 |
 |
| Korean J Intern Med > Volume 39(4); 2024 > Article |
|
Abstract
The detection of early colorectal cancer (CRC) is increasing through the implementation of screening programs. This increased detection enhances the likelihood of minimally invasive surgery and significantly lowers the risk of recurrence, thereby improving patient survival and reducing mortality rates. T1 CRC, the earliest stage, is treated endoscopically in cases with a low risk of lymph node metastasis (LNM). The advantages of endoscopic treatment compared with surgery include minimal invasiveness and limited tissue disruption, which reduce morbidity and mortality, preserve bowel function to avoid colectomy, accelerate recovery, and improve cost-effectiveness. However, T1 CRC has a risk of LNM. Thus, selection of the appropriate treatment between endoscopic treatment and surgery, while avoiding overtreatment, is challenging considering the potential for complete resection, LNM, and recurrence risk.
T1 colorectal cancer (CRC), as defined in the TNM classification, is characterized by carcinoma invasion through the muscularis mucosae and into the submucosa without entry into the muscularis propria, regardless of lymph node involvement [1,2]. The current Japanese classification uses a more nuanced categorization of T1 CRC, subdividing T1 CRC into T1a and T1b according to the extent of submucosal invasion (i.e., whether the submucosal invasion is within or exceeds 1,000 ┬Ąm) [3].
The primary treatment approach for T1 CRC involves surgery with lymph node dissection. However, recent research indicates that specific cases of T1 CRC with a low risk of lymph node metastasis (LNM) can be effectively managed through endoscopic resection (ER) [4]. Successful endoscopic management of malignant polyps is dependent upon accurate prediction of T1 CRC cases with a low risk of LNM and the selection of a high-probability ER method for achieving complete en bloc resection of malignant polyps.
White light endoscopy (WLE) is a fundamental technique for the detection and differentiation of neoplastic polyps. A meta-analysis showed the sensitivity of WLE for predicting T1 CRC was relatively low (0.21ŌĆō0.46); this was inferior to the sensitivities of narrow-band imaging (NBI) and magnifying chromoendoscopy (CE). However, its specificity was relatively high (0.81ŌĆō1.0) [5]. Thus, malignant polyps should be suspected when using WLE. The keys to prediction or observation of malignant polyps include their morphology, size, and location [6]. Therefore, although WLE may be less sensitive than other T1 CRC detection methods, its high specificity makes it valuable for the initial identification and suspicion of malignant polyps.
Lesion size is closely associated with the risk of CRC [7,8]. In a retrospective study of 755 polyps measuring Ōēź 6 mm, larger polyp size was associated with higher rates of malignancy: 0%, 0.9%, 6.1%, and 38.1% for polyps measuring 6ŌĆō9, 10ŌĆō19, 20ŌĆō29, and Ōēź 30 mm, respectively [7]. Furthermore, a meta-analysis of studies concerning laterally spreading tumors (LSTs) revealed that the proportion of malignancy increased according to lesion size: 4.6%, 9.2%, and 16.5% for lesions measuring 10ŌĆō19, 20ŌĆō29, and Ōēź 30 mm, respectively [9]. Both the US Multi-Society Task Force (USMSTF) on CRC [10] and the Clinical Guidelines of the European Society of Gastrointestinal Endoscopy [11] recommend using advanced imaging for polyps measuring Ōēź 10 mm to assess the malignancy risk and depth of submucosal invasion (DSI).
Morphological features play key roles in the prediction of T1 CRC. Several studies have identified specific morphological features as indicators of submucosal invasive cancer (SMIC); such features include a demarcated depressed area, ulceration fold convergence, induration, loss of lobulation, excavation, the chicken skin sign, stalk swelling, the non-lifting sign, and spontaneous or contact bleeding [12-15]. A multicenter prospective study of 2,123 cases of T1 CRC showed that the non-lifting sign, the chicken skin sign, depressed areas, induration, and ulceration were significantly associated with deep SMIC, defined as DSI Ōēź 1 mm (1,000 ┬Ąm) [15]. Additionally, a retrospective study of 64 cases of T1 CRC showed that demarcated depressed areas, stalk swelling, and fullness are more common in deep SMIC than in superficial SMIC [12].
As originally proposed by Kudo et al. [16,17], LSTs are non-pedunculated adenomatous lesions measuring Ōēź 10 mm with Paris 0-II and 0-Is classifications [10]. They are classified into granular (LST-G) and non-granular (LST-NG) types based on endoscopic morphology. LST-G is subdivided into homogeneous granular (HG) and nodular mixed (NM) types, whereas LST-NG is divided into the flat elevated (FE) and pseudo-depressed (PD) types (Fig. 1). The risk of malignancy in LSTs increases according to tumor size and subtype [9,18,19]. Tumor size is associated with a higher SMIC rate in LST-NG than in LST-G [18]. A meta-analysis showed that LST-G-HG has a low probability (0.5%) of SMIC, whereas the LST-G-NM, LST-NG-FE, and LST-NG-PD types have increased risks of SMIC: 10.5%, 4.9%, and 31.6%, respectively [9]. The risk of SMIC within LSTs increases when large nodules, depressions, or submucosal tumor-like elevations are present [20,21]. It is also important to be cautious about multifocal submucosal invasion. A comprehensive analysis of 2,822 LSTs revealed that LST-NG-PD has a significantly higher multifocal invasion rate (46.9%) compared with the LST-G-NM (7.9%) and LST-NG-FE (11.8%) types [18].
The relationship between the location and risk of SMIC is unclear, but a meta-analysis showed that most LST-G-HG and LST-NG-FE types are located in the proximal colon (73% and 71%, respectively), whereas the LST-G-NM and LSTNG-PD types are more evenly distributed throughout the colon. LSTs containing SMIC are more frequently located in the distal colon, rather than the proximal colon [9].
Imaging-enhanced endoscopy is an important advancement that offers various contrast enhancement methods via dye, optical, or electronic techniques. Imaging-enhanced endoscopy is broadly categorized into dye- and electronic-based CE. Notable examples of electronic-based CE include NBI, i-scan, and flexible spectral imaging color enhancement (FICE). Imaging-enhanced endoscopy plays a crucial role in detecting and distinguishing neoplastic lesions, as well as predicting the DSI of T1 CRC [22,23]. The USMSTF recommends the proficient use of electronic-based CE, such as NBI, i-scan, FICE, or blue light imaging, as well as dye-based CE. It also emphasizes the importance of proficiency in the endoscopic recognition of DSI [1].
NBI is one of the most widely used endoscopic methods for characterization of colorectal polyps. A simple classification system is essential for international standardization of the NBI observation criteria. The Colon Tumor NBI Interest Group, which comprises endoscopists from Japan, the United States, and Europe, developed the NBI International Colorectal Endoscopic (NICE) classification [24], which classifies colorectal tumors into three types via close observation using high-resolution colonoscopy, regardless of magnification. The primary advantage of this system is that it simplifies the NBI classification, facilitating learning and clinical application. In particular, NICE type 3 is characterized by distorted or missing vessels or the distortion or absence of a surface pattern. The predictive accuracy of deep SMIC for this type exceeds 95% [25,26]. Thus, these findings can reliably guide clinicians who are considering surgery.
However, NICE type 2 is excessively broad and includes histological patterns ranging from adenoma to deep SMIC. Thus, the Japanese NBI expert team (JNET classification) divided NICE type 2 into more detailed subtypes: JNET types 2A and 2B [26]. Type 2B was subdivided into types 2B-low and 2B-high by magnifying endoscopy [27], allowing more accurate histopathological prediction. These modifications are important because accurate prediction of DSI can determine the optimal treatment modality, such as ER or surgery, and reduce underestimates or overtreatments. JNET type 2A histological findings mostly comprise adenoma, whereas JNET 2B-low histological findings [26] with uniformly distributed irregular vessels are consistent with ~98% of adenomas or superficial SMIC and ~2% of deep SMIC [27]. Thus, these findings suggest that ER is feasible (Fig. 2). However, JNET type 2B-high histological findings with irregular heterogeneously distributed vessels are consistent with ~40% of high-grade adenomas or superficial SMIC and ~60% of deep SMIC [27]. These findings suggest that ER is not sufficient. Therefore, suspected cases of JNET type 2B-high should be carefully evaluated to determine the suitability of ER or surgery (Fig. 3). Dye-based CE is an established technique for predicting histological diagnoses and DSI in patients with T1 CRC. This technique involves the use of dyes, such as indigo carmine with or without crystal violet, applied via magnifying endoscopy to highlight the surface pattern of the colonic mucosa. This application is followed by the observation and interpretation of pit patterns, which are categorized according to the Kudo classification [28].
The Kudo classification defines type VI as an irregular pit pattern in terms of shape, size, and arrangement; type VN is considered nonstructural, characterized by the absence of a pit pattern [29,30]. A retrospective analysis of 272 colorectal neoplasms showed that dysplasia, superficial SMIC, and deep SMIC were associated with 57.9%, 11.4%, and 30.7% of type VI pit patterns, respectively. Conversely, dysplasia, superficial SMIC, and deep SMIC were associated with 0%, 4.3%, and 95.7% of type VN pit patterns, respectively [31]. Therefore, surgery should be considered for neoplasms categorized as type VN under the Kudo classification; a careful decision should be made regarding the suitability of ER or surgery for Kudo classification type VI neoplasms. Dyebased CE has several limitations, including a cumbersome time-consuming application process, the cost of the dye solution, and the toxicity-related prohibition of crystal violet use in some countries. Additionally, magnifying endoscopes are not consistently used in clinical practice worldwide.
In contrast, NBI offers a more convenient option because it is easily activated by a button on the endoscope. Accordingly, experts often recommend the initial use of NBI for predicting DSI in cases of suspected or diagnosed CRC. However, in challenging cases (e.g., JNET type 2B-high lesions) where the decision between ER and surgery is ambiguous, experts suggest the use of magnifying dye-based CE. This approach allows more precise evaluation of DSI, aiding the selection of the most appropriate treatment strategy [23,27]. Recently, research has been actively conducted on enhancing the prediction of LNM in T1 CRC by applying artificial intelligence to various endoscopic imaging methods, making it a promising field for future developments [32].
The efficacy of endoscopic treatment of T1 CRC is well-established [33-35]. A meta-analysis found no significant differences in 5-year outcomes, such as overall survival (79.6% vs. 82.1%, hazard ratio [HR], 1.10; 95% confidence interval [CI], 0.84ŌĆō1.45), recurrence-free survival (96.0% vs. 96.7%, HR, 1.28; 95% CI, 0.87ŌĆō1.88), or disease-specific survival (94.8% vs. 96.5%; HR, 1.09; 95% CI, 0.67ŌĆō1.78), between ER and primary surgery for endoscopically resectable T1 CRC. A significantly smaller proportion of patients who underwent ER experienced procedure-related adverse events compared with patients who underwent primary surgery (2.3% vs. 10.9%, p < 0.001) [4].
An important aspect of ER in cases of diagnosed or suspected T1 CRC is that the lesion must be resected en bloc to ensure precise assessment of the resected specimen margin, enabling evaluation of the completeness of lesion removal. In contrast, piecemeal resection increases the risk of local recurrence and makes it difficult to determine whether the lesion has been completely resected. This difficulty can lead to unnecessary surgery, along with complicated treatment and surveillance plans. Thus, the ER method should be carefully selected based on strict standards.
EMR is widely used to remove polyps, typically ranging in size from small (6ŌĆō9 mm) to intermediate (10ŌĆō19 mm). EMR ensures safe effective resection through injection into the submucosal layer, which lifts the lesion and separates it from the muscular layer. This approach reduces the risk of thermal injury and perforation while enhancing the feasibility of en bloc resection [36]. However, EMR usually is not recommended for en bloc resection of non-pedunculated polyps > 20 mm because of snare size limitations and the challenges associated with effective resection at the center of larger lesions [11,37].
The rate of incomplete resection increases according to lesion size. A prospective study of 346 neoplastic polyps removed by 11 endoscopists showed that the incomplete resection rate increased with increasing lesion size: 5.8%, 9.4%, 13.4%, and 23.3% for 5ŌĆō7, 8ŌĆō9, 10ŌĆō14, and 15ŌĆō20 mm, respectively [38]. Another prospective study of 102 intermediate (10ŌĆō20 mm) sessile colorectal polyps removed by EMR demonstrated an en bloc resection rate (EBR) of 75% and a complete resection rate (CRR) of 50% [39]. Therefore, EMR does not consistently ensure en bloc resection or complete resection as the lesion size approaches 20 mm for lesions < 20 mm. Accordingly, for CRCs slightly smaller than 20 mm or with suspected comorbid submucosal fibrosis, or any other case where EMR may not readily achieve en bloc resection, modified EMR or endoscopic submucosal dissection (ESD) can be considered for more reliable en bloc and complete resection [40,41].
EPMR is a method for the removal of large lesions that cannot be captured by a snare, which involves cutting them into several pieces. At the lesion boundary, 2ŌĆō3 mm of normal mucosa is removed and en bloc resection is attempted to the maximum possible extent for an area with suspected malignancy. The remaining lesion is then sequentially removed using a snare [42,43]. Snares measuring 10ŌĆō15 mm are usually recommended. Experts also recommend beginning resection from the most inaccessible and difficult area [42].
In a meta-analysis of lesions measuring > 20 mm, the local recurrence rate was approximately sevenfold greater when the lesion was removed by piecemeal resection than when it was removed by en bloc resection [44]. Furthermore, colorectal tumor resection into > 5 pieces was associated with a high risk of recurrence [45]. In that study, the time to recurrence among cases with resection into > 5 pieces was significantly shorter than the time for cases with resection into < 4 pieces (3.8 ┬▒ 1.9 mo vs. 7.9 ┬▒ 5.0 mo, p < 0.05). EPMR is not recommended for the resection of diagnosed or suspected T1 CRC because of the associated difficulty in evaluating resection margins and the high risk of recurrence. However, if a lesion with SMIC can be resected by EPMR, the suspected carcinoma area should be resected en bloc to the maximum possible extent and follow-up should be carefully scheduled considering the potential for recurrence [42-44,46,47].
Modified EMR is an advanced ER technique intended to overcome the limitations of conventional EMR. It involves using an endoscopic snare and modifications to enhance the likelihood of en bloc resection, even for large lesions, or to achieve deeper submucosal resection.
UEMR is a resection technique that involves submerging the lesion in water. Air is aspirated from the lumen, which is then filled with water. Next, the lesion is removed by snaring without injection of the submucosal layer. This approach is based on the notion that after water immersion, the muscularis propria of the colon remains circular; the mucosal surface tends to involute inwards and assume a collapsed state. Simultaneously, the lesion is submerged in water, causing the mucosa and submucosa to float away from the muscularis propria because of the underwater buoyancy created by the fat density of the submucosal tissue. This combined effect allows safer, more effective lesion resection [48,49].
In a multicenter randomized control trial (RCT), both UEMR and EMR showed high efficacy in removing small non-pedunculated colorectal polyps (4ŌĆō9 mm), with similar EBR (94.4% for UEMR vs. 91.5% for EMR) and CRR (83.1% for UEMR vs. 87.3% for EMR), and no significant differences in complication rates [50]. Another RCT involving small polyps (6ŌĆō9 mm) showed that the rates of incomplete resection in the UEMR and EMR groups were low and did not significantly differ (2% vs. 1.9%, p = 0.91) [51].
In a prospective RCT of intermediate (10ŌĆō20 mm), sessile, colorectal lesions involving 210 polyps, the EBR and CRR were significantly higher in the UEMR group (89% and 69%) than in the EMR group (75% and 50%), but there were no significant differences in adverse events between the groups [39]. In a retrospective study of large lesions (20ŌĆō30 mm) that compared a UEMR group (125 lesions) with an ESD group (306 lesions), the EBR and CRR were significantly lower in the UEMR group (61% and 36%, respectively) than in the ESD group (99% and 86%, respectively) [52]. In another single-center RCT that compared UEMR with EMR for 20ŌĆō40 mm lesions, UEMR demonstrated superior EBR and CRR (33% and 32%, respectively) compared with EMR (18% and 16%, respectively) [53]. However, both groups had very low EBR and CRR.
UEMR can be considered for small or intermediate T1 CRC. However, it is not a reliable method for achieving en bloc or complete resection of T1 CRCs measuring Ōēź 20 mm.
ASEMR, or tip-in EMR, involves making a small incision proximal to the lesion using the tip of a snare after sufficient submucosal injection. The snare tip is anchored within the submucosal layer of the incision site. Subsequently, the snare is opened to capture and resect the entire lesion. This technique has a favorable EBR for large flat lesions because anchoring of the snare tip prevents slippage from the proximal part, ensuring stable capture of the lesion [54-57].
A prospective RCT compared the efficacies of ASEMR and EMR in 82 lesions measuring 15ŌĆō25 mm [58]. The EBRs for ASEMR and EMR were 90% and 73.1%, respectively. However, that study was conducted at a single center with a relatively small sample size; it did not include detailed comparisons of EBR and CRR for ASEMR and EMR in each size range (i.e., 15ŌĆō20 and 20ŌĆō25 mm). A large-scale retrospective study of 709 lesions measuring 20ŌĆō30 mm showed that both EBR (87.4% vs. 97.8%, p < 0.001) and CRR (69.9% vs. 87.3%, p < 0.01) were significantly lower in the ASEMR group than the ESD group; this result remained statistically significant after propensity matching [59]. A multicenter retrospective study analyzing the ASEMR results for a series of 141 consecutive lesions revealed a mean lesion size, overall EBR, and CRR of 19.8 mm, 81.6%, and 70.2%, respectively; the CRR significantly decreased with increasing lesion size (82.8% for lesions < 20 mm, 55.3% for lesions 21ŌĆō30 mm, and 50.0% for lesions > 30 mm; p = 0.002) [57].
Based on these results, caution is needed in the ASEMR-mediated removal of T1 CRC lesions measuring > 20 mm because the rate of incomplete resection may increase.
EMR-P, also known as EMR with circumferential mucosal incision, was developed to enhance the en bloc resection of large, flat, colorectal lesions. In this technique, the tumor is either fully or partially incised along its circumference using a snare tip or endoscopic knife after injection of the submucosal layer. Subsequently, the lesion is captured and resected using the snare (Fig. 4).
A retrospective single-center study evaluated the efficacy of EMR-P for colorectal lesions measuring > 20 mm (mean 22.3 ┬▒ 3.9 mm); it showed that EMR-P achieved high EBR (94.1%) and CRR (76.5%) [60]. Another retrospective study reviewed 523 non-pedunculated colorectal tumors measuring Ōēź 20 mm that underwent ER; the respective EBR and CRR values were 42.9% and 32.9% for EMR (mean tumor size 21.7 ┬▒ 3.5 mm), 65.2% and 59.4% for EMR-P (23.5 ┬▒ 5.6 mm), and 92.7% and 87.6% for ESD (28.9 ┬▒ 12.7 mm) [61]. In a retrospective multicenter study of lesions measuring < 20 mm, the EMR-P group had significantly higher rates of EBR (98.0% vs. 85.7%, p = 0.004) and CRR (87.8% vs. 67.3%, p < 0.001) than the EMR group after propensity score matching; however, the mean procedure time was significantly longer (11.8 ┬▒ 7.5 vs. 2.8 ┬▒ 1.7 min, p < 0.001). For lesions measuring Ōēź 20 mm, EMR-P had significantly higher EBR (88.6% vs. 48.5%, p < 0.001) and CRR (71.4% vs. 42.9%, p = 0.02), compared with EMR [62].
EMR-P tends to have a relatively higher probability of en bloc resection for lesions measuring Ōēź 20 mm, compared with other modified EMR techniques. However, the EBR and CRR are lower for EMR-P than for ESD. The variation in EBR and CRR across studies suggests that endoscopist confidence and proficiency affect the outcome. Therefore, EMR-P could be considered for some T1 CRC lesions measuring Ōēź 20 mm, depending on these factors.
ESD is an advanced ER technique used to remove large lesions with endoscopic knives, enabling en bloc resection regardless of tumor size. Colorectal ESD is typically indicated for lesions that require en bloc resection but may be challenging to manage with snare EMR [41]. EBR and CRR are significantly higher with ESD than with EMR [19], UEMR [52], ASEMR [59], or EMR-P for large colorectal lesions [60,61]. The EBR for ESD was not affected by lesion size. It remained consistently high (> 90%) in all subgroups; it was 92.8% for lesions measuring > 20 mm and 91.9% for lesions measuring > 30 mm [19] (Fig. 5). However, both EBR and CRR tend to be lower for ESD in non-Asian countries (81.2% and 71.3%, respectively) compared with Asian countries (93.0% and 85.6%, respectively) [63]. Recently, the use of pocket creation or traction-assisted ESD, which enhance submucosal layer exposure during submucosal dissection, has shown promising results for challenging cases, such as lesions with submucosal fibrosis, lesions requiring deep vertical margins, or instances of locally recurring/residual lesions [64-67].
Nonetheless, ESD has not been broadly adopted worldwide because of technical difficulties, long procedure time, and a high risk of complications [68-71]. In one meta-analysis, the overall perforation rate and delayed bleeding rate of ESD were 5.2% and 2.7%, respectively [63]. Another meta-analysis showed that ESD had a significantly higher perforation risk than EMR (pooled incidence 5.9% vs. 1.2%) [19].
EFTR is advantageous for efforts to secure a sufficient vertical-free margin for T1 CRC. Although it cannot be used to resect lymph nodes, its indications are identical to ESD. The most popular EFTR device is the full-thickness resection device (FTRD; Ovesco Endoscopy, T├╝bingen, Germany). In an analysis of data from the Multicenter Dutch EFTR registry that included consecutive EFTR procedures involving an FTRD for T1 CRC, the primary resection group (n = 132) had a median lesion size of 15 mm (12ŌĆō16 mm); technical success was achieved in 89.4% of EFTR-amenable cases, whereas R0 resection was achieved in 82% of such cases. In the secondary treatment group, which included cases of previously incomplete ER (n = 198) with a median lesion size of 10 mm (7ŌĆō15 mm), technical success was achieved in 85.4% of EFTR-amenable cases; R0 resection was achieved in 88.0% of such cases [72]. A meta-analysis of EFTR for colorectal lesions incorporated data from 14 studies involving 1,936 patients. The mean procedure duration was 45.4 ┬▒ 11.4 min. The pooled technical success rate was 87.6%, and the R0 resection rate was 78.8%. Procedure-associated adverse events occurred in 12.2% of cases, and the recurrence rate was 12.6% over a mean weighted follow-up of 20.1 ┬▒ 3.8 weeks. The R0 resection rate was significantly lower, and the overall procedural-associated adverse event rate was significantly higher, for lesions measuring > 20 mm than for lesions measuring Ōēż 20 mm [73].
EFTR with an FTRD has a high success rate for primary treatment of T1 CRC tumors measuring < 20 mm, as well as cases with a history of incomplete ER or concomitant severe fibrosis.
After endoscopic removal of T1 CRC, it is important to evaluate whether the resection was curative when considering the need for additional surgery. The final classification of ER of T1 CRC as curative (i.e., associated with a low risk of LNM) or non-curative depends on histological findings concerning the resected specimen. Guidelines from the USMSTF [1], Korean Society [74], and Japanese Society for Cancer of the Colon and Rectum (JSCCR) [37] indicate that curative resection should be assessed based on the resection margin status, DSI, lymphovascular invasion, histological grade, and tumor budding. Widely accepted criteria for curative ER include en bloc and histologically complete resection with tumor-free horizontal and vertical margins (R0); the absence of lymphatic or vascular invasion; a low to moderate histological grade, excluding poor histological types (poorly differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous carcinoma); superficial DSI (< 1,000 ┬Ąm); and low-grade tumor budding (single tumor cell or a cell cluster of Ōēż 4 tumor cells) [75] at the site of deepest invasion [1,37,74,76].
The decision to curatively resect a pedunculated malignant polyp is controversial compared with such a decision for non-pedunculated malignant polyps [77,78]. Malignant pedunculated polyps are typically categorized using the Haggitt classification [79], which is based on the invasion level. This system classifies polyps as level 1 (invasive adenocarcinoma confined to the polyp head, but penetrating through the muscularis mucosae), level 2 (invasion of the neck), level 3 (infiltration of the stalk), and level 4 (invasion of submucosa below the stalk, but not reaching the muscularis propria).
In a retrospective multicenter study involving 384 malignant pedunculated polyps, the overall incidence of LNM was 3.5%; the incidence of LNM in patients with head invasion was 0.0% (0/101), compared with 6.2% (8/129) among patients with stalk invasion [4]. In another study involving 141 malignant pedunculated polyps, the LNM rate was 0% in head or stalk invasion with DSI < 3,000 ┬Ąm in the absence of lymphatic invasion [80]. In a third study, the overall incidence of LNM was 6% for malignant pedunculated polyps, although no LNM was observed when the SMIC was limited to the polyp head, neck, and stalk (levels 1, 2, and 3) [81]. The risk of LNM significantly increased to 27% when the SMIC reached the base of the stalk (level 4).
The DSI is particularly relevant to non-pedunculated malignant polyps, whereas the resection margin is important for pedunculated polyps [1,77]. In one study, when the resection margin was Ōēż 1 mm in the absence of other unfavorable histological factors (e.g., poor histological grade [3] or lymphatic or vascular invasion), the rate of adverse outcomes such as recurrence, local cancer, or LNM was 19.7% [82]. However, when the margin was > 1 mm and no other unfavorable histological factors were present, the rate of adverse outcomes was 0%.
The American Society for Gastrointestinal Endoscopy [83] and USMSTF [1] recommend surgery for pedunculated polyps with unfavorable histological features, such as a cancer-free margin < 1 mm, poor histological differentiation, vascular or lymphatic invasion, submucosal invasion below the stalk of the polyp, or deep wall invasion.
In cases of T1 CRC, DSI Ōēź 1,000 ┬Ąm (1 mm) is a controversial independent high-risk factor for LNM. In a study that involved 724 non-pedunculated T1 CRC lesions, the rate of LNM was 0% for DSI < 1,000 ┬Ąm, whereas it was 12.5% for DSI Ōēź 1000 ┬Ąm; it subsequently increased (1,000 Ōēż X < 1,500 ┬Ąm: 11.5% LNM and 1,500 Ōēż X < 2,000 ┬Ąm: 12.2% LNM) [81]. Several studies have demonstrated that DSI Ōēź 1,000 ┬Ąm (odds ratio 2.1 to 5.4) is a risk factor for LNM in T1 CRC [80,84,85]. However, a recent meta-analysis of eight studies involving 1,146 patients revealed that DSI Ōēź 1,000 ┬Ąm was the sole risk factor for LNM in the absence of other histological high-risk factors, such as poor histological grade, lymphovascular invasion, or tumor budding; the results showed that the absolute risk of LNM for DSI Ōēź 1,000 ┬Ąm was 2.6% [86]. These findings suggest that DSI Ōēź 1,000 ┬Ąm in the absence of all other high-risk features is associated with a relatively low risk of LNM. Indeed, challenges are associated with the measurement of DSI [87]. These challenges include the deformed status of the muscularis mucosa and interobserver agreement regarding its status, with a kappa value of 0.67, indicating substantial but not perfect agreement [88].
Assessments of the resection margin are controversial. R0 resection is typically defined as the absence of tumor invasion within 1 mm of the transection line, whereas R1 resection is defined as the absence of tumor invasion within Ōēż 1 mm. This criterion is based on findings that the risk of local residual or recurrent cancer is 0ŌĆō2% in tumors with a free resection margin > 1 mm [82,89,90]; this risk increases to Ōēż 16% in cases with a resection margin Ōēż 1 mm [82,89]. However, recent evidence challenges this notion, demonstrating similar risks of residual disease in patients with free resection margins between 0.1 and 1 mm and patients with margins > 1 mm in the absence of other histological risk factors [91].
When piecemeal resection of T1 CRC results in a negative vertical margin but unclear lateral margin, in the absence of other histological high-risk factors for LNM, a difficult decision arises. The selection of secondary ER/EFTR, short-term surveillance, or additional surgery requires careful consideration because there is a lack of definitive information regarding residual disease. The JSCCR guideline recommends surgical resection when the vertical margin is positive. However, when piecemeal resection results in a positive horizontal margin, the guideline suggests endoscopic surveillance for approximately 6 months considering the increased risk of local recurrence [37].
When piecemeal resection is performed, especially in cases where only a single fragment exhibited malignancy, the resection margin could be accurately evaluated. However, most piecemeal resections hinder accurate histological assessment of submucosal or lymphovascular invasion. This limitation is particularly concerning in lesions classified as LST-NG, where piecemeal resection may not allow adequate analysis in cases of multifocal invasion [92,93]. Previous studies have demonstrated a significant association between EPMR and an increased risk of local recurrence [94-96]. Therefore, the Chinese national guidelines and general consensus recommend colectomy with regional lymph node dissection when the specimen is fragmented, which would limit pathological evaluation [97,98].
After piecemeal resection of T1 CRC, additional surgery should be prioritized. However, this decision can be individualized based on factors such as the estimated oncologic benefit of surgery, the operative risk, the endoscopistŌĆÖs confidence in achieving complete resection, and the patientŌĆÖs preferences.
There is some concern that pre-surgical ER affects patient outcomes. Some studies have evaluated the outcomes of ER followed by secondary surgery for T1 CRC. A retrospective study analyzed 191 patients with high-risk T1 CRC after ER [99]. More than 90% of the patients successfully underwent laparoscopic surgery, rather than open surgery. Another retrospective study of 852 patients compared the long-term outcomes of T1 CRC primary surgery in 388 patients and secondary surgery in 464 patients over a median follow-up interval of 57.0 months. The rates of cancer recurrence (2.8% vs. 1.5%, p = 0.180) and locoregional (0.3% vs. 0.6%) and distant recurrence (2.5% vs. 0.9%, p = 0.105) did not significantly differ between the primary and secondary surgery groups. Further analyses of recurrence-free survival rates according to nodal stage and the number of high-risk histological features also showed no differences between the groups [100]. A recent retrospective study revealed that 5-year recurrence-free survival did not significantly differ between radical surgery with prior ER and radical surgery alone in matched data (96.9% vs. 95.5%, p = 0.596) or in the unadjusted model (97.2% vs. 96.8%, p = 0.930). ER before surgery did not increase the cost of radical surgery [101]. A meta-analysis showed no significant differences in recurrence-free survival between additional T1 CRC surgery after ER and primary surgery (HR, 1.27; 95% CI, 0.85ŌĆō1.89) [4]. Thus, ER before surgery for high-risk T1 CRC does not appear to affect patient outcomes.
In conclusion, T1 CRC should initially be suspected according to endoscopic findings. The risk of LNM should be assessed to determine the most suitable treatment, endoscopic or surgical, based on this risk. If endoscopic treatment is chosen, a method that allows complete en bloc resection of the lesion should be selected. The pathology should be evaluated, and the decision for additional surgery should be made carefully, considering the risk of LNM and the patientŌĆÖs overall condition.
Figure┬Ā1.
Endoscopic images of the four endoscopic laterally spreading tumor subtypes: (A) homogenous granular; (B) granular nodular mixed; (C) non-granular flat elevated; and (D) non-granular pseudo-depressed.
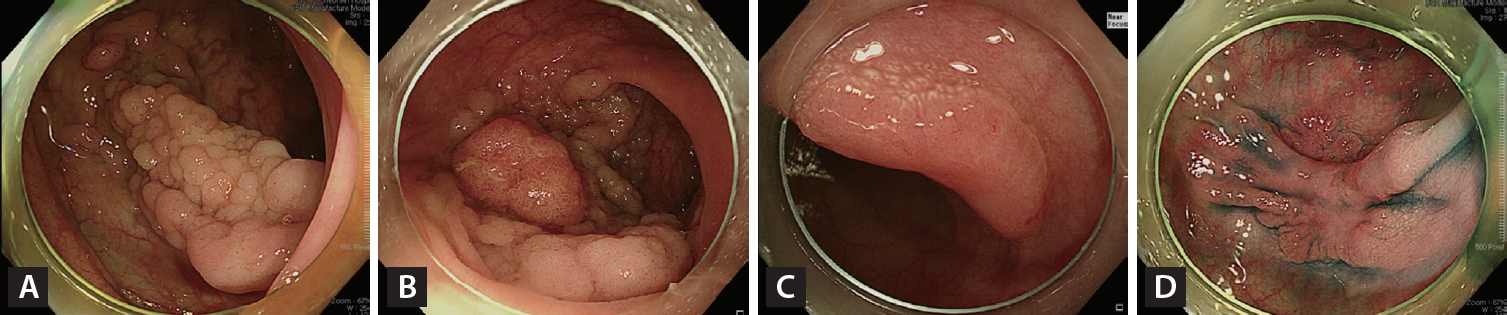
Figure┬Ā2.
Endoscopic curative resection. (A) A 20-mm non-granular pseudo-depressed laterally spreading tumor located in the transverse colon. (B) Irregular and uniform vascular and surface patterns were observed (JNET type 2B-low) on narrow-band imaging with near-focus. (C) Circumferential incision and submucosal dissection of the lesion using an endoscopic knife after submucosal injection. (D) Mucosal defect after completion of ESD. (E) Histopathological examination confirmed complete resection (R0) (22 ├Ś 17 mm, tubulovillous adenoma with low-grade dysplasia).
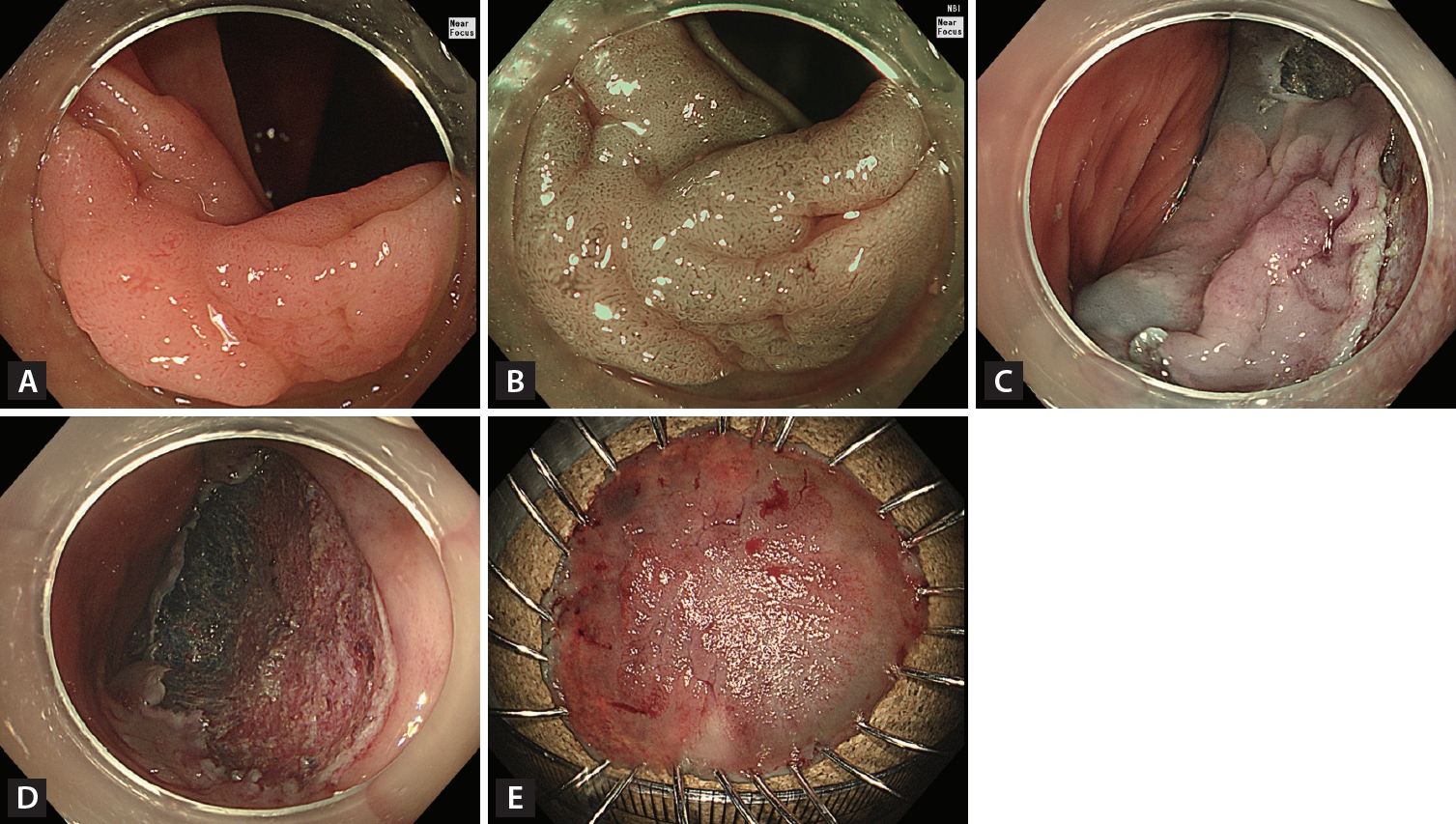
Figure┬Ā3.
Endoscopic non-curative resection. (A) A 20-mm non-granular pseudo-depressed laterally spreading tumor located in the transverse colon. (B) Irregular and heterogeneous vascular and surface patterns were observed (JNET type 2B-high) on narrow-band imaging with near-focus. (C) Circumferential incision and submucosal dissection of the lesion using an endoscopic knife after submucosal injection. (D) Mucosal defect after completion of endoscopic submucosal dissection. (E) Histopathological examination confirmed incomplete resection (20 ├Ś 17 mm adenocarcinoma well-differentiated, depth of submucosal invasion Ōēź 2,000 ╬╝m, no lymphatic invasion, with carcinoma involvement in the vertical resection margin).
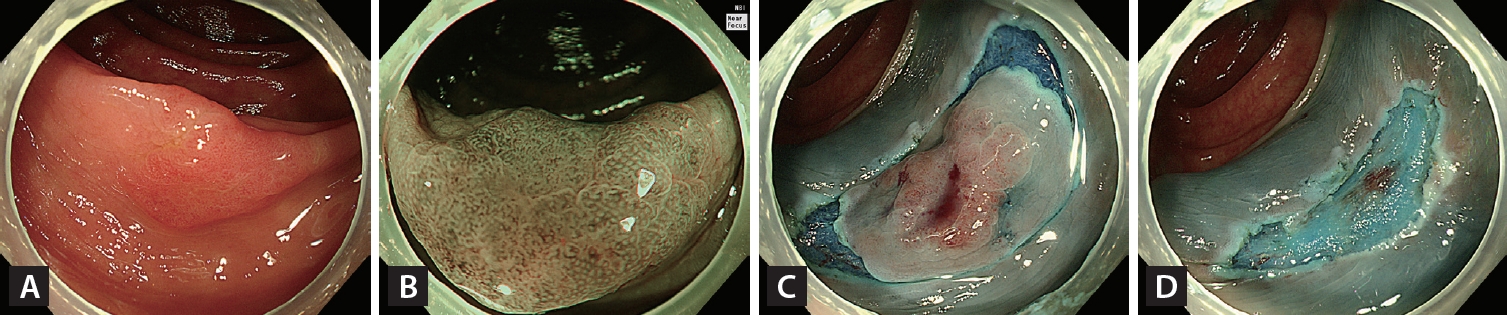
Figure┬Ā4.
Endoscopic mucosal resection with precutting. (A) A 16-mm non-granular flat elevated laterally spreading tumor located in the ascending colon. (B) Irregular and uniform vascular and surface patterns were observed on narrow-band imaging with near-focus (JNET type 2B-low). (C) Complete circumferential incision of the lesion using a snare tip after submucosal injection. (D) Visual inspection of the iatrogenic polypectomy ulcer after snare-mediated mucosal resection of the lesion.
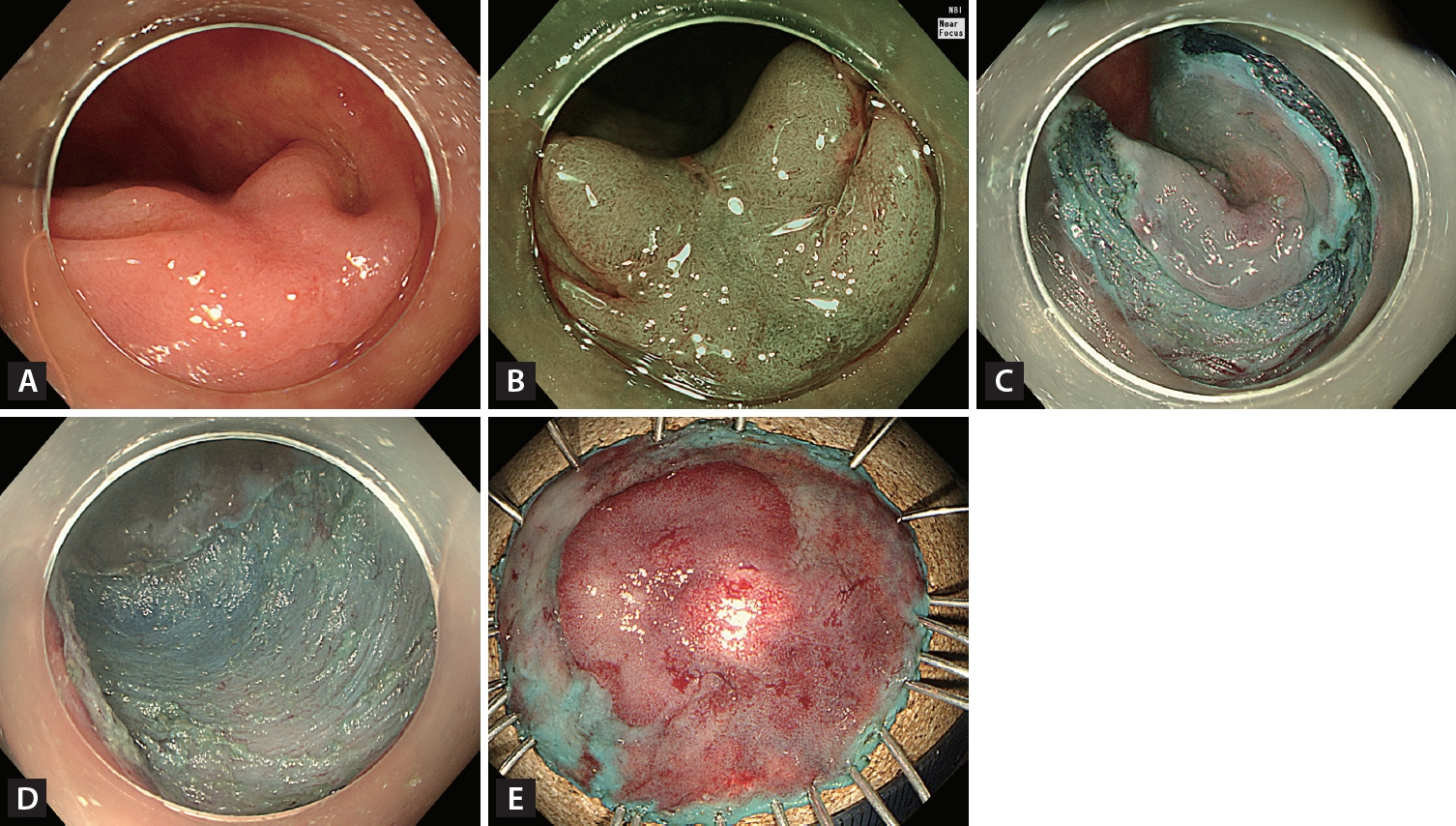
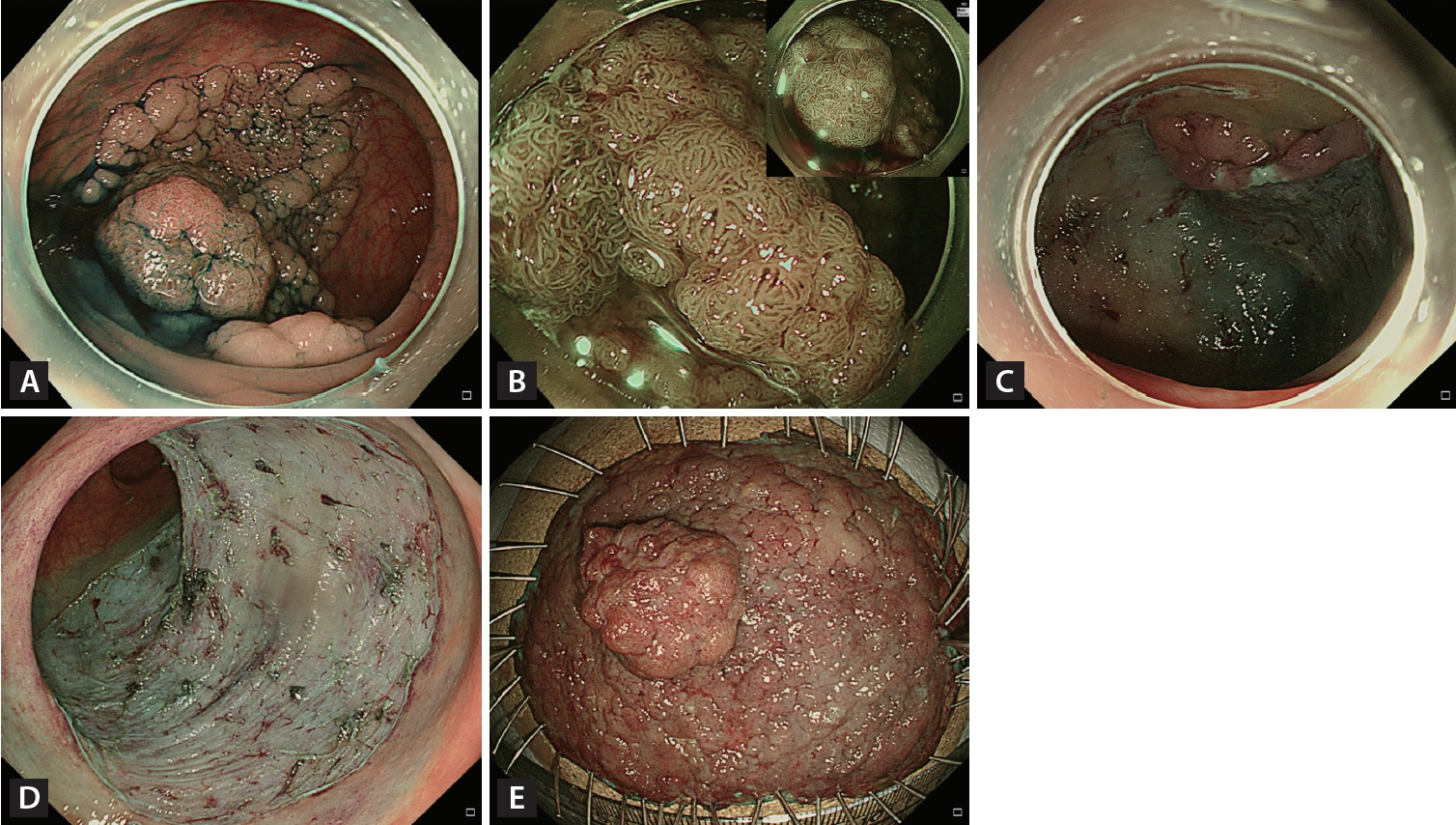
Figure┬Ā5.
Endoscopic submucosal dissection (ESD). (A) A 70-mm granular nodular mixed laterally spreading tumor located in the rectosigmoid junction. (B) Most of the lesion had a regular vascular and surface pattern (JNET type 2A) on narrow-band imaging with near-focus, but the large nodule had an irregular and uniform vascular pattern (JNET type 2B-low). (C) Circumferential incision and submucosal dissection of the lesion using an endoscopic knife after submucosal injection. (D) Mucosal defect after completion of ESD. (E) Histopathological examination confirmed complete resection (R0) (70 ├Ś 55 mm villous adenoma with high-grade dysplasia).
REFERENCES
1. Shaukat A, Kaltenbach T, Dominitz JA, et al. Endoscopic recognition and management strategies for malignant colorectal polyps: recommendations of the US Multi-Society Task Force on colorectal cancer. Am J Gastroenterol 2020;115:1751ŌĆō1767.


3. Japanese Society for Cancer of the Colon and Rectum. Japanese classification of colorectal, appendiceal, and anal carcinoma: the 3d english edition [secondary publication]. J Anus Rectum Colon 2019;3:175ŌĆō195.



4. Yeh JH, Tseng CH, Huang RY. Long-term outcomes of primary endoscopic resection vs surgery for T1 colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2020;18:2813ŌĆō2823.e5.


5. Backes Y, Moss A, Reitsma JB, Siersema PD, Moons LM. Narrow band imaging, magnifying chromoendoscopy, and gross morphological features for the optical diagnosis of T1 colorectal cancer and deep submucosal invasion: a systematic review and meta-analysis. Am J Gastroenterol 2017;112:54ŌĆō64.



6. Saraiva S, Rosa I, Fonseca R, Pereira AD. Colorectal malignant polyps: a modern approach. Ann Gastroenterol 2022;35:17ŌĆō27.



7. Pickhardt PJ, Hain KS, Kim DH, Hassan C. Low rates of cancer or high-grade dysplasia in colorectal polyps collected from computed tomography colonography screening. Clin Gastroenterol Hepatol 2010;8:610ŌĆō615.


8. Kudo S, Kashida H, Tamura T, et al. Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer. World J Surg 2000;24:1081ŌĆō1090.



9. Bogie RMM, Veldman MHJ, Snijders LARS, et al. Endoscopic subtypes of colorectal laterally spreading tumors (LSTs) and the risk of submucosal invasion: a meta-analysis. Endoscopy 2018;50:263ŌĆō282.


10. Kaltenbach T, Anderson JC, Burke CA, et al. Endoscopic removal of colorectal lesions-recommendations by the US Multi-Society Task Force on colorectal cancer. Gastroenterology 2020;158:1095ŌĆō1129.


11. Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2017;49:270ŌĆō297.


12. Park W, Kim B, Park SJ, et al. Conventional endoscopic features are not sufficient to differentiate small, early colorectal cancer. World J Gastroenterol 2014;20:6586ŌĆō6593.



13. Ikehara H, Saito Y, Matsuda T, Uraoka T, Murakami Y. Diagnosis of depth of invasion for early colorectal cancer using magnifying colonoscopy. J Gastroenterol Hepatol 2010;25:905ŌĆō912.


14. Bugajski M, Kaminski MF, Orlowska J, et al. Suspicious macroscopic features of small malignant colorectal polyps. Scand J Gastroenterol 2015;50:1261ŌĆō1267.


15. Puig I, L├│pez-Cer├│n M, Arnau A, et al. Accuracy of the narrow-band imaging international colorectal endoscopic classification system in identification of deep invasion in colorectal polyps. Gastroenterology 2019;156:75ŌĆō87.

16. Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy 1993;25:455ŌĆō461.


17. Kudo SE, Takemura O, Ohtsuka K. Flat and depressed types of early colorectal cancers: from East to West. Gastrointest Endosc Clin N Am 2008;18:581ŌĆō593, xi.


18. Ishigaki T, Kudo SE, Miyachi H, et al. Treatment policy for colonic laterally spreading tumors based on each clinicopathologic feature of 4 subtypes: actual status of pseudo-depressed type. Gastrointest Endosc 2020;92:1083ŌĆō1094.e6.


19. Russo P, Barbeiro S, Awadie H, Lib├ónio D, Dinis-Ribeiro M, Bourke M. Management of colorectal laterally spreading tumors: a systematic review and meta-analysis. Endosc Int Open 2019;7:E239ŌĆōE259.



20. Yamada M, Saito Y, Sakamoto T, et al. Endoscopic predictors of deep submucosal invasion in colorectal laterally spreading tumors. Endoscopy 2016;48:456ŌĆō464.


21. Saito Y, Ono A, Garc├Ła VAJ, et al. Diagnosis and treatment of colorectal tumors: differences between Japan and the West and future prospects. DEN Open 2021;2:e66.




22. Nagai M, Suzuki S, Minato Y, et al. Detecting colorectal lesions with image-enhanced endoscopy: an updated review from clinical trials. Clin Endosc 2023;56:553ŌĆō562.




23. Lee HH, Lee BI. Image-enhanced endoscopy in lower gastrointestinal diseases: present and future. Clin Endosc 2018;51:534ŌĆō540.




24. Tanaka S, Sano Y. Aim to unify the narrow band imaging (NBI) magnifying classification for colorectal tumors: current status in Japan from a summary of the consensus symposium in the 79th Annual Meeting of the Japan Gastroenterological Endoscopy Society. Dig Endosc 2011;23 Suppl 1:131ŌĆō139.


25. Tanaka S, Hayashi N, Oka S, Chayama K. Endoscopic assessment of colorectal cancer with superficial or deep submucosal invasion using magnifying colonoscopy. Clin Endosc 2013;46:138ŌĆō146.



26. Sumimoto K, Tanaka S, Shigita K, et al. Clinical impact and characteristics of the narrow-band imaging magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Gastrointest Endosc 2017;85:816ŌĆō821.


27. Sumimoto K, Tanaka S, Shigita K, et al. Diagnostic performance of Japan NBI Expert Team classification for differentiation among noninvasive, superficially invasive, and deeply invasive colorectal neoplasia. Gastrointest Endosc 2017;86:700ŌĆō709.


28. Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc 1996;44:8ŌĆō14.


29. Bianco MA, Rotondano G, Marmo R, et al. Predictive value of magnification chromoendoscopy for diagnosing invasive neoplasia in nonpolypoid colorectal lesions and stratifying patients for endoscopic resection or surgery. Endoscopy 2006;38:470ŌĆō476.


30. Tanaka S, Kaltenbach T, Chayama K, Soetikno R. High-magnification colonoscopy (with videos). Gastrointest Endosc 2006;64:604ŌĆō613.


31. Kanao H, Tanaka S, Oka S, et al. Clinical significance of type V(I) pit pattern subclassification in determining the depth of invasion of colorectal neoplasms. World J Gastroenterol 2008;14:211ŌĆō217.



32. Li JW, Wang LM, Ichimasa K, Lin KW, Ngu JC, Ang TL. Use of artificial intelligence in the management of T1 colorectal cancer: a new tool in the arsenal or is deep learning out of its depth? Clin Endosc 2024;57:24ŌĆō35.




33. Park EY, Baek DH, Lee MW, Kim GH, Park DY, Song GA. Long-term outcomes of T1 colorectal cancer after endoscopic resection. J Clin Med 2020;9:2451.



34. Lopez A, Bouvier AM, Jooste V, et al. Outcomes following polypectomy for malignant colorectal polyps are similar to those following surgery in the general population. Gut 2019;68:111ŌĆō117.


35. Cooper GS, Xu F, Barnholtz Sloan JS, Koroukian SM, Schluchter MD. Management of malignant colonic polyps: a population-based analysis of colonoscopic polypectomy versus surgery. Cancer 2012;118:651ŌĆō659.



36. ASGE Technology Committee; Hwang JH, et al.; Konda V. Endoscopic mucosal resection. Gastrointest Endosc 2015;82:215ŌĆō226.


37. Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 2020;25:1ŌĆō42.




38. Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology 2013;144:74ŌĆō80.e1.


39. Yamashina T, Uedo N, Akasaka T, et al. Comparison of underwater vs conventional endoscopic mucosal resection of intermediate-size colorectal polyps. Gastroenterology 2019;157:451ŌĆō461.e2.


40. Jung Y, Hwangbo Y, Cho YS, et al. Is colorectal endoscopic submucosal dissection safe and effective for 15-19-mm tumors? Int J Colorectal Dis 2023;38:206.



41. Tanaka S, Kashida H, Saito Y, et al. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2020;32:219ŌĆō239.



42. Jideh B, Bourke MJ. How to perform wide-field endoscopic mucosal resection and follow-up examinations. Gastrointest Endosc Clin N Am 2019;29:629ŌĆō646.


43. Saito Y, Sakamoto T, Fukunaga S, Nakajima T, Kiriyama S, Matsuda T. Endoscopic submucosal dissection (ESD) for colorectal tumors. Dig Endosc 2009;21 Suppl 1:S7ŌĆōS12.


44. Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014;46:388ŌĆō402.


45. Komeda Y, Watanabe T, Sakurai T, et al. Risk factors for local recurrence and appropriate surveillance interval after endoscopic resection. World J Gastroenterol 2019;25:1502ŌĆō1512.



46. Sekiguchi M, Matsuda T, Hotta K, Saito Y. Post-polypectomy surveillance: the present and the future. Clin Endosc 2022;55:489ŌĆō495.




47. Kim SY, Kwak MS, Yoon SM, et al. Korean guidelines for postpolypectomy colonoscopic surveillance: 2022 revised edition. Clin Endosc 2022;55:703ŌĆō725.




48. Binmoeller KF, Weilert F, Shah J, Bhat Y, Kane S. "Underwater" EMR without submucosal injection for large sessile colorectal polyps (with video). Gastrointest Endosc 2012;75:1086ŌĆō1091.


49. Nett A, Binmoeller K. Underwater endoscopic mucosal resection. Gastrointest Endosc Clin N Am 2019;29:659ŌĆō673.


50. Zhang Z, Xia Y, Cui H, et al. Underwater versus conventional endoscopic mucosal resection for small size non-pedunculated colorectal polyps: a randomized controlled trial : (UEMR vs. CEMR for small size non-pedunculated colorectal polyps). BMC Gastroenterol 2020;20:311.


51. Yen AW, Leung JW, Wilson MD, Leung FW. Underwater versus conventional endoscopic resection of nondiminutive nonpedunculated colorectal lesions: a prospective randomized controlled trial (with video). Gastrointest Endosc 2020;91:643ŌĆō654.e2.



52. Inoue T, Nakagawa K, Yamasaki Y, et al. Underwater endoscopic mucosal resection versus endoscopic submucosal dissection for 20-30 mm colorectal polyps. J Gastroenterol Hepatol 2021;36:2549ŌĆō2557.



53. Nagl S, Ebigbo A, Goelder SK, et al. Underwater vs conventional endoscopic mucosal resection of large sessile or flat colorectal polyps: a prospective randomized controlled trial. Gastroenterology 2021;161:1460ŌĆō1474.e1.


54. Chien H, Imai K, Hotta K, et al. Tip-in EMR for R0 resection for a large flat colonic tumor. Gastrointest Endosc 2016;84:743.


55. Jacques J, Legros R, Charissoux A, et al. Anchoring the snare tip by means of a small incision facilitates en bloc endoscopic mucosal resection and increases the specimen size. Endoscopy 2017;49(S 01):E39ŌĆōE41.


56. Sato Y, Ozawa SI, Yasuda H, et al. Tip-in endoscopic mucosal resection for large colorectal sessile polyps. Surg Endosc 2021;35:1820ŌĆō1826.



57. Pioche M, Wallenhorst T, Lepetit H, et al. Endoscopic mucosal resection with anchoring of the snare tip: multicenter retrospective evaluation of effectiveness and safety. Endosc Int Open 2019;7:E1496ŌĆōE1502.



58. Imai K, Hotta K, Ito S, et al. Tip-in endoscopic mucosal resection for 15- to 25-mm colorectal adenomas: a single-center, randomized controlled trial (STAR Trial). Am J Gastroenterol 2021;116:1398ŌĆō1405.


59. Takada K, Hotta K, Imai K, et al. Tip-in EMR as an alternative to endoscopic submucosal dissection for 20- to 30-mm nonpedunculated colorectal neoplasms. Gastrointest Endosc 2022;96:849ŌĆō856.e3.


60. Yang DH, Kwak MS, Park SH, et al. Endoscopic mucosal resection with circumferential mucosal incision for colorectal neoplasms: comparison with endoscopic submucosal dissection and between two endoscopists with different experiences. Clin Endosc 2017;50:379ŌĆō387.




61. Lee EJ, Lee JB, Lee SH, Youk EG. Endoscopic treatment of large colorectal tumors: comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc 2012;26:2220ŌĆō2230.



62. Yoshida N, Inoue K, Dohi O, et al. Efficacy of precutting endoscopic mucosal resection with full or partial circumferential incision using a snare tip for difficult colorectal lesions. Endoscopy 2019;51:871ŌĆō876.


63. Fuccio L, Hassan C, Ponchon T, et al. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc 2017;86:74ŌĆō86.e17.


64. Yoshida N, Naito Y, Yasuda R, et al. The efficacy of the pocket-creation method for cases with severe fibrosis in colorectal endoscopic submucosal dissection. Endosc Int Open 2018;6:E975ŌĆōE983.



65. Faller J, Jacques J, Oung B, et al. Endoscopic submucosal dissection with double clip and rubber band traction for residual or locally recurrent colonic lesions after previous endoscopic mucosal resection. Endoscopy 2020;52:383ŌĆō388.


66. Ide D, Ohya TR, Ishioka M, et al. Efficacy of the pocket-creation method with a traction device in endoscopic submucosal dissection for residual or recurrent colorectal lesions. Clin Endosc 2022;55:655ŌĆō664.




67. Yang DH. Combination of endoscopic submucosal dissection techniques, a practical solution for difficult cases. Clin Endosc 2022;55:626ŌĆō627.




68. Ma MX, Bourke MJ. Endoscopic submucosal dissection in the West: current status and future directions. Dig Endosc 2018;30:310ŌĆō320.



69. Rashid MU, Alomari M, Afraz S, Erim T. EMR and ESD: indications, techniques and results. Surg Oncol 2022;43:101742.


70. Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015;81:583ŌĆō595.


71. Gweon TG, Yang DH. Management of complications related to colorectal endoscopic submucosal dissection. Clin Endosc 2023;56:423ŌĆō432.




72. Zwager LW, Bastiaansen BAJ, van der Spek BW, et al. Endoscopic full-thickness resection of T1 colorectal cancers: a retrospective analysis from a multicenter Dutch eFTR registry. Endoscopy 2022;54:475ŌĆō485.

73. Dolan RD, Bazarbashi AN, McCarty TR, Thompson CC, Aihara H. Endoscopic full-thickness resection of colorectal lesions: a systematic review and meta-analysis. Gastrointest Endosc 2022;95:216ŌĆō224.e18.


74. Park CH, Yang DH, Kim JW, et al. Clinical practice guideline for endoscopic resection of early gastrointestinal cancer. Clin Endosc 2020;53:142ŌĆō166.




75. Lugli A, Kirsch R, Ajioka Y, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol 2017;30:1299ŌĆō1311.



76. Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29(Suppl 4):iv263.


77. Ciocalteu A, Gheonea DI, Saftoiu A, Streba L, Dragoescu NA, Tenea-Cojan TS. Current strategies for malignant pedunculated colorectal polyps. World J Gastrointest Oncol 2018;10:465ŌĆō475.



78. Ichimasa K, Kudo SE, Miyachi H, et al. Current problems and perspectives of pathological risk factors for lymph node metastasis in T1 colorectal cancer: systematic review. Dig Endosc 2022;34:901ŌĆō912.



79. Haggitt RC, Glotzbach RE, Soffer EE, Wruble LD. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology 1985;89:328ŌĆō336.


80. Kitajima K, Fujimori T, Fujii S, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 2004;39:534ŌĆō543.



81. Nivatvongs S, Rojanasakul A, Reiman HM, et al. The risk of lymph node metastasis in colorectal polyps with invasive adenocarcinoma. Dis Colon Rectum 1991;34:323ŌĆō328.


82. Cooper HS, Deppisch LM, Gourley WK, et al. Endoscopically removed malignant colorectal polyps: clinicopathologic correlations. Gastroenterology 1995;108:1657ŌĆō1665.


83. ASGE Standards of Practice Committee, Fisher DA, Shergill AK, et al. Role of endoscopy in the staging and management of colorectal cancer. Gastrointest Endosc 2013;78:8ŌĆō12.

84. Oh JR, Park B, Lee S, et al. Nomogram development and external validation for predicting the risk of lymph node metastasis in T1 colorectal cancer. Cancer Res Treat 2019;51:1275ŌĆō1284.




85. Ha RK, Han KS, Sohn DK, et al. Histopathologic risk factors for lymph node metastasis in patients with T1 colorectal cancer. Ann Surg Treat Res 2017;93:266ŌĆō271.




86. Zwager LW, Bastiaansen BAJ, Montazeri NSM, et al. Deep submucosal invasion is not an independent risk factor for lymph node metastasis in T1 colorectal cancer: a meta-analysis. Gastroenterology 2022;163:174ŌĆō189.


87. Kouyama Y, Kudo SE, Miyachi H, et al. Practical problems of measuring depth of submucosal invasion in T1 colorectal carcinomas. Int J Colorectal Dis 2016;31:137ŌĆō146.




88. Miyachi H, Kudo SE, Ichimasa K, et al. Management of T1 colorectal cancers after endoscopic treatment based on the risk stratification of lymph node metastasis. J Gastroenterol Hepatol 2016;31:1126ŌĆō1132.



89. Butte JM, Tang P, Gonen M, et al. Rate of residual disease after complete endoscopic resection of malignant colonic polyp. Dis Colon Rectum 2012;55:122ŌĆō127.


90. Bujanda L, Cosme A, Gil I, Arenas-Mirave JI. Malignant colorectal polyps. World J Gastroenterol 2010;16:3103ŌĆō3111.



91. Gijsbers KM, van der Schee L, van Veen T, et al. Impact of Ōēź 0.1-mm free resection margins on local intramural residual cancer after local excision of T1 colorectal cancer. Endosc Int Open 2022;10:E282ŌĆōE290.



92. Toyoshima N, Abe S, Saito Y. In addition to free deep margins, R0 resection should be required for T1 colorectal cancers to inform further surgical resection. Endosc Int Open 2022;10:E291ŌĆōE292.



93. Ito T, Eishi Y, Kobayashi D, Akashi T, Koike M, Ohashi K. A risk stratification for nodal metastasis in T1 colorectal cancer after successful therapeutic endoscopy. Gastrointest Endosc 2022;96:131ŌĆō134.


94. Dang H, Dekkers N, le Cessie S, et al. Risk and time pattern of recurrences after local endoscopic resection of T1 colorectal cancer: a meta-analysis. Clin Gastroenterol Hepatol 2022;20:e298.


95. Park EY, Baek DH, Song GA, Kim GH, Lee BE, Park DY. Long-term outcomes of endoscopically resected laterally spreading tumors with a positive histological lateral margin. Surg Endosc 2020;34:3999ŌĆō4010.



96. Sakamoto T, Matsuda T, Otake Y, Nakajima T, Saito Y. Predictive factors of local recurrence after endoscopic piecemeal mucosal resection. J Gastroenterol 2012;47:635ŌĆō640.



97. National Health Commission of the PeopleŌĆÖs Republic of China; Society of Oncology, Chinese Medical Association. National Health Commission guidelines for diagnosis and treatment of colorectal cancer 2023 in China (English version). Chin J Cancer Res 2023;35:197ŌĆō232.



98. Bartel MJ, Brahmbhatt BS, Wallace MB. Management of colorectal T1 carcinoma treated by endoscopic resection from the Western perspective. Dig Endosc 2016;28:330ŌĆō341.


99. Iguchi K, Mushiake H, Aoyama T, et al. Additional surgical resection after endoscopic resection for patients with highrisk T1 colorectal cancer. In Vivo 2019;33:1243ŌĆō1248.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



