison from the Lymphoma Epidemiology of Outcomes Consortium for Real World Evidence (LEO CReWE) study to a clinical trial of mosunetuzumab in relapsed or refractory follicular lymphoma. Haematologica 2024;109:2177–2185.




177. McGough SF, Shamas N, Wang J, et al. Comparative effectiveness between mosunetuzumab monotherapy clinical trial and real-world data in relapsed/refractory follicular lymphoma in third or subsequent lines of systemic therapy. Leuk Lymphoma 2023;64:2269–2278.


178. Chao MP, Alizadeh AA, Tang C, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 2010;142:699–713.



179. Advani R, Flinn I, Popplewell L, et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N Engl J Med 2018;379:1711–1721.



180. Advani R, Bartlett NL, Smith SM, et al. The first-in-class anti-CD47 antibody Hu5F9-G4 + rituximab induces durable responses in relapsed/refractory DLBCL and indolent lymphoma: interim phase 1B/2 results. Hematol Oncol 2019;37(S2):89–90.


181. Patel K, Zonder JA, Sano D, et al. CD47-blocker TTI-622 shows single-agent activity in patients with advanced relapsed or refractory lymphoma: update from the ongoing first-in-human dose escalation study. Blood 2021;138(Supplement 1):3560.


182. Béguelin W, Popovic R, Teater M, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 2013;23:677–692.



183. Italiano A, Soria JC, Toulmonde M, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol 2018;19:649–659.


184. Morschhauser F, Tilly H, Chaidos A, et al. Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol 2020;21:1433–1442.



185. Passerini V, Jurinovic V, Bolen CR, et al. An EZH2 gene expression signature is predictive of differential efficacy of chemotherapy irrespective of EZH2 mutation status in patients with follicular lymphoma treated within the gallium trial. Blood 2021;138(Supplement 1):39.


186. Romero P, Richart L, Aflaki S, et al.
EZH2 mutations in follicular lymphoma distort H3K27me3 profiles and alter transcriptional responses to PRC2 inhibition. Nat Commun 2024;15:3452.




187. Salles G, Park SI, Phillips TJ, et al. Tazemetostat in combination with lenalidomide and rituximab in patients with relapsed/refractory follicular lymphoma: updated phase 1b results of symphony-1 with 22.5 months follow-up. Blood 2023;142(Supplement 1):3035.


188. Isshiki Y, Porazzi P, Chen X, et al. EZH2 inhibitors enhance CART cell quality, efficacy, in vivo homing, tumor cell binding and killing of fully syngeneic primary B cell lymphomas, as well as reprogramming lymphoma cells to a highly immunogenic and T cell adherent phenotype. Blood 2023;142(Supplement 1):432.


189. Porazzi P, Isshiki Y, Ghilardi G, et al. Inhibition of EZH2 improves CART19 immunotherapy by reprogramming lymphoma tumor cells and enhancing T-cell functionality. Blood 2023;142(Supplement 1):1018.


190. Kirschbaum M, Frankel P, Popplewell L, et al. Phase II study of vorinostat for treatment of relapsed or refractory indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. J Clin Oncol 2011;29:1198–1203.



191. Godfrey J, Mei M, Chen L, et al. Results from a phase I trial of pembrolizumab plus vorinostat in relapsed/refractory B-cell non-Hodgkin lymphoma. Haematologica 2024;109:533–542.




192. Chen R, Frankel P, Popplewell L, et al. A phase II study of vorinostat and rituximab for treatment of newly diagnosed and relapsed/refractory indolent non-Hodgkin lymphoma. Haematologica 2015;100:357–362.



193. Ogura M, Ando K, Suzuki T, et al. A multicentre phase II study of vorinostat in patients with relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Br J Haematol 2014;165:768–776.




194. Batlevi CL, Crump M, Andreadis C, et al. A phase 2 study of mocetinostat, a histone deacetylase inhibitor, in relapsed or refractory lymphoma. Br J Haematol 2017;178:434–441.




195. Ribrag V, Kim WS, Bouabdallah RL, et al. Safety and efficacy of abexinostat, a pan-histone deacetylase inhibitor, in non-Hodgkin lymphoma and chronic lymphocytic leukemia: results of a phase II study. Haematologica 2017;102:903–909.



196. Heward J, Konali L, D’Avola A, et al. KDM5 inhibition offers a novel therapeutic strategy for the treatment of KMT2D mutant lymphomas. Blood 2021;138:370–381.


197. Loeffler M, Kreuz M, Haake A, et al. Genomic and epigenomic co-evolution in follicular lymphomas. Leukemia 2015;29:456–463.



198. Irish JM, Czerwinski DK, Nolan GP, Levy R. Altered B-cell receptor signaling kinetics distinguish human follicular lymphoma B cells from tumor-infiltrating nonmalignant B cells. Blood 2006;108:3135–3142.




199. Irish JM, Myklebust JH, Alizadeh AA, et al. B-cell signaling networks reveal a negative prognostic human lymphoma cell subset that emerges during tumor progression. Proc Natl Acad Sci U S A 2010;107:12747–12754.



200. Myklebust JH, Brody J, Kohrt HE, et al. Distinct patterns of B-cell receptor signaling in non-Hodgkin lymphomas identified by single-cell profiling. Blood 2017;129:759–770.




201. Amin R, Mourcin F, Uhel F, et al. DC-SIGN-expressing macrophages trigger activation of mannosylated IgM B-cell receptor in follicular lymphoma. Blood 2015;126:1911–1920.




202. Linley A, Krysov S, Ponzoni M, Johnson PW, Packham G, Stevenson FK. Lectin binding to surface Ig variable regions provides a universal persistent activating signal for follicular lymphoma cells. Blood 2015;126:1902–1910.



203. Pal Singh S, Dammeijer F, Hendriks RW. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol Cancer 2018;17:57.


204. Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 2010;115:2578–2585.




205. Andorsky DJ, Kolibaba KS, Assouline S, et al. An open-label phase 2 trial of entospletinib in indolent non-Hodgkin lymphoma and mantle cell lymphoma. Br J Haematol 2019;184:215–222.




206. Coffey GP, Feng J, Betz A, et al. Cerdulatinib pharmacodynamics and relationships to tumor response following oral dosing in patients with relapsed/refractory B-cell malignancies. Clin Cancer Res 2019;25:1174–1184.



207. Gordon LI, Kaplan JB, Popat R, et al. Phase I study of TAK-659, an investigational, dual SYK/FLT3 inhibitor, in patients with B-Cell lymphoma. Clin Cancer Res 2020;26:3546–3556.



208. Song Y, Cao J, Zhang Q, et al. Phase I study of the Syk inhibitor sovleplenib in relapsed or refractory mature B-cell tumors. Haematologica 2024;109:2165–2176.




209. Gopal AK, Schuster SJ, Fowler NH, et al. Ibrutinib as treatment for patients with relapsed/refractory follicular lymphoma: results from the open-label, multicenter, phase II DAWN study. J Clin Oncol 2018;36:2405–2412.


210. Bartlett NL, Costello BA, LaPlant BR, et al. Single-agent ibrutinib in relapsed or refractory follicular lymphoma: a phase 2 consortium trial. Blood 2018;131:182–190.




211. Nastoupil LJ, Hess G, Pavlovsky MA, et al. Phase 3 SELENE study: ibrutinib plus BR/R-CHOP in previously treated patients with follicular or marginal zone lymphoma. Blood Adv 2023;7:7141–7150.




212. Fowler NH, Coleman M, Stevens DA, et al. Acalabrutinib alone or in combination with rituximab (R) in follicular lymphoma (FL). J Clin Oncol 2018;36(15 Suppl):7549.

213. Strati P, Agajanian R, Lossos IS, et al. Acalabrutinib in combination with rituximab and lenalidomide in patients with relapsed or refractory follicular lymphoma: Results of the phase 1b open-label study (ACE-LY-003). Br J Haematol 2024;Dec. 12. [Epub]. 10.1111/bjh.19951.
214. Tam CS, Quach H, Nicol A, et al. Zanubrutinib (BGB-3111) plus obinutuzumab in patients with chronic lymphocytic leukemia and follicular lymphoma. Blood Adv 2020;4:4802–4811.




215. Zinzani PL, Mayer J, Flowers CR, et al. ROSEWOOD: a phase II randomized study of zanubrutinib plus obinutuzumab versus obinutuzumab monotherapy in patients with relapsed or refractory follicular lymphoma. J Clin Oncol 2023;41:5107–5117.


216. Hu N, Wang F, Sun T, et al. Follicular lymphoma-associated BTK mutations are inactivating resulting in augmented AKT activation. Clin Cancer Res 2021;27:2301–2313.




217. Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med 2014;370:2286–2294.



218. Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 2014;370:1008–1018.



219. Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-Kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol 2017;35:3898–3905.


220. Flinn IW, Miller CB, Ardeshna KM, et al. DYNAMO: a phase II study of duvelisib (IPI-145) in patients with refractory indolent non-Hodgkin lymphoma. J Clin Oncol 2019;37:912–922.


221. Soumerai JD, Diefenbach CS, Jagadeesh D, et al. Safety and efficacy of zandelisib plus zanubrutinib in previously treated follicular and mantle cell lymphomas. Br J Haematol 2024;204:1762–1770.



222. Wang H, Feng J, Liu Y, et al. Phase II study of novel orally PI3Kα/δ inhibitor TQ-B3525 in relapsed and/or refractory follicular lymphoma. Signal Transduct Target Ther 2024;9:99.




223. Phillips TJ, Michot JM, Ribrag V. Can next-generation PI3K inhibitors unlock the full potential of the class in patients with B-Cell lymphoma? Clin Lymphoma Myeloma Leuk 2021;21:8–20e3.


224. Martynchyk A, Hawkes EA. Learnings from phosphatidylinositol 3-kinase inhibitors in lymphoma-Moving to a model where less can be more. Br J Haematol 2024;204:1582–1584.


225. Davids MS, Roberts AW, Seymour JF, et al. Phase I first-inhuman study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol 2017;35:826–833.



226. Zinzani PL, Flinn IW, Yuen SLS, et al. Venetoclax-rituximab with or without bendamustine vs bendamustine-rituximab in relapsed/refractory follicular lymphoma. Blood 2020;136:2628–2637.


227. Smith SM, van Besien K, Karrison T, et al. Temsirolimus has activity in non-mantle cell non-Hodgkin’s lymphoma subtypes: The University of Chicago phase II consortium. J Clin Oncol 2010;28:4740–4746.



228. Bennani NN, LaPlant BR, Ansell SM, et al. Efficacy of the oral mTORC1 inhibitor everolimus in relapsed or refractory indolent lymphoma. Am J Hematol 2017;92:448–453.



229. Ortega-Molina A, Deleyto-Seldas N, Carreras J, et al. Oncogenic Rag GTPase signaling enhances B cell activation and drives follicular lymphoma sensitive to pharmacological inhibition of mTOR. Nat Metab 2019;1:775–789.




230. Kumar EA, Korfi K, Bewicke-Copley F, et al. CREBBP histone acetyltransferase domain mutations predict response to mTOR inhibition in relapsed/refractory follicular lymphoma. Br J Haematol 2024;205:1804–1809.


231. Jurczak W, Zinzani PL, Gaidano G, et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Ann Oncol 2018;29:1266–1272.



232. Morschhauser F, Flinn IW, Advani R, et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet Haematol 2019;6:e254–e265.


233. Diefenbach C, Kahl BS, McMillan A, et al. Polatuzumab vedotin plus obinutuzumab and lenalidomide in patients with relapsed or refractory follicular lymphoma: a cohort of a multicentre, single-arm, phase 1b/2 study. Lancet Haematol 2021;8:e891–e901.


234. Sehn LH, Kamdar M, Herrera AF, et al. Randomized phase 2 trial of polatuzumab vedotin (pola) with bendamustine and rituximab (BR) in relapsed/refractory (r/r) FL and DLBCL. J Clin Oncol 2018;36(15 Suppl):7507.

235. Hamadani M, Radford J, Carlo-Stella C, et al. Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma. Blood 2021;137:2634–2645.




236. Oerlemans S, Issa DE, van den Broek EC, et al. Impact of therapy and disease-related symptoms on health-related quality of life in patients with follicular lymphoma: results of the population-based PHAROS-registry. Eur J Haematol 2014;93:229–238.

237. Johnson PC, Bailey A, Ma Q, et al. Quality of life evaluation in patients with follicular cell lymphoma: a real-world study in Europe and the United States. Adv Ther 2024;41:3342–3361.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



