 |
 |
| Korean J Intern Med > Volume 39(5); 2024 > Article |
|
Abstract
Background/Aims
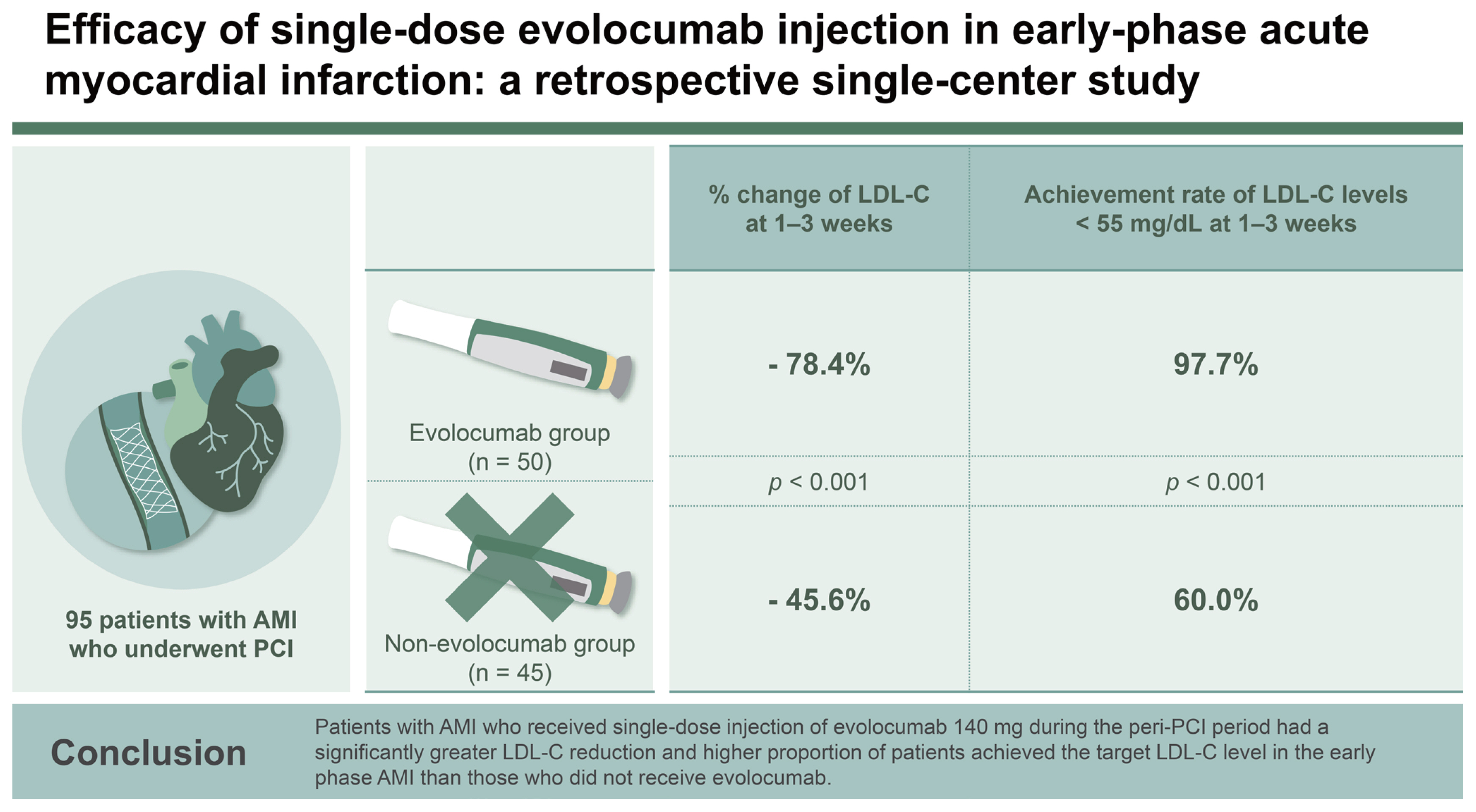
Achieving rapid reduction of low-density lipoprotein cholesterol (LDL-C) levels below 55 mg/dL in patients with acute myocardial infarction (AMI) can be challenging with statins alone. This single-center, retrospective study aimed to assess the impact of single-dose injection of evolocumab 140 mg on LDL-C levels during the peri-percutaneous coronary intervention (PCI) period in patients with AMI.
Methods
A total of 95 patients with AMI who underwent PCI were divided into the evolocumab (n = 50) and non-evolocumab (n = 45) groups.
Results
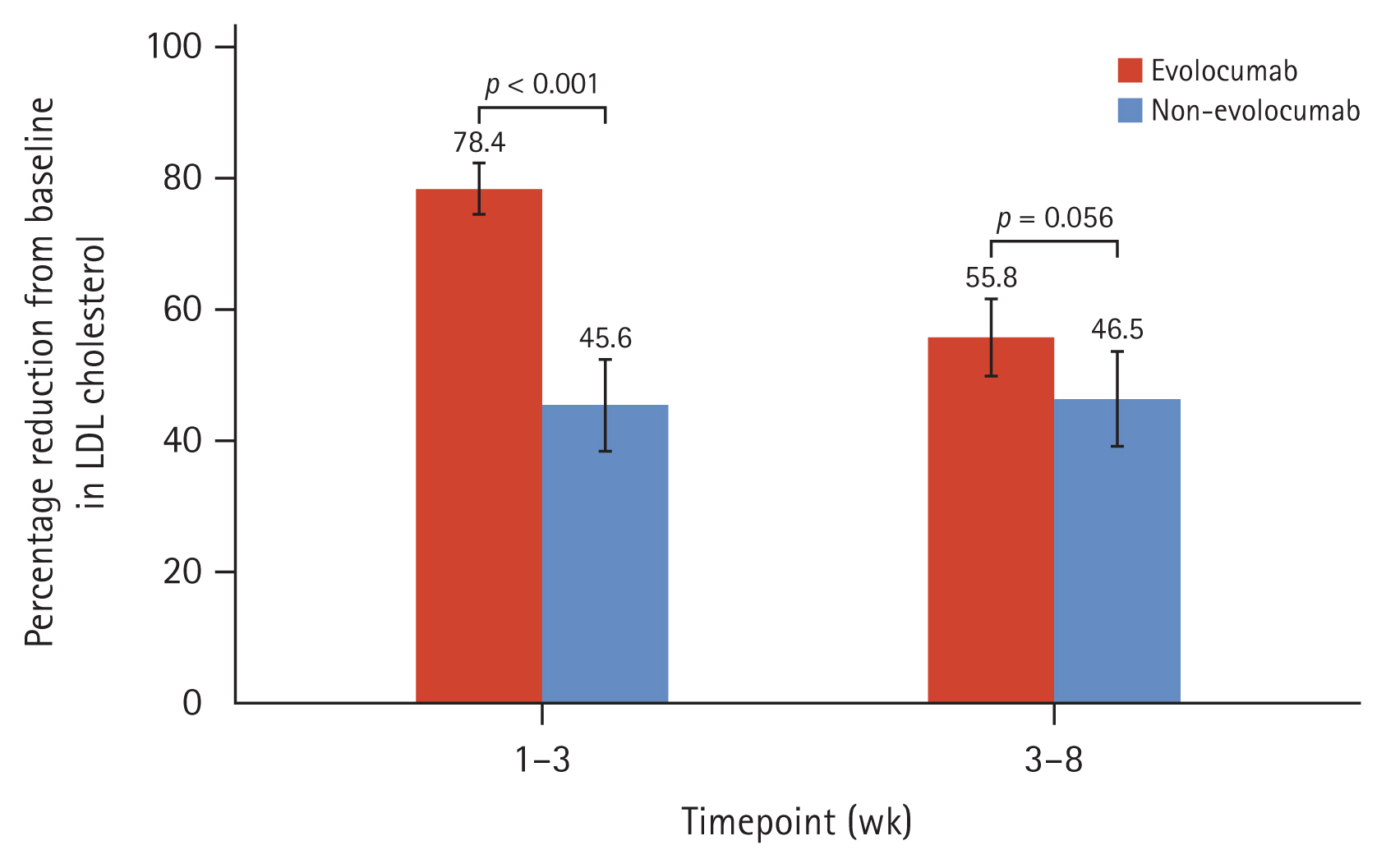
The percentage change of LDL-C level at 1ŌĆō3 weeks from baseline was 78.4 ┬▒ 13.4% reduction in the evolocumab group versus 45.6 ┬▒ 22.6% in the non-evolocumab group, with a mean difference of ŌłÆ33.5% between the groups (95% CI: ŌłÆ42.6 to ŌłÆ24.5%; p < 0.001). The achievement rate of LDL-C levels below 55 mg/dL at 1ŌĆō3 weeks was significantly higher in the evolocumab group than in the non-evolocumab group (97.7% vs. 60.0%, p < 0.001).
Management of elevated low-density lipoprotein cholesterol (LDL-C) levels has been identified as a key strategy for patients with acute myocardial infarction (AMI), and is associated with a significant reduction in cardiovascular mortality [1,2]. Guidelines recommend the early initiation of high-intensity statins to rapidly achieve an LDL-C target level of < 55 mg/dL in this population, followed by the administration of ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors if statin monotherapy proves ineffective [3,4]. Novel PCSK9 inhibitors, such as evolocumab and alirocumab, have recently gained attention for their potential to not only induce plaque regression but also promote plaque stabilization [5ŌĆō8]. Moreover, early administration of PCSK9 inhibitors has been shown to rapidly reduce LDL-C levels in high-risk patients. However, limited data are available on the effect of PCSK9 inhibitors in patients with AMI who have undergone percutaneous coronary intervention (PCI), particularly in the early phase of AMI [7,8]. Therefore, this study aimed to evaluate the efficacy and safety of evolocumab single-dose injection during the peri-PCI period in patients with AMI.
This retrospective study was conducted at Yongin Severance Hospital and considered 111 patients with AMI who received treatment between February 2022 to November 2022. A total of 95 patients who underwent PCI were included in the final analysis, after excluding four patients who underwent coronary artery bypass grafting, three who received medical treatment, three who died within 1 week of AMI event, and six for whom LDL-C level data was unavailable at the time of admission. The enrolled patients were divided into two groups: the evolocumab group (n = 50), those who were subcutaneously injected with single-dose evolocumab 140 mg within 24 hours before or after the PCI, regardless of the initial LDL-C level, and the non-evolocumab group (n = 45), those who were not injected with evolocumab (Supplementary Fig. 1). In both groups, the changes in the lowest LDL-C level between 1ŌĆō3 weeks and 3ŌĆō8 weeks were investigated. In the evolocumab group, the lowest LDL-C levels among 1ŌĆō3 days and 4ŌĆō6 days were additionally investigated. Regarding efficacy outcomes, the primary endpoint was the percent change of LDL-C level at 1ŌĆō3 weeks from the baseline level. The secondary endpoint was the achievement rate of LDL-C level < 55 mg/dL at 1ŌĆō3 weeks. As safety endpoints, changes in aspartate aminotransferase (AST)/alanine aminotransferase (ALT) and high-sensitivity C-reactive protein (hs-CRP) levels were investigated. Regarding clinical outcomes, death, myocardial infarction, stroke, bleeding, and symptoms of myalgia or fatigue over 6 months were also investigated.
The study protocol was approved by the Institutional Review Board (IRB) of Yongin Severance Hospital (approval number: 9-2023-0124), and the study received an exemption from the requirement of informed consent owing to its retrospective nature. All experiments were performed in accordance with the Declaration of Helsinki.
All data are reported as means ┬▒ standard deviations for continuous variables and as frequencies (percentages) for categorical variables. We compared the two groups by conducting independent-samples t-tests and chi-square tests for continuous and categorical variables, respectively. The primary outcomes were analyzed using a linear mixed model with repeated measures, with baseline values, treatment group, time points, and interaction between the treatment group and time points as fixed effects, and patients as a random effect. Statistical significance was set at p < 0.05. All statistical analyses were performed with R software (version 4.3.0; R Foundation for Statistical Computing, Vienna, Austria).
We compared the baseline characteristics and outcomes of the two groups (Table 1). The average age of all the patients was 61.4 ┬▒ 13.1 years, males accounted for 84.2% of the total cohort, and 35.8% of patients showed ST elevation of myocardial infarction. Comparing the baseline demographic and clinical characteristics of two groups, the evolocumab group had a younger mean age of 57.9 ┬▒ 11.2 years compared to that of the non-evolocumab group, which was 65.3 ┬▒ 14.1 years (p = 0.006). The evolocumab group demonstrated a slightly higher BMI as opposed to the non-evolocumab group (25.8 ┬▒ 3.4 kg/m2 vs. 24.1 ┬▒ 3.0 kg/m2, p = 0.011). The proportion of patients with drug-eluting stents and the total number of stents per patient did not significantly differ between the two groups, nor did the use of medication upon discharge. Most patients in both groups were discharged on aspirin and P2Y12 inhibitors, predominantly prasugrel. The intensity of statin therapy at discharge was not significantly different between the two groups, with high-dose statins being used in above 60% of cases in each. Additionally, ezetimibe was used by all patients.
Baseline laboratory test results are summarized in Table 2. The total, triglyceride, LDL-C, and non-high-density lipoprotein cholesterol levels were significantly higher in the evolocumab group. The groups did not significantly differ in terms of hemoglobin A1c level, glucose level, hemoglobin, platelet count, AST, ALT, or hs-CRP. The creatinine level was higher in the non-evolocumab group, which resulted in a lower estimated glomerular filtration rate. The level of peak troponin T after PCI between the two groups was not statistically significant.
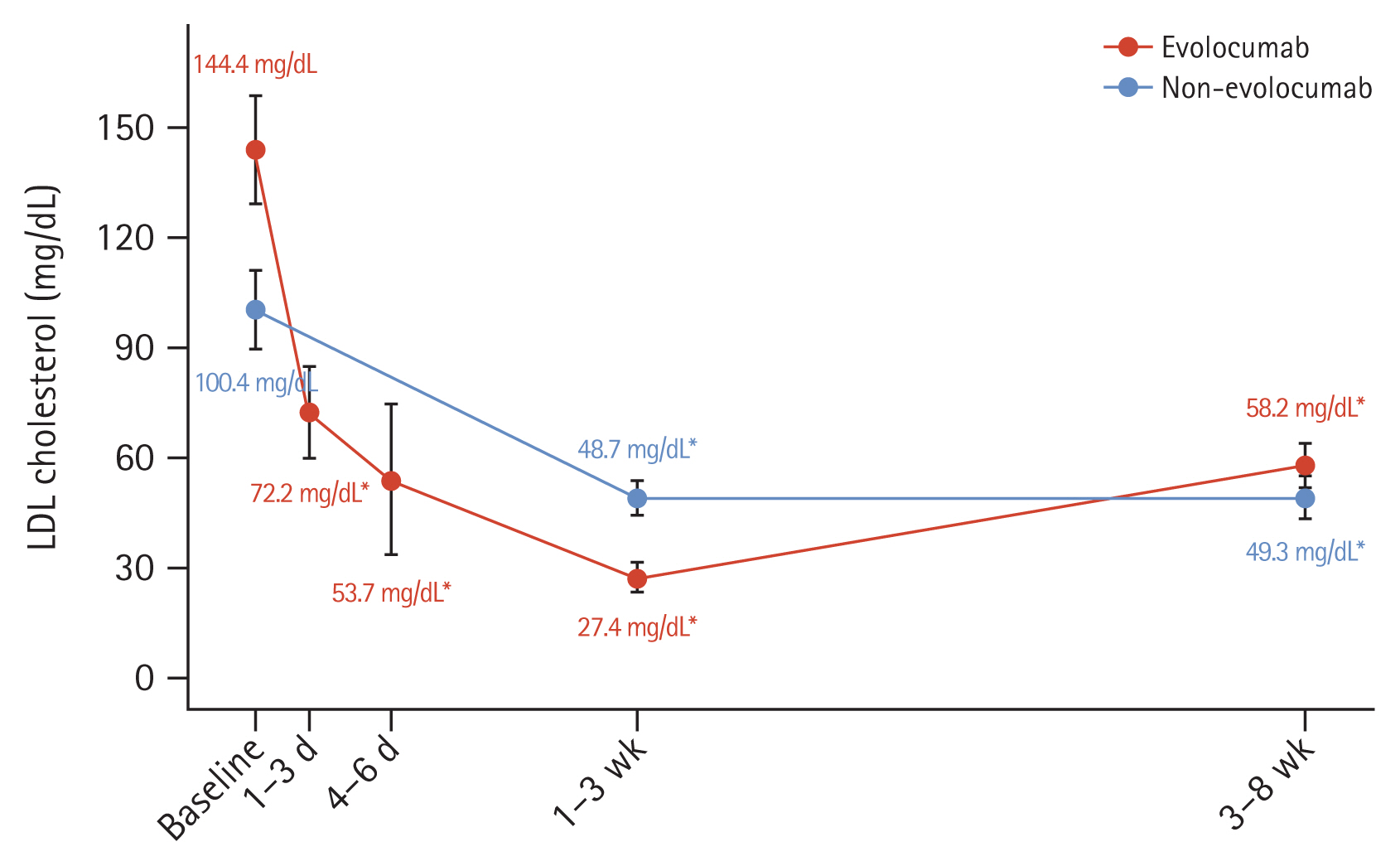
The median interval between hospital arrival and single-dose injection of evolocumab 140 mg was 3.0 hours (interquartile range: 2.0ŌĆō5.0 hours). There were no statistically significant differences in the median day between 1ŌĆō3 weeks and 3ŌĆō8 weeks when the LDL-C level was measured from PCI. The initial LDL-C levels were higher in the evolocumab group than in the non-evolocumab group (144.4 ┬▒ 53.6 mg/dL vs. 100.4 ┬▒ 37.8 mg/dL, p < 0.001). In the evolocumab group, average LDL-C level was 72.7 mg/dL (95% confidence interval [CI]: 72.1ŌĆō98.7 mg/dL) after 1ŌĆō3 days and the 53.7 mg/dL (95% CI: 37.8ŌĆō65.1 mg/dL) after 4ŌĆō6 days (Fig. 1). The LDL-C level at 1ŌĆō3 weeks was significantly lower in the evolocumab group than in the non-evolocumab group (27.4 ┬▒ 14.1 mg/dL vs. 48.7 ┬▒ 15.4 mg/dL, p = 0.003). The absolute change in decline at 1ŌĆō3 weeks from baseline was ŌłÆ112.4 ┬▒ 49.9 mg/dL in the evolocumab group and ŌłÆ50.0 ┬▒ 33.4 mg/dL in the non-evolocumab group. Regarding the change in LDL-C level from baseline, significant reductions were observed at 1ŌĆō3 and 3ŌĆō8 weeks in both groups (both p < 0.001) (Fig. 1, Table 3). The percentage change in the LDL-C level at 1ŌĆō3 weeks after baseline was ŌłÆ78.4 ┬▒ 13.4% in the evolocumab group and ŌłÆ45.6 ┬▒ 22.6% in the non-evolocumab group, with a mean difference of ŌłÆ33.5% between the groups (95% CI: ŌłÆ42.6 to ŌłÆ24.5%; p < 0.001; Fig. 2, Table 3). The achievement rate of LDL-C level < 55 mg/dL at 1ŌĆō3 weeks was higher in the evolocumab group than in the non-evolocumab group (97.7% vs. 60.0%, p < 0.001), although the rate of achieving LDL-C levels < 70 mg/dL did not differ between the groups at 1ŌĆō3 weeks (Table 3). As for the LDL-C level at 3ŌĆō8 weeks, the absolute LDL-C levels (58.2 ┬▒ 23.3 mg/dL vs. 49.3 ┬▒ 20.9 mg/dL, p = 0.172) did not differ between two groups, but absolute change from baseline (ŌłÆ86.1 ┬▒ 47.6 mg/dL vs. ŌłÆ51.1 ┬▒ 36.6, p < 0.001) and % change from baseline (ŌłÆ55.8 ┬▒ 21.4% vs. ŌłÆ46.5 ┬▒ 25.1%, p = 0.033) were higher in evolocumab group. The rates of reaching LDL-C levels below 55 mg/dL (48.0% vs. 73.3%, p = 0.004) were higher in the non-evolocumab group, but that below 70 mg/dL (80.0% vs. 84.4%, p = 0.473) did not differ between the groups (Fig. 1, 2, Table 3).
In terms of safety outcomes, there was no significant difference in AST/ALT abnormality, defined as more than three times the upper limit of the normal range, or in hs-CRP levels at 1ŌĆō3 weeks and 3ŌĆō8 weeks between the two groups, respectively (Supplementary Table 1). Regarding the clinical outcomes, the event of death, myocardial infarction, stroke, and bleeding at 6 months did not significantly differ between the groups. Symptoms of myalgia and fatigue were also similar in both the groups (Supplementary Table 2).
This study provided unique insights into LDL-C changes in AMI patients who underwent PCI, specifically in terms of the impact of single-dose evolocumab 140 mg combined with moderate- or high-intensity statins/ezetimibe in the early phase of AMI. Despite the baseline LDL-C level being higher in the evolocumab group than in the non-evolocumab group, the percentage reduction was higher in the evolocumab group at 1ŌĆō3 weeks than in the non-evolocumab group (ŌłÆ78.4% vs. ŌłÆ45.6%, p < 0.001), and 97.7% of patients in the evolocumab group had an LDL-C level below 55 mg/dL at 1ŌĆō3 weeks.
The current European and American guidelines both recommend a stepwise approach for the treatment of patients who experienced AMI, starting with the initiation of high-intensity statin therapy, followed by the addition of ezetimibe, and considering PCSK9 inhibitor treatment if the target LDL-C level is not reached [3,4]. As a result, although many early clinical events may occur after AMI, especially in patients with higher LDL-C levels, PCSK9 inhibitors are added only several months after AMI events in real-world practice. Furthermore, the proportion of patients with AMI taking PCSK9 inhibitors reportedly accounts for less than 1% of patients with an LDL-C level above 55 mg/dL in clinical practice [9]. However, an in vivo study has revealed that PCSK9 levels increase in the early stages of myocardial ischemia. If the PCSK9 level is inhibited during myocardial ischemia, size of the myocardial infarction, post-infarction inflammation, and cardiac dysfunction after ischemia/reperfusion injury are reduced [10]. Clinical data suggest that the inhibition of PCSK9 not only plays a role in reducing LDL-C levels but also improves endothelial function in the early stages of AMI [11]. Furthermore, rapid administration of PCSK9 inhibitors for very low LDL-C levels has been proven to promote plaque stabilization and regression through studies using coronary intravascular modalities [5,6]. Additionally, patients with the lowest LDL-C levels achieved via PCSK9 inhibitors within 4 weeks of AMI exhibit better long-term clinical outcomes [12]. Patients who have had an AMI have a higher risk of repeat cardiovascular events during this initial period. Despite issues of cost and accessibility, PCSK9 inhibitors have been used and shown in the early stages in pilot studies to address this risk [7,8]. Those studies revealed that PCSK9 inhibitors used alongside high-intensity statins rapidly lowered LDL-C levels to within the target level. In those studies, however, the percentage of the ezetimibe usage proportions were less than 5%. Ezetimibe has already been spotlighted as overcoming the limitations of high-dose statins and enhancing clinical outcomes [13]. The addition of ezetimibe alone to statins results in further reduction of 15ŌĆō22% in the LDL-C level [14]. Moreover, in the 3-year follow-up period, moderate-intensity statins with ezetimibe had no inferiority in clinical outcomes as regards lowering LDL-C level and improved the compliance rate as side effects were reduced compared with the use of high-intensity statins alone [15].
In this study, both moderate- (33.7% of cases) and high- (66.3% of cases) intensity statins were used; interestingly, all the patients initially received ezetimibe after AMI events. Despite a single injection of evolocumab 140 mg during the peri-PCI period, the LDL-C level was reduced by 78.4%, and the rate of achieving an LDL-C level below 55 mg/dL was 97.7% in the early period after AMI, whereas the percentage reduction in LDL-C level decreased to only 48.0% at 3ŌĆō8 weeks after AMI. Choi et al. [16] reported the single injection of evolocumab 140 mg reduced LDL-C level from the baseline to 2 weeks by 58.5% in Korean patients with angina who underwent PCI. In the non-evolocumab group, who used only moderate- or high-dose stains and ezetimibe, the target achievement at both time points was approximately 60ŌĆō70%. In our retrospective analysis, the addition of evolocumab to moderate- or high-intensity statins and ezetimibe was associated with a potent and rapid reduction in the LDL-C level in the early stages after AMI. Therefore, this triple combination therapy in the very early stages after AMI might promote the improvement of clinical outcomes via the stabilization of vulnerable plaques in the future, as previously demonstrated via intravascular imaging modalities [5,6]. Although data on the long-term benefits of PCSK9 inhibitors immediately after an AMI are limited, starting PCSK9 inhibitors and ezetimibe alongside non-high-intensity statins in the early stages after AMI for patients treated with PCI may enhance patient compliance and long-term outcomes.
Our study had some limitations. First, owing to its retrospective nature and limited sample size, the patients prescribed evolocumab tended to have a higher initial LDL-C level than those not prescribed evolocumab. Thus, we used a linear mixed model to adjust for this confounding factor. Second, in the evolocumab group, after the lowest LDL-C level in the first 1ŌĆō3 weeks, a rebound phenomenon can be seen in 3ŌĆō8 weeks. This could be seen as a limitation of single injection, and due to cost and insurance restrictions. Therefore, further research should be considered to confirm the impact of rebound phenomenon. Third, despite the notable LDL-C level decrease following the single injection of evolocumab 140 mg in AMI patients undergoing PCI, this study does not sufficiently predict the long-term benefits of the clinical outcomes, possibly owing to the small sample size and short-term follow up. Therefore, long-term outcomes of early PCSK9 inhibitor use by patients who have had an AMI should be studied in a large-scale randomized controlled trial.
In conclusion, in patients who underwent AMI with PCI, single-dose injection of evolocumab 140 mg during the peri-PCI showed a significantly greater LDL-C reduction, and a higher proportion of patients achieved an LDL-C target level below 55 mg/dL at 1ŌĆō3 weeks than those who did not receive evolocumab.
1. The LDL-C level at 1ŌĆō3 weeks was 78.4% ┬▒ 13.4% lower than baseline in the evolocumab group versus 45.6 ┬▒ 22.6% in the non-evolocumab group, with a statistically significant mean difference of ŌłÆ33.5% between the groups.
2. The achievement rate of a LDL-C level below 55 mg/dL at 1ŌĆō3 weeks was significantly higher in the evolocumab group (97.7%) than that in the non-evolocumab group (60.0%).
Acknowledgments
The authors thank all the staff working in the cardiac catheterization laboratory at the Yongin Severance Hospital for their commitment to this study.
Notes
CRedit authorship contributions
Yongcheol Kim: conceptualization, methodology, resources, investigation, formal analysis, writing - original draft, writing - review & editing, visualization; Ji Woong Roh: conceptualization, methodology, investigation, data curation, formal analysis, writing - original draft, writing - review & editing; Oh-Hyun Lee: investigation, validation, writing - review & editing; Seok-Jae Heo: resources, formal analysis, validation, software, visualization, project administration; Eui Im: validation, software, supervision, project administration; Deok-Kyu Cho: conceptualization, investigation, writing - review & editing, supervision, project administration; Byeong-Keuk Kim: conceptualization, methodology, investigation, writing - review & editing, supervision
Data availability statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Figure┬Ā1
LDL-C levels over time. LDL-C mean values in the two study groups are shown; error bars indicate 95% confidence intervals. LDL-C, low-density lipoprotein cholesterol. The p value was calculated using paired t-test. *p < 0.001 for LDL-C change from baseline.
Figure┬Ā2
Percentage reduction in LDL-C level. Mean percentage changes in LDL-C level from baseline to 1ŌĆō3 and 3ŌĆō8 weeks in both groups are shown; error bars indicate 95% confidence intervals. LDL-C, low-density lipoprotein cholesterol. The p value was calculated using independent samples t-tests.
Table┬Ā1
Baseline characteristics
Table┬Ā2
Baseline laboratory results
Table┬Ā3
LDL-C efficacy outcomes
REFERENCES
1. Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267ŌĆō315.

2. Koskinas KC, Siontis GCM, Piccolo R, et al. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur Heart J 2018;39:1172ŌĆō1180.


3. Collet JP, Thiele H, Barbato E, et al. Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289ŌĆō1367.

4. Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2021;144:e368ŌĆōe454.
5. Nicholls SJ, Kataoka Y, Nissen SE, et al. Effect of Evolocumab on coronary plaque phenotype and burden in statin-treated patients following myocardial infarction. JACC Cardiovasc Imaging 2022;15:1308ŌĆō1321.


6. R├żber L, Ueki Y, Otsuka T, et al. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: the PACMAN-AMI randomized clinical trial. JAMA 2022;327:1771ŌĆō1781.


7. Koskinas KC, Windecker S, Pedrazzini G, et al. Evolocumab for early reduction of LDL cholesterol levels in patients with acute coronary syndromes (EVOPACS). J Am Coll Cardiol 2019;74:2452ŌĆō2462.


8. Mehta SR, Pare G, Lonn EM, et al. Effects of routine early treatment with PCSK9 inhibitors in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: a randomised, double-blind, sham-controlled trial. EuroIntervention 2022;18:e888ŌĆōe896.


9. Ray KK, Molemans B, Schoonen WM, et al. EU-wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol 2021;28:1279ŌĆō1289.



10. Andreadou I, Tsoumani M, Vilahur G, et al. PCSK9 in myocardial infarction and cardioprotection: importance of lipid metabolism and inflammation. Front Physiol 2020;11:602497.



11. Marfella R, Prattichizzo F, Sardu C, et al. Evidence of an anti-inflammatory effect of PCSK9 inhibitors within the human atherosclerotic plaque. Atherosclerosis 2023;378:117180.


12. Giugliano RP, Pedersen TR, Park JG, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet 2017;390:1962ŌĆō1971.


13. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387ŌĆō2397.


14. Vavlukis M, Vavlukis A. Adding ezetimibe to statin therapy: latest evidence and clinical implications. Drugs Context 2018;7:212534.



15. Kim BK, Hong SJ, Lee YJ, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet 2022;400:380ŌĆō390.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement figure 1
Supplement figure 1 Print
Print



