 |
 |
| Korean J Intern Med > Volume 39(6); 2024 > Article |
|
Abstract
Background/Aims
Non-alcoholic fatty liver disease (NAFLD), now the most common chronic liver worldwide, has become a significant public health concern. This study aims to analyze the evolving epidemiology of NAFLD in South Korea.
Methods
We utilized claim data from the Korean National Health Insurance Service from 2010 to 2022 to analyze NAFLDŌĆÖs incidence, prevalence, and progression.
Results
From 2010 to 2022, the incidence and prevalence rates of NAFLD each increased from 1.87% to 4.47% and from 10.49% to 17.13%, respectively. The differences in prevalence rates between urban and rural areas were minimal in 2012 and 2022, yet both areas showed significant increases in the prevalence of NAFLD over the decade. The NAFLD group had a higher prevalence of comorbidities compared to the control group, and the most common comorbid condition was hypertension. Moreover, the ten-year incidence rates of malignancy, heart disease, and stroke in the NAFLD group were 13.42%, 15.72%, and 8.36%, respectively, which were significantly higher than those in the control group. The incidence rates of cirrhosis and hepatocellular carcinoma in NAFLD over 10 years were 2.22% and 0.77%, respectively. The total medical costs of NAFLD patients more than doubled over ten years and were all significantly higher than those of the control group.
Conclusions
A significant increase in NAFLD prevalence and its impact on healthcare utilization was observed in South Korea. With NAFLD leading to serious liver diseases and increased healthcare costs, integrated care strategies that include both medical treatment and lifestyle modifications are essential.
The landscape of liver diseases worldwide has been undergoing a significant shift, markedly influenced by the recent development of effective antiviral interventions, including widespread vaccination programs. These advances have dramatically reduced the prevalence and impact of viral liver diseases such as hepatitis B and C, major causes of liver-related morbidity and mortality worldwide [1,2]. As a result, the focus of liver disease epidemiology has shifted away from viral hepatitis to metabolic liver diseases such as non-alcoholic fatty liver disease (NAFLD), which has become increasingly predominant due to changes in lifestyle and demographic patterns [3ŌĆō5].
NAFLD is the most prevalent chronic liver condition worldwide, characterized by the accumulation of fatty liver cells in individuals who consume little or no alcohol. This disease represents a significant public health concern due to its association with obesity, diabetes, and metabolic syndrome, all of which have been on the rise globally [6]. While NAFLD varies in severity from simple liver steatosis to more severe forms such as non-alcoholic steatohepatitis (NASH), it poses potential risks of advancing to liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [7,8]. Therefore, understanding the epidemiology and characteristics of NAFLD is crucial for the effective management and prevention of NAFLD [9].
In recent years, the medical community has focused on redefining NAFLD with broader terms such as metabolic dysfunction-associated fatty liver disease (MAFLD) or metabolic dysfunction-associated steatotic liver disease (MASLD) [10]. These proposed changes aim to reflect the systemic nature of the disease and its strong association with metabolic dysregulation to further distance [11]. Unlike traditional NAFLD, MAFLD includes criteria such as the presence of overweight or obesity, type 2 diabetes, or evidence of metabolic dysregulation (e.g., high blood pressure, dyslipidemia, insulin resistance) [12]. While similar to MAFLD, MASLD is intended to emphasize the broader association between liver steatosis and metabolic risk factors. MASLD is a concept within the broader umbrella terminology of steatotic liver disease, referring to fatty liver accompanied by metabolic factors while not involving significant alcohol consumption. Keeping in mind that these new terminologies have yet to reach international consensus, it is essential to continue research under the pre-established NAFLD framework while simultaneously redefining NAFLD to ensure continuity and comparability of data across studies. The primary objective of this research is to analyze the evolving epidemiology of NAFLD in South Korea, utilizing comprehensive data from the Korean National Health Insurance Service (NHIS) from 2010 to 2022. This study aims to elucidate the incidence, prevalence, progression, and comorbidities associated with NAFLD to better understand its impact on public health. Additionally, our research seeks to provide empirical data that can guide future clinical practices and health policies in the presence of emerging diagnostic criteria and changing disease patterns.
Our study utilized the claim data of the NHIS in the Republic of Korea (ROK), where 98% of its population benefits from a universal health coverage system. Approximately 46 million patients, which is around 90% of registered residents in ROK, receive health insurance each year, reflecting the immensity of data accumulated by NHIS. To examine the overall pattern of NAFLD changes over 14 years, data from the Korean NHIS was collected from 2010 to 2023. The Institutional Review Board of Soonchunhyang University Bucheon Hospital approved the current study (IRB No. SCHBC 2023-05-007, approval date 23-May-2023). Informed consent was waived by the IRB since only de-identified information was utilized. Our study adhered to the ethical guidelines of the World Medical Association Declaration of Helsinki.
The NAFLD group consisted of patients aged 18 years and above who had been diagnosed with NAFLD or any of its subcategories. Exclusion criteria included the presence of liver diseases other than NAFLD, such as viral hepatitis and alcoholic hepatitis, as well as pre-existing cirrhosis or HCC prior to the NAFLD diagnosis. The diagnosis codes excluded were B15-18, K75.4, E83.K01, K74.3, C22.0, K70, K70.1, K70.9, K76.1, K76.5, and Z22.5 based on International Classification of Diseases Tenth Revision (ICD-10) standards.
Our study also included factors such as the incidence of cirrhosis and HCC, commonly known long-term complications of NAFLD. The control group was defined as the subjects with registered health insurance who had never been diagnosed with NAFLD or viral hepatitis to compare the characteristics of NAFLD patients with those of the general population. Propensity score matching (PSM) analysis was used to match the NAFLD subjects with control subjects of similar demographic characteristics in a 1:2 ratio. The adjusted variables used in PSM were age and sex. This study utilizes health insurance claims data from Korea, where a national health insurance system is in operation, to understand the overall status of the country. The results can be interpreted as generalized outcomes and therefore can be considered age-adjusted findings.
Prevalence was defined as all patients diagnosed with NAFLD in the given year, while the incidence was limited to patients newly diagnosed with NAFLD who had not previously been diagnosed with the disease. NAFLD was defined as a case with a diagnosis code of ICD-10 K760 or K758. Liver cirrhosis was defined by the presence of one of the following ICD-10 codes: K702, K703, K74, K766, or K767. HCC was defined by either the ICD-10 code C22.0 or the ICD-10 reimbursement benefit extension coverage code V193. We assessed the presence of nine comorbidities based on ICD-10 codes, including cerebrovascular disease, coronary heart disease, diabetes, hyperlipidemia, hypertension, rheumatoid arthritis, osteoarthritis, fracture or osteoporosis, and chronic kidney disease. Detailed definitions for each comorbidity and medication can be found in the Supplementary Table.
The data cleaning process involved several steps to ensure the accuracy and reliability of the dataset. First, we verified the accuracy of NAFLD-related diagnosis codes by cross-referencing with clinical records and ensuring consistency across multiple entries. Missing data were addressed using multiple imputation methods. Specifically, for demographic and clinical variables with missing values, we used predictive mean matching to input missing data points. Potential biases in data selection were minimized by employing PSM analysis. This technique matched NAFLD subjects with control subjects of similar demographic characteristics in a 1:2 ratio, adjusting for age and sex. Outliers in continuous variables such as age and medical costs were identified and examined. Outliers that were deemed data entry errors were corrected or removed.
Continuous variables were presented as means with standard deviations, while categorical variables were expressed as percentages unless otherwise specified. Group differences were assessed using the StudentŌĆÖs t-test for continuous variables and the Žć2 test for categorical variables. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org/). A p value of less than 0.05, determined from a two-sided test, was considered indicative of statistical significance.
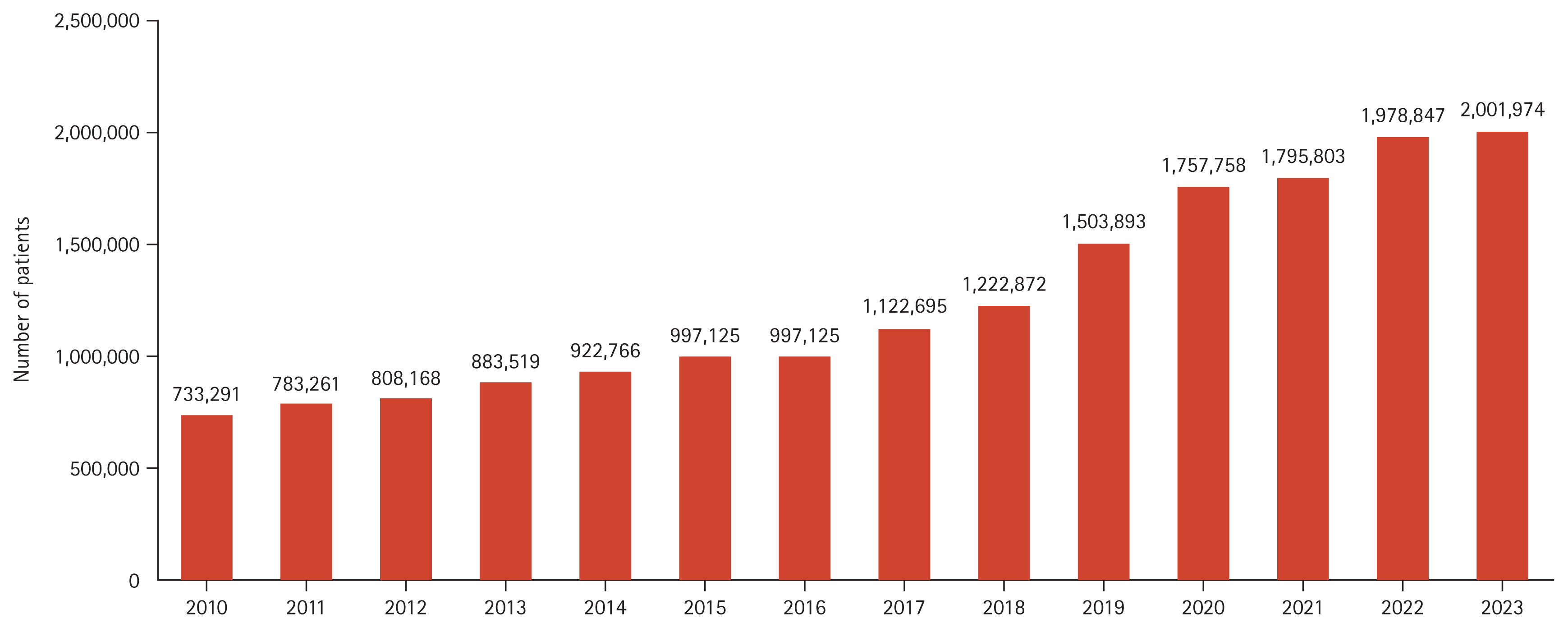
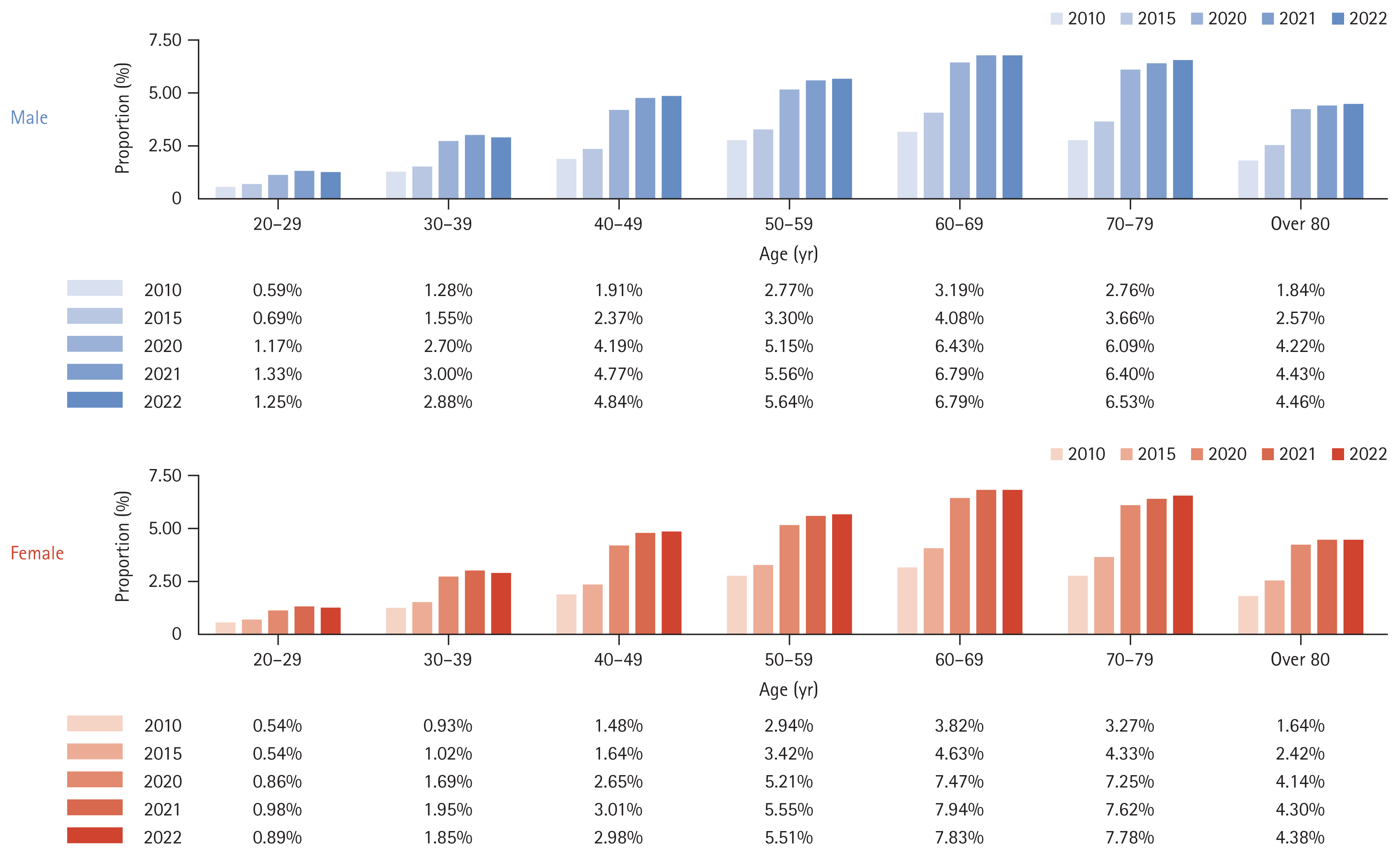
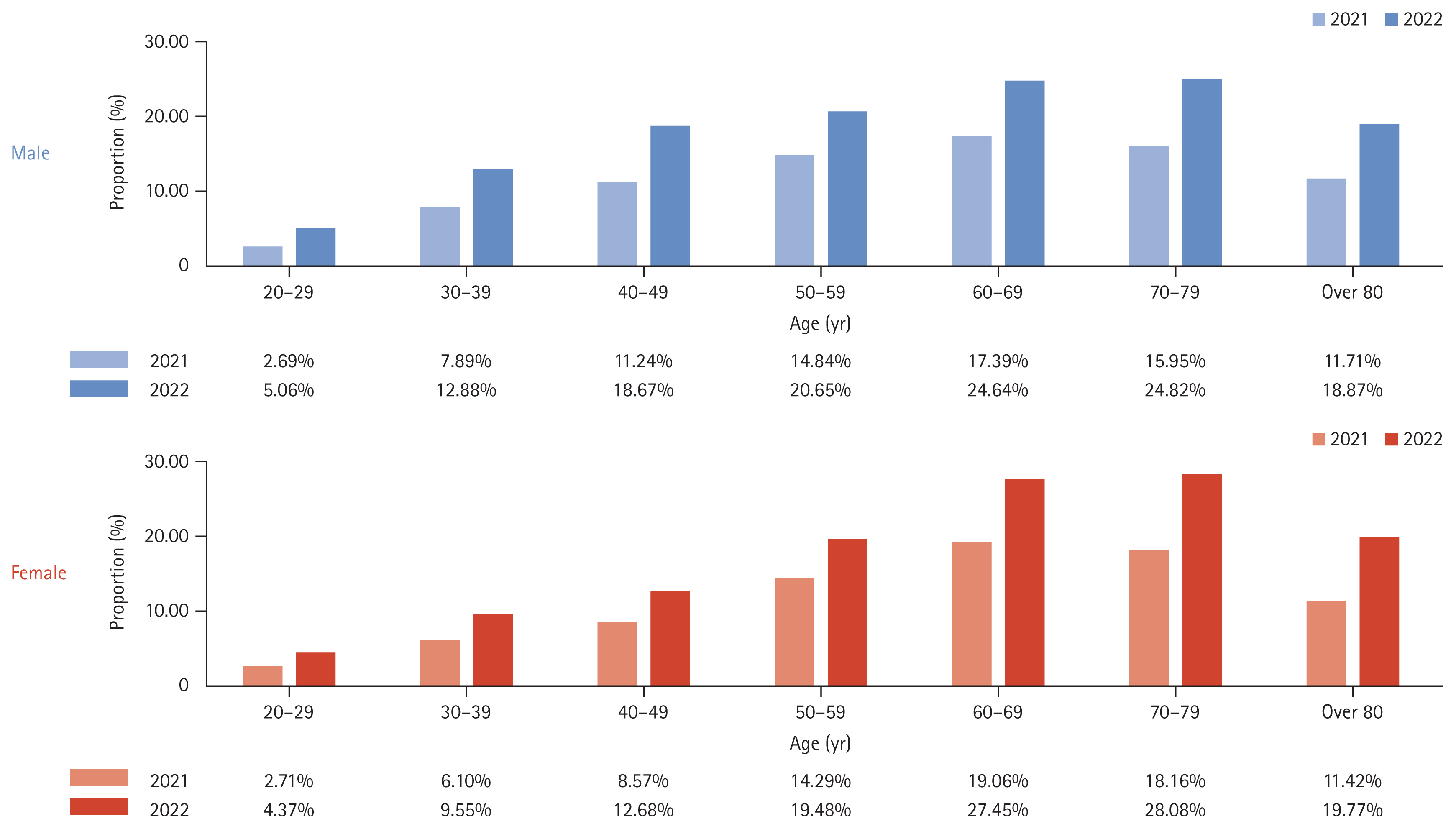
Figure 1 shows the annual change in the number of patients who visited the hospital more than once for NAFLD. The incidence rates of NAFLD according to age and sex are listed in Table 1 and Figure 2. From 2010 to 2022, the incidence of NAFLD in patients who visited hospitals more than once annually rose from 1.87% to 4.47%, affecting all age groups and sex. Specifically, the rates in males and females increased from 1.86% to 4.55% and from 1.87% to 4.38%, respectively. The highest incidence rates were in individuals aged from 60 to 69 years, reaching 6.79% in males and 7.83% in females by 2022.
Over a ten-year period from 2012 to 2022, the prevalence of NAFLD among adults increased from 10.49% in 2012 to 17.13% in 2022 (Table 2). Sex differences were minimal, with males and females showing similar trends. NAFLD was the most prevalent in individuals in their seventies, reaching rates of 24.82% in males and 28.08% in females in 2022 (Fig. 3).
Differences in the prevalence of NAFLD between urban and rural areas were analyzed (Table 3). In 2012, the prevalence rates in urban and rural areas were 11.01% and 10.86%, respectively. A decade later, the prevalence in both urban and rural areas exhibited increases up to 17.15% and 15.95%, respectively. Thus, while the differences in prevalence rates between urban and rural areas of South Korea were minimal in both 2012 and 2022, both areas showed significant increases in the prevalence of NAFLD over the decade. The incidence rates of NAFLD over the decade in both urban and rural settings were also compared, demonstrating a consistent increase from 1.92% to 4.50% in urban areas and from 1.92% to 4.05% in rural areas.
Comorbidities were assessed in the NAFLD and matched control groups using 1:2 PSM based on sex and age (Table 4). Regardless of the period, the NAFLD group had a higher prevalence of comorbidities compared to the control group. In 2012, the most common comorbid condition among NAFLD patients was hypertension, present in 34.49% of the cases, followed by osteoarthritis, diabetes, and fractures or osteoporosis, respectively seen in 26.46%, 14.58%, and 13.07% of all NAFLD subjects. Similarly, the prevalence of comorbidities in 2022 followed the same order: hypertension, osteoarthritis, diabetes, and fractures or osteoporosis, with respective prevalences of 42.26%, 25.68%, 19.10%, and 15.15%, indicating an overall increase in the proportion of associated comorbidities compared to the figures in 2012.
When classified according to the number of concomitant diseases, the NAFLD group often had two or more concomitant diseases compared to the control group (Table 5). Among subjects with two or more comorbidities, hypertension and diabetes were the most common, as seen in a prevalence of 3.87% in 2012. The proportion of patients with two or more comorbidities increased in 2022 compared to 2012. Moreover, 178 and 688 subjects had four or more comorbidities in 2012 and 2022, respectively.
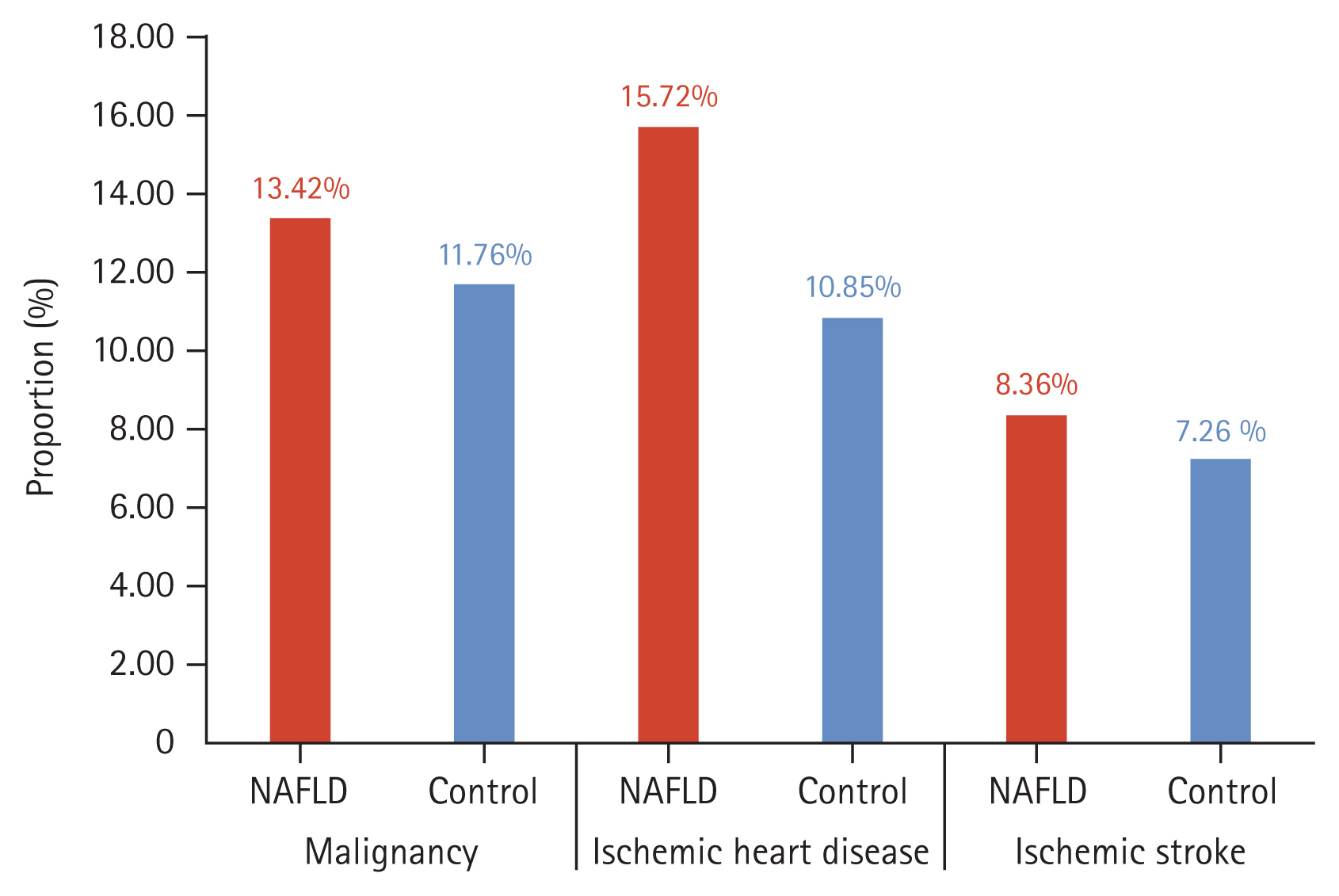
Next, we compared the incidence of malignancy, ischemic heart disease, and ischemic stroke between the NAFLD and control groups over a ten-year period (Fig. 4). The incidence rates in the NAFLD group were 13.42% for malignancy, 15.72% for ischemic heart disease, and 8.36% for ischemic stroke. These rates were significantly higher than those in the control group, which were 11.76% for malignancy, 10.85% for ischemic heart disease, and 7.26% for ischemic stroke.
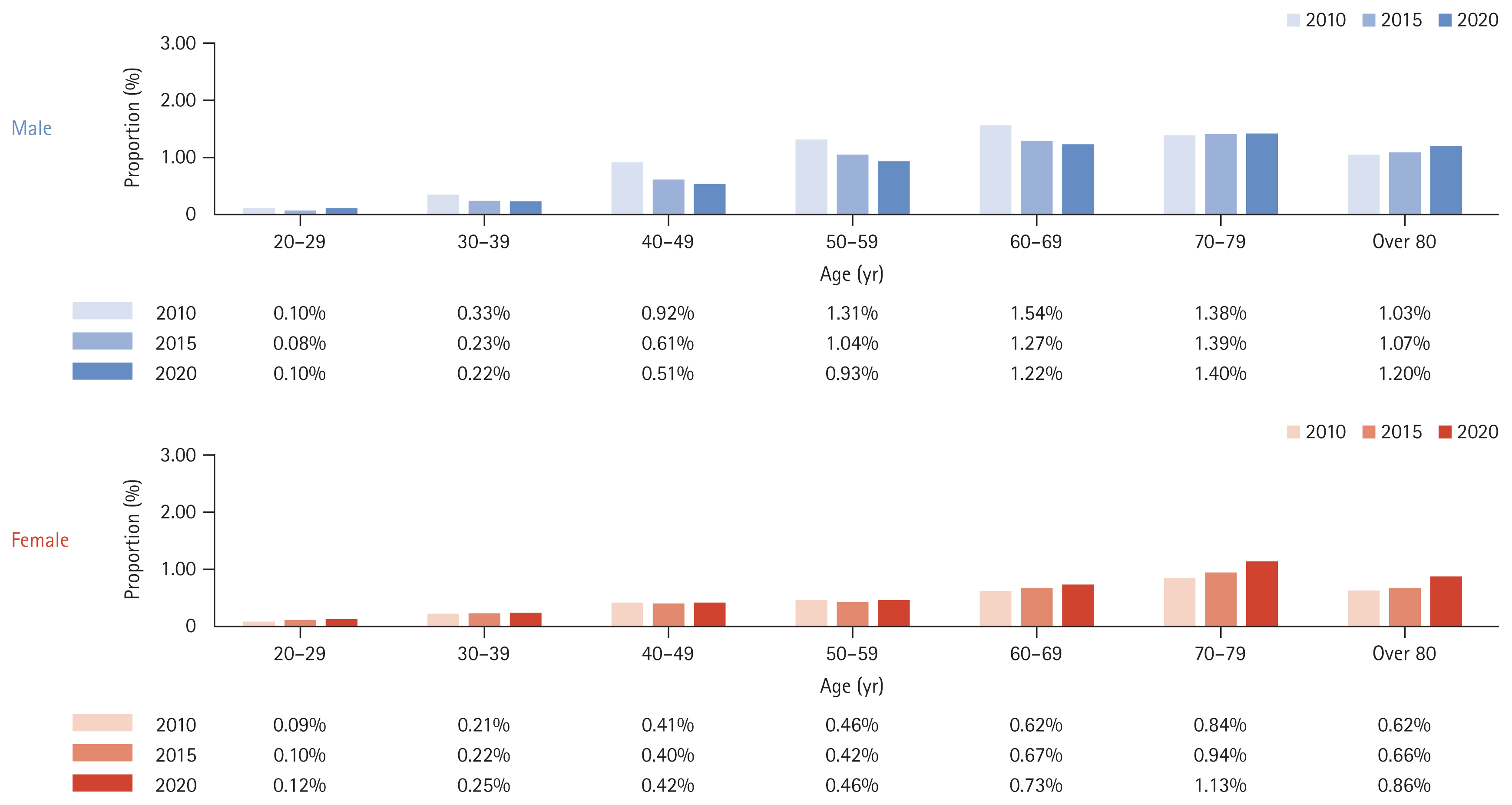
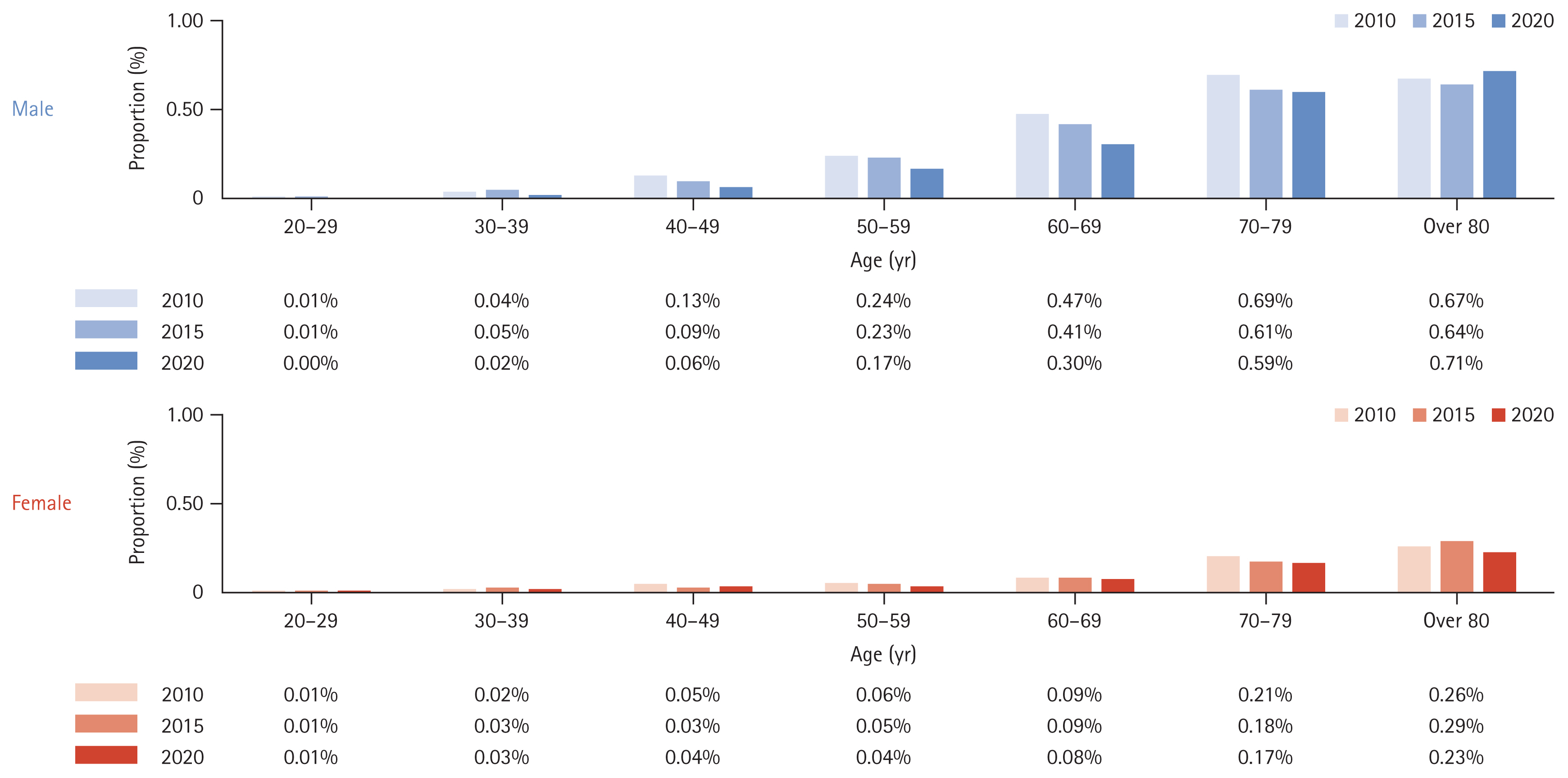
We analyzed the incidence rates of cirrhosis and HCC over ten years among people diagnosed with NAFLD in 2010 (Table 6). The incidence rates of cirrhosis and HCC over ten years were 2.22% and 0.77%, respectively. The incidence of liver cirrhosis (Fig. 5) or HCC (Fig. 6) was higher in males than in females and increased proportionally with age.
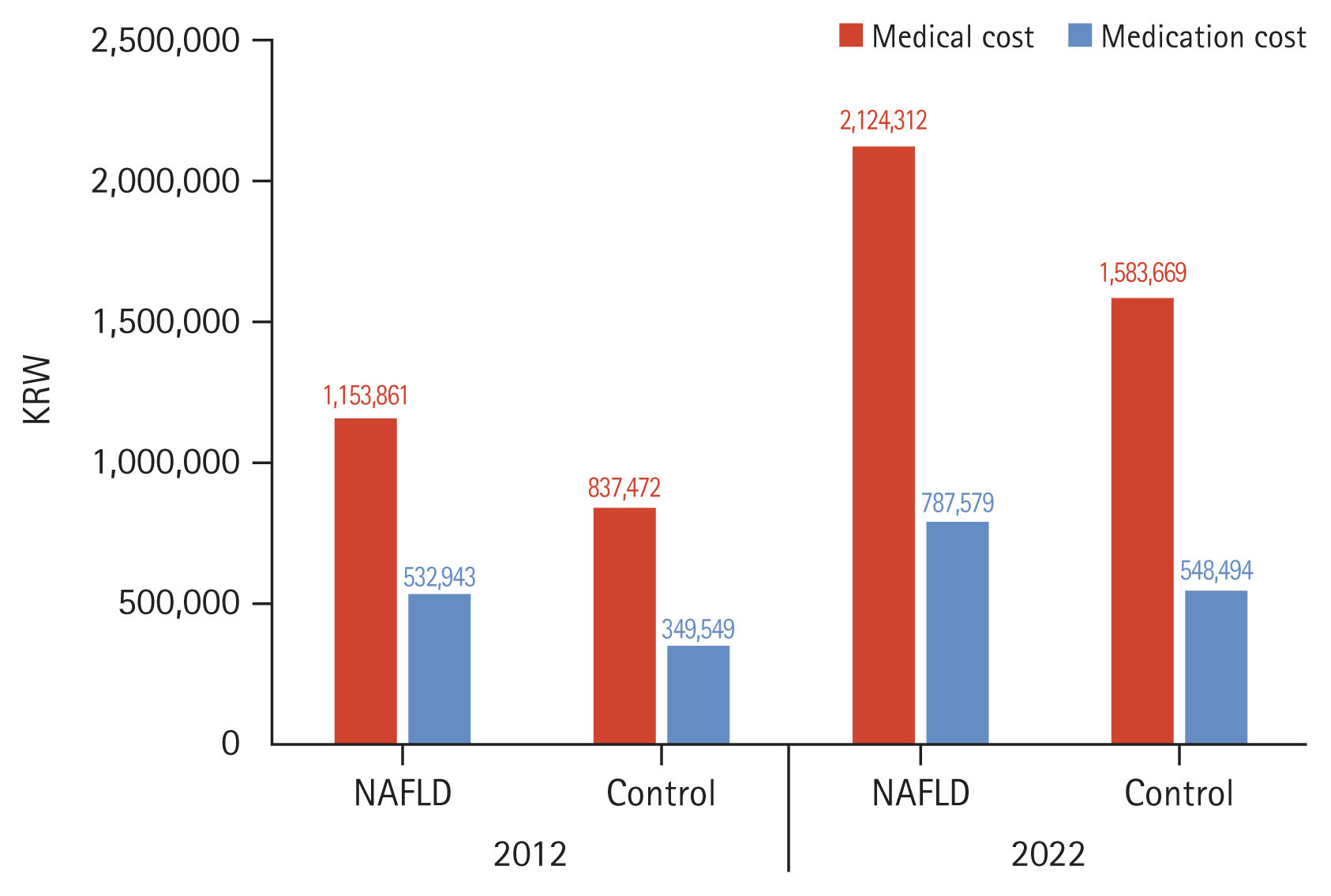
Lastly, healthcare utilization and medical costs for NAFLD patients were compared with their matched control subjects, utilizing 1:2 PSM based on sex and age (Table 7, Fig. 7). In 2012, the mean medical cost for the NAFLD group was 1,153,861 KRW, compared to 837,472 KRW for the control group. The medication costs were 532,943 KRW for the NAFLD group and 349,549 KRW for the control group. Though medical and medication costs increased overall in both groups in 2022, the rate of increase was higher in the NAFLD group compared to that in the control group. Medication costs for the NAFLD group increased to 787,579 KRW, while those for the control group were 548,494 KRW. Such figures indicate a substantial rise in both medical and medication costs from 2012 to 2022 for patients with NAFLD compared to those in the matched controls. Additionally, the number of outpatient visits in the NAFLD group was significantly higher than that of the control group both in 2012 and 2022.
Our study provided foundational, extensive data on the incidence, prevalence, and progression of NAFLD in South Korea. First, our analysis revealed an annual incidence rate of 4.47%, which aligns closely with the recently published meta-analysis results in Asia of 4.5% [13ŌĆō15]. Concerning the prevalence, previous studies had reported a rate of 34.6% [16], while our study found a significantly lower rate of 17.13%. This discrepancy can be attributed to differences in the diagnostic criteria for NAFLD. In prior studies, NAFLD had been diagnosed based on ultrasound results at health check-ups [15ŌĆō17], whereas in our study, the diagnosis was determined by the ICD-10-based diagnostic codes. Such a difference might have led to an underestimation of the prevalence rate in our study.
Furthermore, domestic reports on the natural history of NAFLD are particularly scarce, specifically those concerning the progression to cirrhosis or liver cancer. In our study, the percentage of progression to cirrhosis for ten years was 2.22%, compared to figures reported in Western countries of 3% over 7.6 years [18] and 1.2% over twenty years [19]. Additionally, the progression from NAFLD to HCC in our study was 0.77% over ten years, whereas another study reported a rate of up to 2.6% per year in cases of non-alcoholic fatty liver-related cirrhosis [20,21].
Our analysis also reveals distinct epidemiological patterns of NAFLD between urban and rural areas, suggesting that geographical differences play a crucial role in the prevalence and progression of the disease. Initially, the prevalence rates of NAFLD in urban and rural settings were similar in 2012, but in 2022, urban areas showed a slightly higher prevalence than rural areas. This could be attributed to differences in lifestyle factors such as dietary habits, access to healthcare, and socioeconomic status, which tend to vary significantly between urban and rural populations. Socio-economic status impacts health outcomes, with urban areas exhibiting more disparity that affects access to healthcare and education. Rural areas, though often having traditional diets, are increasingly adopting processed foods, contributing to rising NAFLD rates. Dietary habits also differ, with urban residents consuming more high-fat and high-sugar foods, while rural diets have been traditionally healthier. However, the trend toward processed food consumption is growing in rural areas. Lifestyle factors, such as physical activity, further influence these patterns. Urban lifestyles tend to be more sedentary, whereas rural residents typically engage in more physical labor, though this phenomenon is changing with increasing sedentary activities in rural areas. Urbanization trends are causing rural lifestyles to resemble urban ones, leading to similar NAFLD prevalence rates.
The impact of NAFLD on public health is profound and expanding. As our data demonstrates, NAFLD not only progresses to more severe liver conditions such as cirrhosis and HCC but also significantly burdens the healthcare system on a national scale. This impact is evidenced by the rising medical and medication costs and increased number of hospital visits in patients with NAFLD compared to those in matched control subjects. Thus, we suggest the urgent need for targeted public health interventions to address the rising prevalence of NAFLD. Policymakers should consider implementing comprehensive public health campaigns focused on educating the population about the risks of NAFLD and promoting healthy dietary and lifestyle choices. Screening programs for early detection of NAFLD, particularly in high-risk groups, could significantly improve outcomes through timely intervention. Additionally, lifestyle intervention initiatives, such as community-based physical activity programs and nutritional counseling, are essential to mitigate the progression of NAFLD.
Another finding of our study is that while the prevalence of NAFLD showed minimal sex differences, the incidence of HCC originating from NAFLD exhibited an evident gender disparity, with males being at higher risk. This discrepancy can be explained by the well-documented fact that the male gender is a significant risk factor for HCC [22]. While NAFLD prevalence does not significantly differ between men and women, the progression from NAFLD to HCC is notably higher in men. Studies have consistently shown that men are 2.5 times more likely to develop HCC from NAFLD compared to women [23]. This increased risk in men could be due to various factors, including differences in hormone levels, genetic predispositions, and lifestyle factors [24]. For example, obesity, a primary risk factor for NAFLD, has been associated with a higher incidence of HCC in men compared to women. One report indicated that while 30,200 cases of HCC occur annually in obese women, the number rises to 54,600 cases in obese men [25].
Our study has several limitations. First, our study acknowledges the potential for underestimating NAFLD prevalence due to reliance on ICD-10 codes for diagnosis, which may not capture all cases as effectively as imaging or histological methods. In our study, the prevalence of NAFLD in 2022 was 17.13%, which differed somewhat from previous studies. Previous large-scale studies in South Korea have reported varying prevalence rates based on different diagnostic methods. For instance, a single institution study using transient elastography diagnosed hepatic steatosis with a prevalence of 42.9% among 3,033 health check-up recipients [26]. A study of 589 living liver donors in South Korea reported a histologically diagnosed NAFLD prevalence of 51% [27]. Another study using magnetic resonance imaging to diagnose hepatic steatosis in 2,170 health check-up recipients found a prevalence of 27.7% [28]. Additionally, a recent analysis of the Korea National Health and Nutrition Examination Survey (KNHANES) data (1998ŌĆō2001, 2016ŌĆō2017; n = 25,893) reported an increase in NAFLD prevalence from 18.6% in the earlier period (1998ŌĆō2001) to 21.5% in the later period (2016ŌĆō2017) [29]. While studies using ICD-10 codes may be less accurate than those using ultrasound or liver biopsy, using ICD-10 codes allows for the analysis of a large, representative population over an extended period, providing valuable epidemiological insights and enabling the assessment of comorbidities and healthcare utilization patterns. Another notable limitation of our study is its inability to fully incorporate the recent criteria for MAFLD and MASLD. Though it may seem the pre-established NAFLD framework in our study limits the applicability of our findings to the evolving understanding of the disease, it is noteworthy that these new terminologies have yet to reach international consensus; thus, simultaneously utilizing the pre-established NAFLD framework and redefining NAFLD may ensure continuity and comparability of data across multiple studies.
In conclusion, our study highlights the escalating prevalence and impact of NAFLD in South Korea, suggesting NAFLD as an impending public health challenge. Combating such multifactorial liver diseases requires not only continuous surveillance of the epidemiological trends of NAFLD but also a concerted effort towards integrated care strategies that encompass both medical treatment and lifestyle modifications.
1. The landscape of liver diseases is shifting towards metabolic liver diseases like NAFLD due to effective antiviral therapies reducing viral hepatitis impact.
2. The incidence of NAFLD in South Korea has increased from 1.87% to 4.47% over a twelve-year period, affecting all age groups and sex.
3. Prevalence rates of NAFLD among adults rose from 10.49% in 2012 to 17.13% in 2022, with minimal sex differences.
4. Urban and rural areas similarly show increasing trends in NAFLD prevalence, suggesting lifestyle factors contribute to the diseaseŌĆÖs rise.
5. NAFLD patients exhibit a higher prevalence of comorbidities like hypertension and diabetes compared to controls, with rising medical costs and healthcare utilization.
6. NAFLD progression to cirrhosis and HCC over a ten-year period was 2.22% and 0.77%, respectively, highlighting the diseaseŌĆÖs severe implications.
7. Continuous surveillance and integrated care strategies are essential to address the escalating prevalence and impact of NAFLD as a significant public health challenge in South Korea.
Notes
CRedit authorship contributions
Jae Woo Park: formal analysis, writing - original draft; Jeong-Ju Yoo: formal analysis, writing - original draft; Dong Hyeon Lee: investigation; Young Chang: investigation; Hoongil Jo: investigation; Young Youn Cho: investigation; Sangheun Lee: investigation; Log Young Kim: conceptualization, writing - review & editing; Jae Young Jang: conceptualization, writing - review & editing
Figure┬Ā4
Incidence of malignancy, ischemic heart disease, and ischemic stroke between the non-alcoholic fatty liver disease (NAFLD) and control groups.
Figure┬Ā7
Comparison of healthcare utilization between non-alcoholic fatty liver disease (NAFLD) and control group.
Table┬Ā1
Incidence of NAFLD
Table┬Ā2
Prevalence of NAFLD
Table┬Ā3
Differences in NAFLD depending on residence
| 2012 | 2022 | |||
|---|---|---|---|---|
|
|
|
|||
| Incidence (%) | Prevalence (%) | Incidence (%) | Prevalence (%) | |
| Urban area | 1.92 | 11.01 | 4.50 | 17.15 |
|
|
||||
| Rural area | 1.92 | 10.86 | 4.05 | 15.95 |
Table┬Ā4
Comparison of comorbidities between NAFLD and control group
Table┬Ā5
Comparison of the number of comorbidities between NAFLD and control group
Table┬Ā6
Development of liver cirrhosis and hepatocellular carcinoma during 10-year follow-up: 2010 (target population), 2011ŌĆō2021 (10 years follow-up)
Table┬Ā7
Comparison of healthcare utilization between NAFLD and control group
REFERENCES
1. Huang DQ, Terrault NA, Tacke F, et al. Global epidemiology of cirrhosis - aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol 2023;20:388ŌĆō398.




2. Eris T, Hassan M, Hikal Y, et al. Changes in the etiology of chronic liver disease by referral to a FibroScan center: Increasing prevalence of the nonalcoholic fatty liver disease. Hepatol Forum 2023;4:7ŌĆō13.



3. Wong RJ, Singal AK. Trends in liver disease etiology among adults awaiting liver transplantation in the United States, 2014ŌĆō2019. JAMA Netw Open 2020;3:e1920294.


4. Kim DY. Changing etiology and epidemiology of hepatocellular carcinoma: Asia and worldwide. J Liver Cancer 2024;24:62ŌĆō70.




5. Daher D, Dahan KSE, Singal AG. Non-alcoholic fatty liver disease-related hepatocellular carcinoma. J Liver Cancer 2023;23:127ŌĆō142.




6. Oh JH, Jun DW. Clinical impact of five cardiometabolic risk factors in metabolic dysfunction-associated steatotic liver disease (MASLD): insights into regional and ethnic differences. Clin Mol Hepatol 2024;30:168ŌĆō170.




7. Orci LA, Sanduzzi-Zamparelli M, et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin Gastroenterol Hepatol 2022;20:283ŌĆō292e10.


8. Le MH, Le DM, Baez TC, et al. Global incidence of adverse clinical events in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Clin Mol Hepatol 2024;30:235ŌĆō246.




9. Yoo SH, Kim SS, Kim SG, et al. Current status of ultrasonography in national cancer surveillance program for hepatocellular carcinoma in South Korea: a large-scale multicenter study. J Liver Cancer 2023;23:189ŌĆō201.




10. Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol 2023;79:1542ŌĆō1556.

11. Shiha G, Korenjak M, Eskridge W, et al. Redefining fatty liver disease: an international patient perspective. Lancet Gastroenterol Hepatol 2021;6:73ŌĆō79.


12. Gofton C, Upendran Y, Zheng MH, George J. MAFLD: how is it different from NAFLD? Clin Mol Hepatol 2023;29(Suppl):S17ŌĆōS31.




13. Chang Y, Jung HS, Cho J, et al. Metabolically healthy obesity and the development of nonalcoholic fatty liver disease. Am J Gastroenterol 2016;111:1133ŌĆō1140.



14. Lee MJ, Kim EH, Bae SJ, et al. Age-related decrease in skeletal muscle mass is an independent risk factor for incident nonalcoholic fatty liver disease: a 10-year retrospective cohort study. Gut Liver 2019;13:67ŌĆō76.



15. Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999ŌĆō2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2019;4:389ŌĆō398.


16. Bae JC, Cho YK, Lee WY, et al. Impact of nonalcoholic fatty liver disease on insulin resistance in relation to HbA1c levels in nondiabetic subjects. Am J Gastroenterol 2010;105:2389ŌĆō2395.



17. Choi SY, Kim D, Kim HJ, et al. The relation between non-alcoholic fatty liver disease and the risk of coronary heart disease in Koreans. Am J Gastroenterol 2009;104:1953ŌĆō1960.



18. Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113ŌĆō121.


19. Dunn W, Xu R, Wingard DL, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol 2008;103:2263ŌĆō2271.



20. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021;18:223ŌĆō238.




21. Jang W, Lee HW, Lee JS, et al. Clinical characteristics and prognosis of Korean patients with hepatocellular carcinoma with respect to etiology. J Liver Cancer 2022;22:158ŌĆō166.




22. Lonardo A, Ballestri S, Chow PKH, Suzuki A. Sex disparity in hepatocellular carcinoma owing to NAFLD and non-NAFLD etiology: epidemiological findings and pathobiological mechanisms. Hepatoma Res 2020;6:83.

23. Pinyopornpanish K, Khoudari G, Saleh MA, et al. Hepatocellular carcinoma in nonalcoholic fatty liver disease with or without cirrhosis: a population-based study. BMC Gastroenterol 2021;21:394.




24. Smiriglia A, Lorito N, Serra M, Perra A, Morandi A, Kowalik MA. Sex difference in liver diseases: how preclinical models help to dissect the sex-related mechanisms sustaining NAFLD and hepatocellular carcinoma. iScience 2023;26:108363.



25. Nevola R, Tortorella G, Rosato V, et al. Gender differences in the pathogenesis and risk factors of hepatocellular carcinoma. Biology (Basel) 2023;12:984.



26. Lee HW, Kim BK, Kim SU, et al. Prevalence and predictors of significant fibrosis among subjects with transient elastography-defined nonalcoholic fatty liver disease. Dig Dis Sci 2017;62:2150ŌĆō2158.



27. Lee JY, Kim KM, Lee SG, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol 2007;47:239ŌĆō244.


- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print



