 |
 |
| Korean J Intern Med > Volume 39(4); 2024 > Article |
|
Abstract
Background/Aims
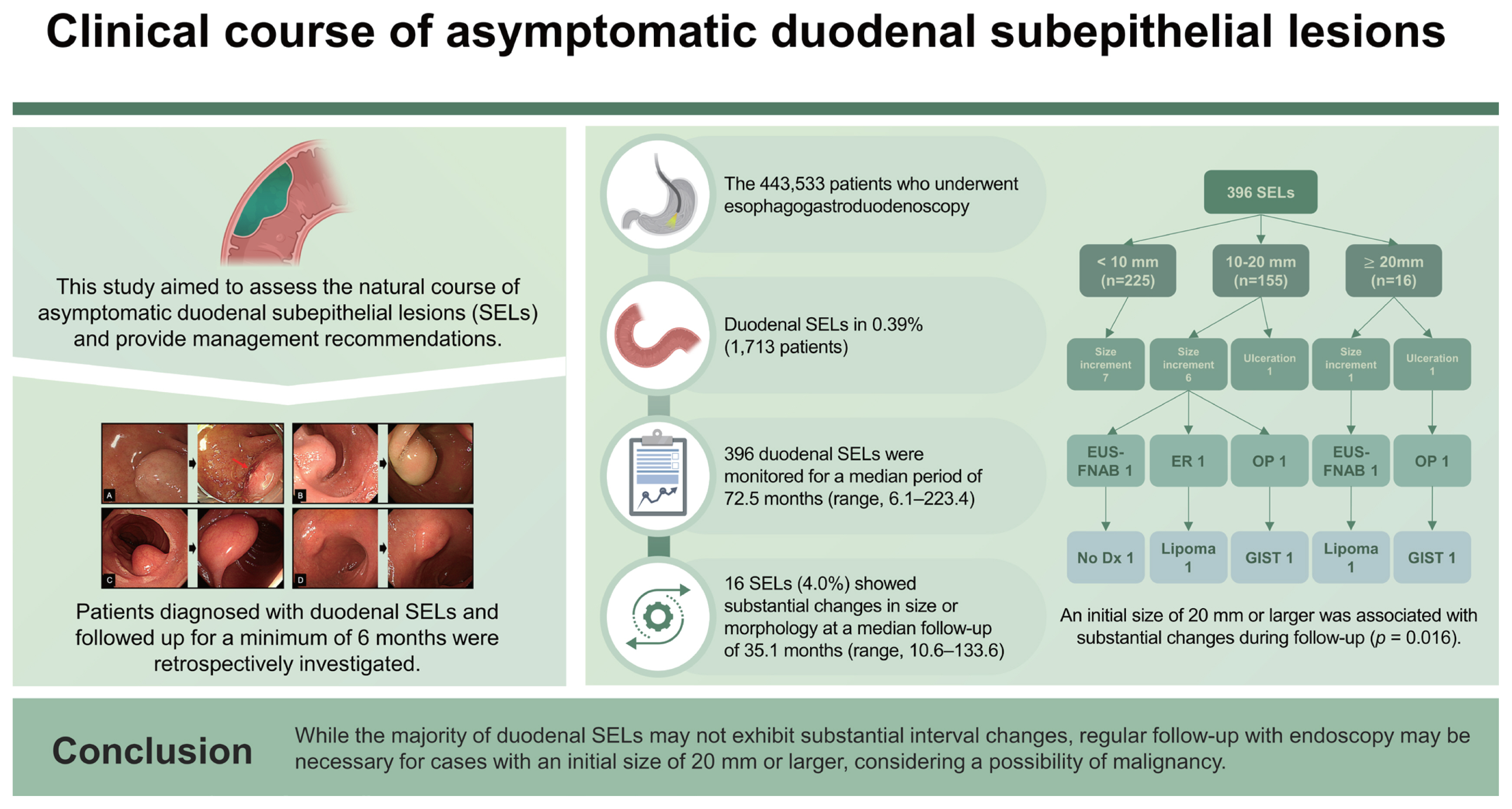
There is limited knowledge regarding the management of duodenal subepithelial lesions (SELs) owing to a lack of understanding of their natural course. This study aimed to assess the natural course of asymptomatic duodenal SELs and provide management recommendations.
Methods
Patients diagnosed with duodenal SELs and followed up for a minimum of 6 months were retrospectively investigated.
Results
Among the 443,533 patients who underwent esophagogastroduodenoscopy between 2008 and 2020, duodenal SELs were identified in 0.39% (1,713 patients). Among them, 396 duodenal SELs were monitored for a median period of 72.5 months (interquartile range, 37.7ŌĆō111.3 mo). Of them, 16 SELs (4.0%) showed substantial changes in size or morphology at a median follow-up of 35.1 months (interquartile range, 21.7ŌĆō51.4 mo). Of these SELs with substantial changes, tissues of two SELs were acquired using endoscopic ultrasound-guided fine needle aspiration biopsy: one was a lipoma and the other was non-diagnostic. Three SELs were surgically or endoscopically removed; two were diagnosed as gastrointestinal stromal tumors, and one was a lipoma. An initial size of 20 mm or larger was associated with substantial changes during follow-up (p = 0.016).
Esophagogastroduodenoscopy (EGD) is commonly used to evaluate gastrointestinal (GI) symptoms and to screen for cancer. During EGD, subepithelial lesions (SELs), previously known as submucosal tumors, are often encountered incidentally by endoscopists. SEL originate as a lesion, including tumor, bulge, or impression from the area underneath the epithelium of the GI tract, including the muscularis mucosae, submucosa, and muscularis propria. SEL can range from benign lesions, such as lipomas, leiomyomas, and duplication cysts, to malignant tumors, including gastrointestinal stromal tumors (GISTs) and neuroendocrine tumors.
SELs are found in 1 in every 300 endoscopies [1ŌĆō3], with a higher frequency in Korea and Japan, where EGD is included in cancer screening [4]. The stomach is the most commonly involved organ, followed by the esophagus [5,6], whereas the duodenum is the least involved organ in the upper GI tract [5,7].
Recent advancements in endoscopic procedures have rendered endoscopic resection (ER) a potential treatment option for upper GI SELs [8ŌĆō10]. However, ER for duodenal lesions is challenging because of the anatomical characteristics of the duodenum, including a relatively thin muscle layer and narrow luminal diameter, which increases the risk of perforation and makes endoscopic maneuverability difficult. Surgical resection of duodenal SELs is also technically challenging because of their close proximity to major systemic and splanchnic vessels, the pancreas, and biliary organs, as well as their retroperitoneal location. Therefore, careful consideration is necessary when selecting duodenal SELs for endoscopic or surgical resections.
While several management plans and guidelines have been proposed for gastric SELs, limited data and information are available for the management of duodenal SELs, owing to a lack of knowledge about their natural course. This study aimed to evaluate the natural course of duodenal SELs and suggest appropriate management strategies.
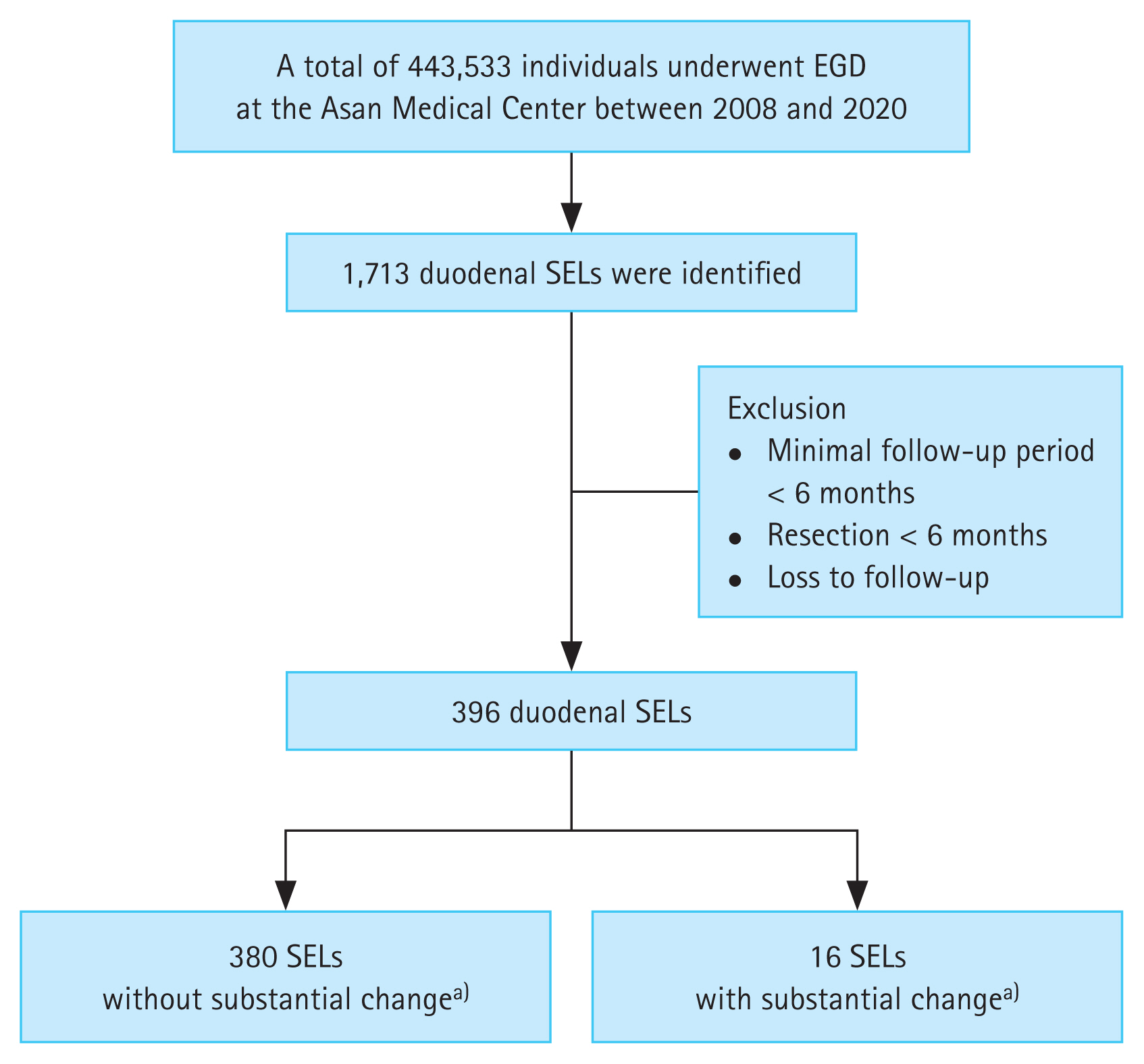
We identified new cases of duodenal SELs diagnosed during 443,533 EGDs between January 2008 and December 2020 by conducting a computerized search of the Asan Biomedical Research Environment, a de-identified clinical data warehouse of the Asan Medical Center, using the terms ŌĆ£duodenal subepithelial tumor,ŌĆØ ŌĆ£duodenal subepithelial lesion,ŌĆØ and ŌĆ£duodenal submucosal tumor.ŌĆØ Patients who were lost to follow-up or who underwent surgery or ER within 6 months were excluded. Finally, 396 cases with duodenal SELs with a minimal follow-up period of 6 months were enrolled (Fig. 1). None of the enrolled duodenal SELs had surface ulcerations at initial diagnosis. The baseline characteristics of the enrolled patients were collected from their medical records.
SELs were defined as bulges or masses covered with normal-appearing mucosa, as observed during endoscopy. All EGDs were performed by board-certified endoscopists, with or without trainees. The characteristics of the SELs, including size, location, and presence of surface ulceration, were evaluated during the initial diagnosis. Among the enrolled 396 duodenal SELs, 70 were further evaluated at the physicianŌĆÖs discretion using endoscopic ultrasound (EUS) to determine the layer of origin and echogenicity pattern. EUS was performed by expert endoscopists (Kim DH, Na HK, Ahn JY, Lee JH, Choi KD, and Song HJ).
A total of 396 patients underwent follow-up EGD or EUS, with a minimum follow-up period of 6 months after the initial diagnosis. Patients without substantial changes in tumor size, echogenicity, or morphology underwent periodic EGD or EUS surveillance every 12 to 24 months. Substantial changes during follow-up were defined as follows: a size increment Ōēź 25% in the longest diameter; surface ulceration; and echogenicity changes suggestive of malignancy, including irregular border, echogenic foci, cystic spaces, and heterogeneity [4]. EUS-guided fine needle aspiration biopsy (EUS-FNAB), ER, or surgery were recommended for patients who show substantial changes during follow-up.
Categorical variables were analyzed using FisherŌĆÖs exact test or the chi-squared test, as indicated, and continuous variables were analyzed using MannŌĆōWhitney U test or StudentŌĆÖs t-test, as indicated. Univariate Cox regression analysis was conducted to identify factors associated with substantial changes in SELs. All statistical analyses were performed using R 4.1.1 (R foundation for Statistical Computing, Vienna, Austria). Two-sided p values < 0.05 were considered as statistically significant.
Table 1 presents the baseline characteristics of 396 patients with asymptomatic duodenal SELs. The mean age was 53.6 years, and 285 of 396 patients were males. The median and interquartile range (IQR) of the follow-up duration were 72.5 months and 37.7ŌĆō111.3 months (range, 6.1ŌĆō223.4 mo), respectively. The initial size of SELs was < 10 mm in 225 cases (56.8%), 10ŌĆō20 mm in 155 cases (39.1%), and Ōēź 20 mm in 16 (4.0%) cases. The median initial size was 8.0 mm (IQR, 6.0ŌĆō10.0 mm). The most common location was the second portion (61.9%), followed by the bulb (36.1%) and the third portion (2.0%). Among the 396 SELs, 70 (17.7%) were evaluated using EUS (Supplementary Table 1, Supplementary Fig. 1), and the most common layer of origin was the submucosal layer (60.0%). There were no significant differences in baseline characteristics between SELs with and without interval changes. Additionally, no statistically significant differences were observed in substantial changes according to the layer of origin, even when compared in pairs (Supplementary Table 2).
Among the 443,533 patients who underwent EGD between January 2008 and December 2020 (Fig. 1), duodenal SELs were identified in 0.39% (1,713 patients).
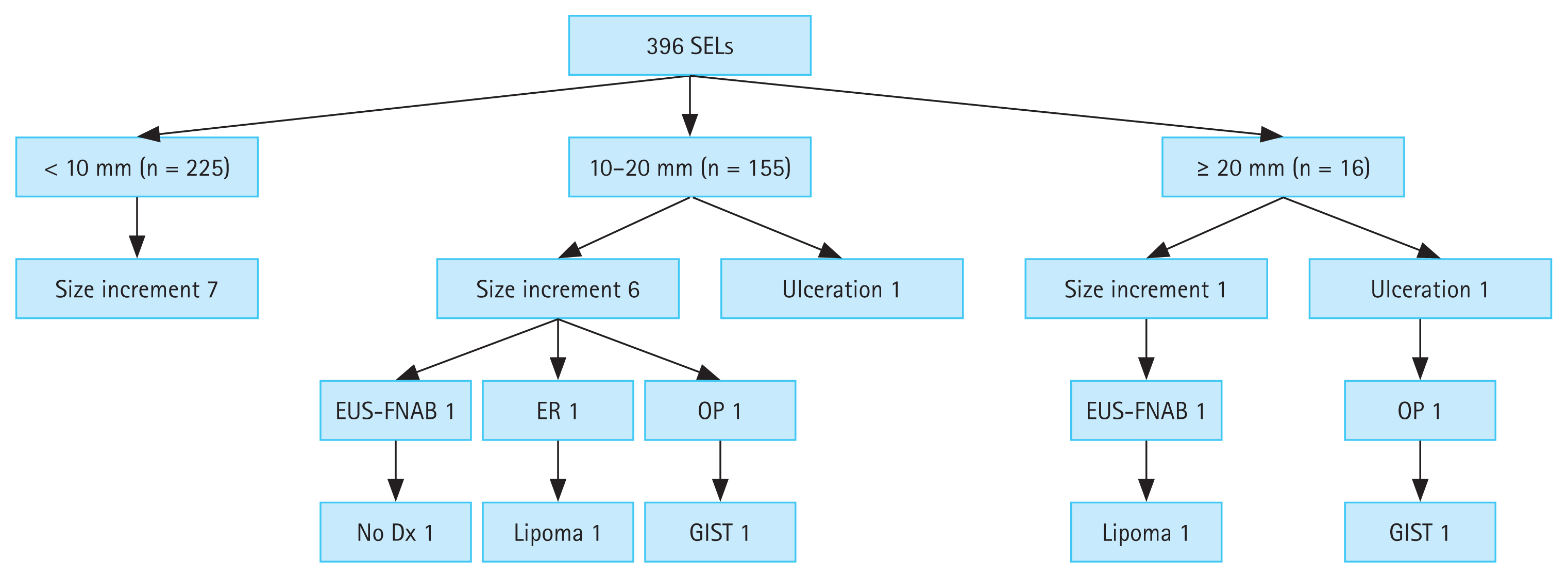
Of the 396 duodenal SELs with a minimum follow-up period of 6 months, 16 (4.0%) showed substantial changes during the follow-up period. Fourteen SELs had size increments, and two SELs showed surface ulceration at a median follow-up duration of 35.1 months (IQR, 21.7ŌĆō51.4 mo; range, 10.6ŌĆō133.6 mo).
Age, sex, location, and the layer of origin were not statistically significant factors associated with substantial changes in SELs (Table 2), while an initial size Ōēź 20 mm was a significant factor (p = 0.016).
Figure 2 shows the flowchart of the 396 duodenal SELs during the follow-up period. Of the 225 SELs with an initial size of < 10 mm, 7 increased in size, but none underwent further evaluation for pathological confirmation due to patient refusal, loss to follow-up, or technical difficulties. Among the 155 SELs with an initial size of 10ŌĆō20 mm, 7 exhibited interval changes (six increased in size and one showed ulcerative changes). EUS-FNAB was performed in one patient, but the sample was non-diagnostic owing to an insufficient amount of specimen acquired. Endoscopic and surgical resections were performed in two patients, resulting in a diagnosis of lipoma and GIST. Among the 16 SELs with an initial size Ōēź 20 mm, two presented interval changes (size increment and ulceration): one was identified as a lipoma using EUS-FNAB, while the other was surgically resected and revealed as a GIST.
Duodenal SELs are usually discovered incidentally during routine EGD and most are small (usually less than 2 cm) and asymptomatic. However, patients with SELs can present with GI bleeding, abdominal pain, or obstruction, for which resection is recommended [2,3,5]. Nevertheless, there are limited data and knowledge about the natural course of asymptomatic duodenal SELs. The European Society of Gastrointestinal Endoscopy stated that there is insufficient evidence to guide recommendations for duodenal SELs and suggested that obtaining a definitive diagnosis is necessary for making further decisions [5]. However, it is difficult to diagnose all asymptomatic duodenal SELs identified during routine EGD in a clinical setting.
In our study, duodenal SELs were identified in 0.39% of the cases (1,713/443,533). Among 396 asymptomatic duodenal SELs with a minimal follow-up period of 6 months, the majority (96.0%) did not exhibit substantial changes in size, morphology, or echogenicity during a median follow-up duration of 72.5 months (IQR, 37.7ŌĆō111.3 mo). On the other hand, 16 (4.0%) SELs showed substantial changes at a median period of 35.1 months (IQR, 21.7ŌĆō51.4 mo): 14 showed size increments and 2 showed surface ulceration. Among these cases, pathological confirmation was obtained for five SELs using EUS-FNAB, ER, or surgery, and only two (0.5%) were diagnosed as GISTs.
Several studies investigated the natural course of upper GI SELs. Gill et al. [11] found that 86.3% (44/51) of upper GI SELs, including two duodenal SELs, demonstrated changes in size and/or echogenicity at a mean period of 29.7 months, whereas none of the duodenal SELs showed changes. Bruno et al. [12] reported that 89.4% (42/47) of upper GI SELs, including four duodenal SELs, did not show changes in size and echogenicity. Lim et al. [7] observed that 96.8% (244/252) of upper GI SELs, including 18 duodenal lesions, showed no changes over a mean duration of 59.1 months. Similarly, Song et al. [13] reported that 96.4% (920/954) of upper GI SELs showed no interval changes during a median period of 47.3 months, and 96.2% (126/131) of duodenal SELs did not exhibit size increments during follow-up, which is consistent with the findings of our study. Kim et al. [14] reported that 85.6% (575/672) of the upper GI SELs did not show changes at a mean period of 68 months, and 90.0% (81/90) of the duodenal SELs did not show any changes. Unfortunately, these studies predominantly concentrated on gastric SELs and had a limited scope in addressing the natural course of duodenal SELs.
EUS is a useful tool for the differential diagnosis of SELs by evaluating the layer of origin, size, and echogenic characteristics [3,15,16]. EUS has demonstrated a sensitivity of 92% for distinguishing SELs from extrinsic compressions [16]. However, differentiating between benign and malignant SELs using EUS alone poses challenges due to its relatively lower sensitivity (64%) and specificity (80%) [2,17]. Furthermore, the interpretation of EUS findings can vary between operators, and there may be poor interobserver agreement, particularly when assessing echogenic features suggestive of malignancy (echogenic foci, cystic spaces, irregular borders, or heterogeneity).
Therefore, pathological confirmation is often necessary for a definite diagnosis of SELs [3,4]. Because SELs are located beneath the epithelium, mucosal biopsies using standard biopsy forceps often fail to acquire tissues [18]. EUS can facilitate tissue acquisition through EUS-FNAB, which is a widely used method for sampling lesions in the GI tract [3,19]. EUS-FNAB allows for the sampling of all GI tract lesions and has reported accuracy rates of 80ŌĆō90% [20ŌĆō25]. However, EUS-FNAB for duodenal SELs can be challenging because of difficulties in maneuvering an angulated scope-tip position in the duodenum [2]. Additionally, the diagnostic yield is poor for duodenal SELs compared to that for gastric lesions [26,27]. Thus, we believe that it is important to predict the malignant potential of SEL on the basis of endoscopic and EUS findings. In our study, an initial size Ōēź 20 mm was a significant factor associated with substantial changes in SELs (p = 0.016), which aligns with previous reports and guidelines.
To date, our study is the largest to focus solely on duodenal SELs rather than on gastric lesions, as previous studies have predominantly concentrated on gastric SELs. In addition, factors such as age, sex, location, and the layer of origin were not statistically significant in relation to substantial changes in SELs (Table 2), whereas an initial size Ōēź 20 mm was a significant factor. Of the 379 duodenal SELs, only two (0.5%) were identified as GISTs. This finding suggests that regular endoscopic follow-ups may be an appropriate strategy for the management of duodenal SELs.
Neuroendocrine tumors are a rare type of neuroendocrine cancer that occur less frequently in the duodenum than in other parts of the digestive tract. Duodenal neuroendocrine tumors are usually small [28], with mean sizes ranging from 7 to 15 mm [29]. Due to their malignant potential, several guidelines advocate resection, regardless of their small size [5,30]. Therefore, even when encountering small duodenal SELs (< 20 mm), careful examination is important to exclude the possibility of neuroendocrine tumors. Neuroendocrine tumors are typically characterized by rounded lesions with a yellowish or reddish color that differ from the surrounding mucosa and are generally diagnosed using endoscopic mucosal forceps biopsy [5].
Our study had several limitations. First, this was a single-center retrospective observational study, which might have limited the generalizability of our results. Second, multiple endoscopists performed EGD and EUS to evaluate SELs. To minimize inter-observer variations, the EGD and EUS images were reviewed by a single investigator. Third, EUS, which can provide valuable information for SEL assessment, was not performed in all cases. Fourth, not all 16 SELs with substantial changes underwent pathological confirmation; tissue acquisition was attempted in only five cases, four of which were diagnosed pathologically as two GISTs and two lipomas. Some patients refused further evaluation or were lost to follow-up. In other cases, technical difficulties were encountered owing to the acute angulation position of EUS or small lesion size. Finally, owing to the retrospective design of this study, certain endoscopic characteristics of SELs, including consistency and mobility, could not be evaluated using EGD images, and therefore, were not available.
In conclusion, the majority of duodenal SELs did not exhibit substantial interval changes during long-term follow-up. Nevertheless, regular endoscopic follow-up is recommended for cases with an initial size of 20 mm or larger, considering a possibility of malignancy.
1. Duodenal subepithelial tumors were identified in 0.39% of the cases (1,713/443,533). Among the 396 asymptomatic subepithelial tumors with a minimal follow-up period of 6 months, only 4.0% (16 cases) showed substantial changes.
2. Univariate Cox regression analysis demonstrated that an initial size Ōēź 20 mm was a significant factor associated with substantial changes (p = 0.016).
3. While the majority of duodenal SETs may not exhibit substantial interval changes, regular follow-up with EGD may be necessary for cases with an initial size of 20 mm or larger, considering a possibility of malignancy.
Notes
CRedit authorship contributions
Seokin Kang: data curation, formal analysis, writing - original draft, writing - review & editing; Kwangbeom Park: data curation, formal analysis, writing - original draft, writing - review & editing; Do Hoon Kim: conceptualization, methodology, writing - original draft, writing - review & editing, supervision, project administration; Yuri Kim: data curation, formal analysis; Hee Kyong Na: methodology; Jeong Hoon Lee: methodology; Ji Yong Ahn: data curation; Kee Wook Jung: data curation; Kee Don Choi: data curation; Ho June Song: data curation; Gin Hyug Lee: data curation; Hwoon-Yong Jung: conceptualization, supervision
Figure┬Ā1
Flowchart of patient enrollment. a)Size increment Ōēź 25%, echogenicity, and ulceration. EGD, esophagogastroduodenoscopy; SEL, subepithelial lesion.
Figure┬Ā2
Flowchart of duodenal SELs during follow-up. SEL, subepithelial lesion; EUS-FNAB, endoscopic ultrasound-guided fine needle aspiration biopsy; ER, endoscopic resection; OP, operation; Dx, diagnosis; GIST, gastrointestinal stromal tumor.
Figure┬Ā3
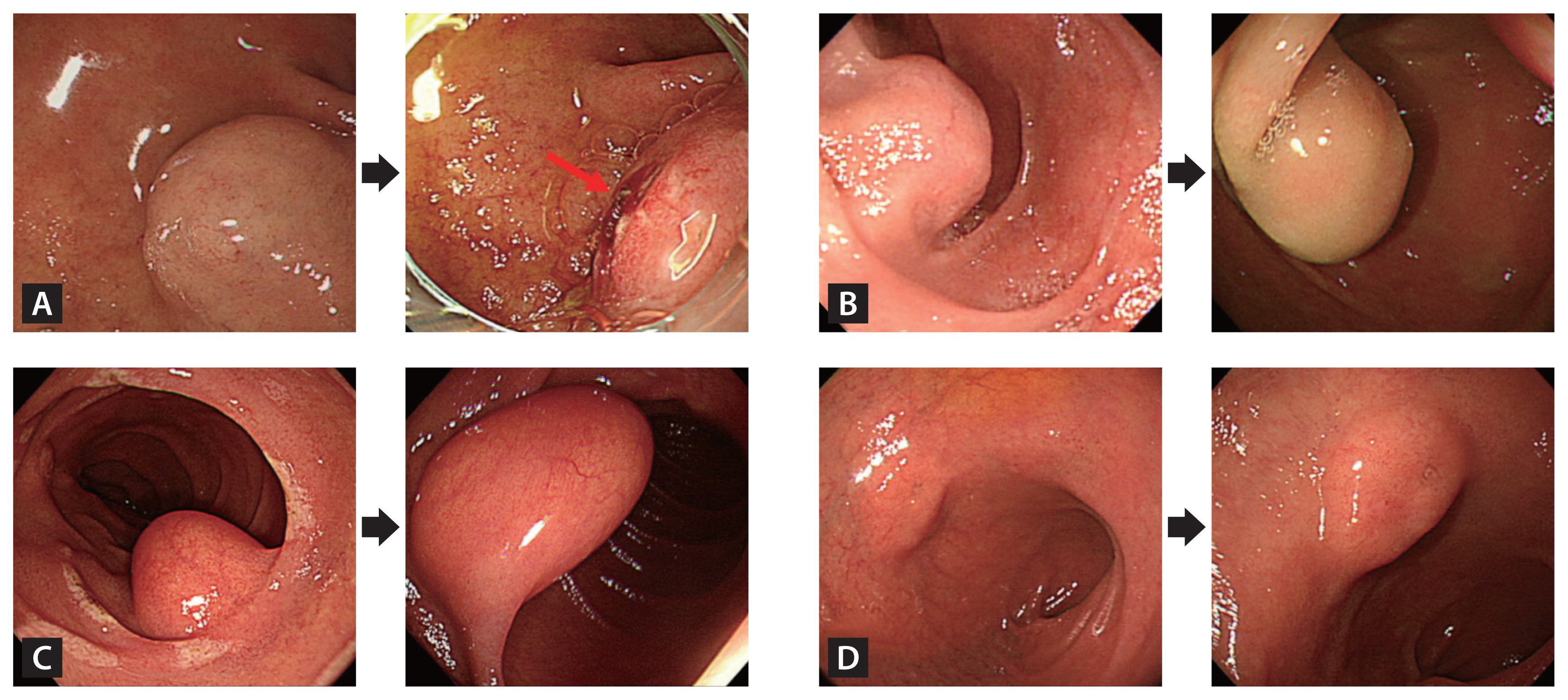
Endoscopic images of duodenal SELs exhibiting substantial interval changes during the follow-up period. (A) The duodenal SEL developed an ulceration (red arrow) at 10.6 months, underwent surgical resection, and was confirmed as a gastrointestinal stromal tumor. (B) The lesion presented size increment at 101.9 months, was surgically resected, and was identified as a gastrointestinal stromal tumor. (C) The lesion showed size increment at 31.5 months, was removed endoscopically, and was confirmed as a lipoma. (D) The lesion exhibited size increment at 12.4 months. An endoscopic ultrasound evaluation indicated a heterotopic pancreas, and it was monitored regularly for 55.2 months without substantial interval changes. SEL, subepithelial lesion.
Table┬Ā1
Baseline characteristics of the enrolled patients
| Variable | Total (n = 396) | Without interval changes (n = 380) | With interval changes (n = 16) | p value |
|---|---|---|---|---|
| Age (yr) | 53.6 ┬▒ 9.8 | 53.5 ┬▒ 9.6 | 54.1 ┬▒ 13.5 | 0.864 |
| Sex (male:female) | 285:111 | 272:108 | 13:3 | 0.576 |
| Follow-up duration (mo) | 72.5 (37.7ŌĆō111.3) | 72.5 (38.0ŌĆō110.6) | 78.6 (34.0ŌĆō129.9) | 0.694 |
| Initial size (mm) | 8.0 (6.0ŌĆō10.0) | 8.0 (5.5ŌĆō10.0) | 11.0 (6.0ŌĆō15.0) | 0.170 |
| ŌĆā< 10 | 225 (56.8) | 218 (57.4) | 7 (43.8) | |
| ŌĆā10ŌĆō20 | 155 (39.1) | 148 (38.9) | 7 (43.8) | |
| ŌĆāŌēź 20 | 16 (4.0) | 14 (3.7) | 2 (12.5) | |
| Location | 0.448 | |||
| ŌĆāBulb | 143 (36.1) | 135 (35.5) | 8 (50.0) | |
| ŌĆāSecond portion | 245 (61.9) | 237 (62.4) | 8 (50.0) | |
| ŌĆāThird portion | 8 (2.0) | 8 (2.1) | 0 (0.0) | |
| Layer of origina) | 0.232 | |||
| ŌĆāMuscularis mucosae | 10 (14.3) | 10 (16.4) | 0 (0.0) | |
| ŌĆāSubmucosa | 42 (60.0) | 37 (60.7) | 5 (55.6) | |
| ŌĆāMuscularis propria | 18 (25.7) | 14 (23.0) | 4 (44.4) | |
| Duration until interval change (mo) | 35.1 (21.7ŌĆō51.4) | |||
| Substantial interval changes | ||||
| ŌĆāSize increment | 14 | |||
| ŌĆāSurface ulceration | 2 |
Table┬Ā2
Factors related to substantial changes
Table┬Ā3
Characteristics of 16 duodenal subepithelial lesions showing substantial changes during the follow-up
REFERENCES
1. Papanikolaou IS, Triantafyllou K, Kourikou A, R├Čsch T. Endoscopic ultrasonography for gastric submucosal lesions. World J Gastrointest Endosc 2011;3:86ŌĆō94.



2. Sharzehi K, Sethi A, Savides T. AGA clinical practice update on management of subepithelial lesions encountered during routine endoscopy: expert review. Clin Gastroenterol Hepatol 2022;20:2435ŌĆō2443e4.


3. Faulx AL, Kothari S, Acosta RD, et al. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest Endosc 2017;85:1117ŌĆō1132.


4. Cho JW, Korean ESD Study Group. Current guidelines in the management of upper gastrointestinal subepithelial tumors. Clin Endosc 2016;49:235ŌĆō240.




5. Deprez PH, Moons LMG, OŌĆÖToole D, et al. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022;54:412ŌĆō429.


6. Ko WJ, Song GW, Cho JY. Evaluation and endoscopic management of esophageal submucosal tumor. Clin Endosc 2017;50:250ŌĆō253.




7. Lim YJ, Son HJ, Lee JS, et al. Clinical course of subepithelial lesions detected on upper gastrointestinal endoscopy. World J Gastroenterol 2010;16:439ŌĆō444.



8. Kim TW, Kim GH, Park DY, et al. Endoscopic resection for duodenal subepithelial tumors: a single-center experience. Surg Endosc 2017;31:1936ŌĆō1946.



9. Granata A, Martino A, Zito FP, et al. Exposed endoscopic full-thickness resection for duodenal submucosal tumors: current status and future perspectives. World J Gastrointest Endosc 2022;14:77ŌĆō84.



10. Li C, Chu Y, Lv L, et al. Safety and efficacy of endoscopic resection for the treatment of duodenal subepithelial lesions. J Gastrointest Oncol 2021;12:856ŌĆō863.



11. Gill KR, Camellini L, Conigliaro R, et al. The natural history of upper gastrointestinal subepithelial tumors: a multicenter endoscopic ultrasound survey. J Clin Gastroenterol 2009;43:723ŌĆō726.

12. Bruno M, Carucci P, Repici A, et al. The natural history of gastrointestinal subepithelial tumors arising from muscularis propria: an endoscopic ultrasound survey. J Clin Gastroenterol 2009;43:821ŌĆō825.

13. Song JH, Kim SG, Chung SJ, Kang HY, Yang SY, Kim YS. Risk of progression for incidental small subepithelial tumors in the upper gastrointestinal tract. Endoscopy 2015;47:675ŌĆō679.


14. Kim D, Cho S, Park SY, et al. Natural course of asymptomatic upper gastrointestinal subepithelial lesion of 2 cm or less in size. J Clin Med 2022;11:7506.



15. Landi B, Palazzo L. The role of endosonography in submucosal tumours. Best Pract Res Clin Gastroenterol 2009;23:679ŌĆō701.


16. R├Čsch T, Kapfer B, Will U, et al. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol 2002;37:856ŌĆō862.

17. Ji JS, Lee BI, Choi KY, et al. Diagnostic yield of tissue sampling using a bite-on-bite technique for incidental subepithelial lesions. Korean J Intern Med 2009;24:101ŌĆō105.



18. Dumonceau JM, Deprez PH, Jenssen C, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated January 2017. Endoscopy 2017;49:695ŌĆō714.


19. Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol 2018;24:2806ŌĆō2817.



20. Larghi A, Fuccio L, Chiarello G, et al. Fine-needle tissue acquisition from subepithelial lesions using a forward-viewing linear echoendoscope. Endoscopy 2014;46:39ŌĆō45.


21. Ca─¤lar E, Hatemi I, Atasoy D, Si┼¤man G, Sent├╝rk H. Concordance of endoscopic ultrasonography-guided fine needle aspiration diagnosis with the final diagnosis in subepithelial lesions. Clin Endosc 2013;46:379ŌĆō383.



22. Jung YS, Lee H, Kim K, Sohn JH, Kim HJ, Park JH. Using forceps biopsy after small submucosal dissection in the diagnosis of gastric subepithelial tumors. J Korean Med Sci 2016;31:1768ŌĆō1774.




23. Lee M, Min BH, Lee H, et al. Feasibility and diagnostic yield of endoscopic ultrasonography-guided fine needle biopsy with a new core biopsy needle device in patients with gastric subepithelial tumors. Medicine (Baltimore) 2015;94:e1622.



24. Han JP, Lee TH, Hong SJ, et al. EUS-guided FNA and FNB after on-site cytological evaluation in gastric subepithelial tumors. J Dig Dis 2016;17:582ŌĆō587.



25. Lee JH, Cho CJ, Park YS, et al. EUS-guided 22-gauge fine needle biopsy for the diagnosis of gastric subepithelial tumors larger than 2 cm. Scand J Gastroenterol 2016;51:486ŌĆō493.


26. Sepe PS, Moparty B, Pitman MB, Saltzman JR, Brugge WR. EUS-guided FNA for the diagnosis of GI stromal cell tumors: sensitivity and cytologic yield. Gastrointest Endosc 2009;70:254ŌĆō261.


27. Pih GY, Kim DH. Endoscopic ultrasound-guided fine needle aspiration and biopsy in gastrointestinal subepithelial tumors. Clin Endosc 2019;52:314ŌĆō320.




28. Borbath I, Pape UF, Deprez PH, et al. ENETS standardized (synoptic) reporting for endoscopy in neuroendocrine tumors. J Neuroendocrinol 2022;34:e13105.



29. Exarchou K, Moore AR, Smart HL, Duckworth CA, Howes N, Pritchard DM. A ŌĆ£watch and waitŌĆØ strategy involving regular endoscopic surveillance is safe for many patients with small, sporadic, grade 1, non-ampullary, non-functioning duodenal neuroendocrine tumours. Neuroendocrinology 2021;111:764ŌĆō774.



- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,351 View
- 237 Download
- Related articles
-
KommerellŌĆÖs diverticulum: a rare cause of esophageal subepithelial lesion2019 November;34(6)
Clinical value of procalcitonin for suspected nosocomial bloodstream infection2018 January;33(1)
Pectus excavatum: a rare cause of gastric subepithelial lesion2018 January;33(1)
Clinical applications of mesenchymal stem cells2013 July;28(4)
Clinical Features of Eosinophilic Bronchitis2002 March;17(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement figure 1
Supplement figure 1 Print
Print


