 |
 |
| Korean J Intern Med > Volume 39(4); 2024 > Article |
|
Abstract
Background/Aims
Methods
Results
Notes
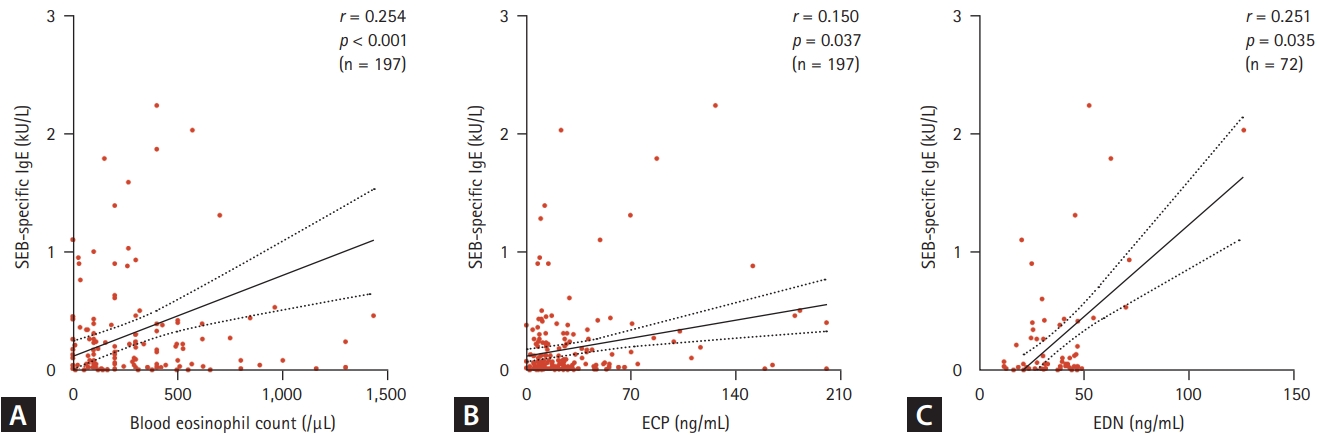
Figure┬Ā1.
Table┬Ā1.
Values are presented as number (%) for categorical variables and as mean ┬▒ standard deviation for continuous variables.
ECP, eosinophil cationic protein; EDN, eosinophil-derived neurotoxin; SEA, staphylococcal enterotoxins A; SEB, staphylococcal enterotoxin B; TSST-1, toxic shock syndrome toxin-1; FEV1, forced expiratory volume in 1 s; PC20, provocation concentration of methacholine required to reduce FEV1 by 20% from the baseline value; ND, no data.
p values were obtained by PearsonŌĆÖs chi-squared test for categorical variables and by StudentŌĆÖs t test for continuous variables.
Table┬Ā2.
Values are presented as number (%) for categorical variables and as mean ┬▒ standard deviation for continuous variables.
TEC, total eosinophil count; ECP, eosinophil cationic protein; EDN, eosinophil-derived neurotoxin; Dp, Dermatophagoides pteronyssinus; Df, Dermatophagoides farinae; FEV1, forced expiratory volume in 1 s; PC20, provocation concentration of methacholine required to reduce FEV1 by 20% from the baseline value; SEA, staphylococcal enterotoxins A; SEB, staphylococcal enterotoxin B; TSST-1, toxic shock syndrome toxin-1; SAgs, staphylococcal superantigens.
SAg-specific IgE levels > 0.35 kU/L were considered positive.
p values were obtained by PearsonŌĆÖs chi-squared test for categorical variables and by StudentŌĆÖs t-test for continuous variables.
Table┬Ā3.
Values are presented as number (%) for categorical variables and as mean ┬▒ standard deviation for continuous variables.
SEA, staphylococcal enterotoxins A; SEB, staphylococcal enterotoxin B; TSST-1, toxic shock syndrome toxin-1; CRS, chronic rhinosinusitis; FEV1, forced expiratory volume in 1 s; PC20, provocation concentration of methacholine required to reduce FEV1 by 20% from the baseline value; SAgs, staphylococcal superantigens.
SAg-specific IgE levels > 0.35 kU/L were considered positive.
p values were obtained by PearsonŌĆÖs chi-squared test for categorical variables and by StudentŌĆÖs t-test for continuous variables.
Table┬Ā4.
Values are presented as mean ┬▒ standard deviation for continuous variables.
SEA, staphylococcal enterotoxins A; SEB, staphylococcal enterotoxin B; TSST-1, toxic shock syndrome toxin-1; Df, Dermatophagoides farinae; Dp, Dermatophagoides pteronyssinus; TEC, total eosinophil count; eos, eosinophils; ECP, eosinophil cationic protein; EDN, eosinophil-derived neurotoxin; SAgs, staphylococcal superantigens.
SAg-specific IgE levels > 0.35 kU/L were considered positive.
p values were obtained by PearsonŌĆÖs chi-squared test for categorical variables and by StudentŌĆÖs t-test for continuous variables.
REFERENCES
-
METRICS

- Related articles
-
Staphylococcal enterotoxin B sensitization in eosinophilic asthma2024 July;39(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


