 |
 |
| Korean J Intern Med > Volume 39(3); 2024 > Article |
|
Abstract
Background
Helicobacter pylori infection, prevalent in more than half of the global population, is associated with various gastrointestinal diseases, including peptic ulcers and gastric cancer. The effectiveness of early diagnosis and treatment in preventing gastric cancer highlights the need for improved diagnostic methods. This study aimed to develop a simple scoring system based on endoscopic findings to predict H. pylori infection.
Methods
A retrospective analysis was conducted on 1,007 patients who underwent upper gastrointestinal endoscopy at Asan Medical Center from January 2019 to December 2021. Exclusion criteria included prior H. pylori treatment, gastric surgery, or gastric malignancies. Diagnostic techniques included rapid urease and 13C-urea breath tests, H. pylori culture, and assessment of endoscopic features following the Kyoto gastritis classification. A new scoring system based on endoscopic findings including regular arrangement of collecting venules (RAC), nodularity, and diffuse or spotty redness was developed for predicting H. pylori infection, utilizing logistic regression analysis in the development set.
Results
The scoring system demonstrated high predictive accuracy for H. pylori infection in the validation set. Scores of 2 and 3 were associated with 96% and 99% infection risk, respectively. Additionally, there was a higher prevalence of diffuse redness and sticky mucus in cases where the initial H. pylori eradication treatment failed.
Helicobacter pylori infection is a prevalent infectious disease affecting more than 50% of the population [1,2]. H. pylori infection is associated with peptic ulcers, gastric hyperplastic polyps, and malignancies, including gastric mucosa-associated lymphoid tissue lymphoma or adenocarcinoma [3,4]. Therefore, early infection diagnosis is vital for eradication and gastric cancer prevention [3,5,6]. In clinical practice, there are various H. pylori infection diagnosis methods including the 13C-urea breath test (UBT), rapid urease test (RUT), H. pylori-specific IgG, stool antigen test, and H. pylori culture. However, if H. pylori infection can be predicted based on endoscopic findings, the diagnostic modality accuracy may increase, improving the diagnostic rate.
Several studies have attempted to distinguish H. pylori status by assessing the endoscopic gastric mucosal morphology with the Kyoto gastritis classification, allowing a more accurate H. pylori status determination [7]. The Kyoto classification score incorporates five endoscopic features: atrophy, intestinal metaplasia, fold enlargement (more than 5 mm), nodularity, and diffuse redness. A score of 0 indicates no infection, whereas 2 or more suggests H. pylori infection. However, the Kyoto classification score is limited by its five categories, the subjective 5 mm fold enlargement, and atrophy potentially progressing with age [8–10]. Therefore, a more practical and straightforward method for predicting infection is essential. We analyzed endoscopic features and developed a simple scoring system to accurately detect H. pylori infection based on endoscopic findings of the Kyoto gastritis classification.
We retrospectively investigated 1,007 upper gastrointestinal endoscopy patients and obtained gastric mucosal tissues for H. pylori culture between January 2019 and December 2021 from Asan Medical Center, Seoul, Korea. Patients with previous H. pylori eradication treatment (n = 216), gastric surgery history (n = 12), familial adenomatous polyposis (n = 1), and advanced gastric cancer (n = 37) or lymphoma (n = 29) malignancies, which interfere with intact gastric mucosal observation, were excluded. We excluded patients who did not undergo further UBT when the results of both the culture and RUT were negative. (n = 222) (Fig. 1). We defined H. pylori-positive patients as those with positive results for the culture, or both positive in RUT, and UBT and H. pylori-negative patients as those with all negative results for the culture, RUT, and UBT. The Institutional Review Board of Asan Medical Center approved this study (approval number: 2022-0605). Informed consent was waived by Institutional Review Board.
The RUT (Chongkeundang Pharma, Seoul, Korea) assessed the two gastric specimens obtained from the antrum and body. Two samples of gastric tissues were separately placed into RUT kits A and B, and a negative result was obtained when there was no color change for 24 hours. After at least 4 hours of fasting, a breath sample was collected, and a testing tablet containing 100 mg 13C-urea (UBIT® tablet; Otsuka Pharmaceutical Co. Ltd., Tokyo, Japan) was given to the patient. A second breath sample was procured 20 min later and was tested for H. pylori infection by the 13C-UBT (Analyzer POCone; Otsuka Electronics Co. Ltd., Osaka, Japan). The cutoff value was 2.5 ppm. The UBT could detect H. pylori infection before eradication treatment and indicate if the treatment was successful.
During endoscopy, the two H. pylori culture specimens were obtained from the antrum and body. For bacterial isolation, the medium used in H. pylori culture plates was Brucella broth agar, which was coated with 5% sheep blood (Becton Dickinson, Franklin Lakes, NJ, USA), trimethoprim (5 μg/mL), vancomycin (10 μg/mL), amphotericin B (5 μg/mL), and polymyxin B (2.5 IU) (Sigma-Aldrich, Darmstadt, Germany). These specimens were incubated at 37°C under micro-ventilation conditions (5% O2, 10% CO2, and 85% N2) for 7 days. The H. pylori colonies collected from each culture were confirmed through gram staining and biochemical methods.
We used the GI-H290 scope (Olympus Medical, Inc., Tokyo, Japan) to examine mucosal lesions, and a senior endoscopist (AJY, more than 15 years of experience) assessed endoscopic findings according to the Kyoto gastritis classification [7]. Atrophy (closed and open), intestinal metaplasia, diffuse redness, spotty redness, mucosal edema, sticky mucus, enlarged folds, nodularity, xanthoma, hyperplastic polyp, regular arrangement of collecting venules (RAC), fundic gland polyp, linear streak, raised erosion, hematin, patchy redness, map-like redness, and multiple whitish flat lesions were assessed based on previous endoscopic images. RAC presence was evaluated in the lesser curvature. We divided the endoscopic findings into non-, current, and past infection. Endoscopic findings associated with non-infection were RAC, raised erosion, fundic gland polyp, linear streak, and hematin. Current infection indicators were open and closed atrophies, spotty redness, diffuse redness, hyperplastic polyp, xanthoma, intestinal metaplasia, edema, nodularity, enlarged folds, and sticky mucus. Past infection was determined by the presence of patchy redness, map-like redness, and multiple whitish flat lesions.
Initial eradication treatment included standard triple therapy (n = 186), bismuth quadruple therapy (n = 22), HDDT (high-dose dual therapy) (n = 17), combination therapy (n = 2), or PBTL (proton pump inhibitor, bismuth, clarithromycin, and levofloxacin) (n = 1) depending on the available antibiotic resistance results. Next, patients took a UBT at least 6 weeks post-treatment to confirm eradication. If the initial eradication therapy failed, a second treatment was recommended with a bismuth-based quadruple therapy when culture results were unavailable or a tailored therapy if culture results were available. If they agreed, patients who failed second-line treatment were treated with a different regimen.
The scoring system assessed three endoscopic parameters with scores ranging from 0 to 3 as follows: diffuse redness or spotty redness, nodularity, and RAC (Supplementary Table 1). To develop the scoring system for predicting H. pylori infection, we divided factors into development (January 2019 to June 2021) and validation sets (July 2021 to December 2021) based on the test date. Initially, since there was a gray area between spotty and diffuse redness when viewed endoscopically, we defined diffuse redness and spotty redness as one factor. For the development set, we performed logistic regression analysis with backward elimination to select factors and predict the H. pylori infection status. In multivariable analysis, we found that diffuse redness or spotty redness and nodularity significantly increased the risk of H. pylori infection, and RAC significantly decreased the risk of H. pylori infection. Previous studies have shown that the presence of RAC, diffuse redness or spotty redness, and nodularity has low inter-observer variability, and they may be easily observed under white light endoscopy [11–14]. Therefore, we selected nodularity, RAC, and spotty redness or diffuse redness as the key indicators (Fig. 2). The risk score was the weighted sum of these predictors, for which weights were the rounded integer value of the quotient of regression coefficients divided by the coefficient of the reference predictor (nodularity). The predicted probability was estimated by a logistic regression equation with the risk score in the validation set. The event probability was one divided by the sum of one and the exponential function of negative one times the sum of negative 0.9884 and 4.4197 times the score squared:
The risk score’s discrimination capability was assessed using the C statistic, and the calibration capability was assessed using a calibration plot and the Hosmer–Lemeshow test in both the development and validation sets.
Statistical analysis was performed with SPSS ver. 22.0 for Windows (IBM Corp., Armonk, NY, USA) and R (version 3.6.1; https://www.R-project.org). All descriptive variables are expressed as the mean with standard deviation representative of the distribution. In addition, each group characteristic was compared using the chi-squared or Fisher’s exact tests for categorical variables and two-sample t-tests for continuous variables. A p value less than 0.05 (represented by “< 0.05”) indicated statistical significance.
This study examined 358 H. pylori-positive and 132 H. pylori-negative patients. Based on endoscopic findings, compared with the H. pylori-negative group, the H. pylori-positive group exhibited a significantly higher prevalence of atrophy (98.6% vs. 81.1%, p < 0.001), intestinal metaplasia (64.0% vs. 45.5%, p < 0.001), diffuse redness (47.5% vs. 3.0%, p < 0.001), spotty redness (77.1% vs. 6.8%, p < 0.001), edema (14.2% vs. 5.3%, p = 0.010), sticky mucus (28.5% vs. 1.5%, p < 0.001), enlarged folds (6.1% vs. 0.0%, p = 0.008), and nodularity (7.5% vs. 0.0%, p = 0.003). On the other hand, compared with the H. pylori-positive group, the H. pylori-negative group had a significantly higher occurrence of RAC (56.1% vs. 1.1%, p < 0.001), fundic gland polyp (12.9% vs. 0.6%, p < 0.001), linear streak (37.9% vs. 0.8%, p < 0.001), and raised erosion (28.8% vs. 2.0%, p < 0.001) (Table 1).
The H. pylori-negative group was further divided into no atrophy (n = 25) and atrophy (n = 107). The mean age of the no atrophy group was lower than that of the atrophy group (48.4 ± 17.3 yr vs. 63.4 ± 11.3 yr). Endoscopic features demonstrated that all 25 patients in the no atrophy group had notably more RAC (100.0% vs. 45.8%, p < 0.001) and linear streaks (84.0% vs. 27.1%, p < 0.001) than those in the atrophy group. The no atrophy group did not exhibit intestinal metaplasia, diffuse redness, spotty redness, edema, sticky mucus, enlarged folds, nodularity, or hyperplastic polyp. On the other hand, 56.1% of patients in the atrophy group had intestinal metaplasia (p < 0.001), and 24.3% of them had xanthoma (p = 0.025) (Supplementary Table 2).
The development set consisted of 349 patients, and the validation set included 141 patients. In our scoring system (0–3), diffuse or spotty redness, nodularity, and RAC loss were worth 1 point each. At a score of 2, 97% of patients in the development and validation sets were H. pylori-positive, and at a score of 3, 100% of patients were H. pylori-positive (Table 2). The scoring system indicated 2%, 45%, 96%, and 99% infection risk at scores of 0, 1, 2, and 3, respectively (Fig. 3).
The performance of the scoring system was assessed using discrimination and calibration metrics (Table 3). Discrimination capability was evaluated using the C statistic, and calibration was assessed using the Hosmer–Lemeshow test. For the development set, the scoring system demonstrated a high level of discrimination, with a C statistic of 0.929 (standard error [SE] = 0.018) and a 95% confidence interval (CI) ranging from 0.894 to 0.963. The calibration of the model showed acceptable agreement with the observed outcomes, as indicated by a Hosmer–Lemeshow χ2 value of 2.000 (degrees of freedom [DF] = 2) and a non-significant p value of 0.368, suggesting no evidence of poor fit. For the temporal validation set, the model maintained strong discrimination with a C statistic of 0.951 (SE = 0.017) and a 95% CI of 0.918–0.984. The calibration test yielded a χ2 value of 0.084 (DF = 2) with a p value of 0.959, indicating a good fit between the predicted and observed event rates over the deciles of risk.
The endoscopic findings of patients who underwent the initial eradication therapy were categorized into the success (n = 198) or failure (n = 30) groups, with an 86% success rate observed with initial eradication treatment. The success group was treated with various eradication regimens, including standard triple therapy (163/186, 87%), bismuth-based quadruple therapy (22/22, 100%), combination therapy (1/2, 50%), HDDT (14/17, 82%), and PBTL (1/1, 100%). Patients with an unsuccessful initial treatment were re-treated with the following regimens: standard triple therapy (0/1, 0%), bismuth-based quadruple therapy (12/14, 86%), HDDT (0/2, 0%), and PAL (proton pump inhibitor, amoxicillin, and levofloxacin) (1/1, 100%). All patients with an unsuccessful second eradication treatment with standard triple and high-dose dual therapies were successfully treated with bismuth-based quadruple therapy. The remaining patients were lost to follow-up or refused further treatment. The proportions of patients with diffuse redness and sticky mucus were significantly higher in the failure group than in the success group (43.9% vs. 66.7%, p = 0.033 and 26.8% vs. 46.7%, p = 0.044, respectively) (Supplementary Table 3).
H. pylori infection causes various gastrointestinal symptoms and contributes to gastric ulcers, cancer, and associated lymphoma; thus, H. pylori eradication improves gastritis and helps prevent gastric ulcers and cancer. The global H. pylori prevalence has surpassed 50%, making eradication treatment crucial to prevent the disease from spreading. This study compared endoscopic findings of H. pylori infection, created a scoring system for predicting infection, and identified endoscopic features that may influence eradication treatment, such as diffuse redness and sticky mucus.
The findings of the Kyoto classification of gastritis indicate H. pylori infection and non-infection. In addition, several studies have suggested that mucosal edema, spotty redness, diffuse redness, nodularity, and RAC loss are associated with H. pylori infection [7,10,15,16]. In our study, these endoscopic features were considerably more apparent in H. pylori-infected patients, whereas uninfected patients showed increased RAC presence, fundic gland polyp, linear streak, and raised erosion. The presence of hematin did not significantly differ between patients in our study, which may be explained by its association with mucosal injury from other factors [17]. Although xanthoma is closely associated with H. pylori infection, our results did not show a significant difference between the two groups [18–20]. It is possible that xanthoma is also associated with other causes, including idiopathic drug-associated atrophy and dyslipidemia [21].
A previous study established that a score of 2 or more in the Kyoto classification system indicates H. pylori infection with an infection rate higher than 90% [22]. This preceding 0 to 8 scoring system examines five endoscopic features: atrophy degree (none, C1: 0, C2, C3: 1, O1–O3: 2), intestinal metaplasia (none: 0, antrum: 1, body: 2), gastric folds enlargement (< 5 mm: 0, ≥ 5 mm: 1), nodularity (absence: 0, presence: 1), and diffuse redness (none: 0, mild: 1, severe: 2). However, by only considering five factors and including subjective scores that cannot be directly measured, its findings may be misleading in clinical practice. Therefore, we established a practical scoring system to assess the most objective factors, which was efficient in predicting H. pylori infection in our internal validation set. A score of 2 suggested possible H. pylori infection with a 96% probability, and a score of 3 indicated that H. pylori infection was almost certain with a 99% probability. Further external validation with more patients and multicenter studies are needed to provide more concrete evidence.
In our study, we found that 50% of H. pylori-negative patients had an open type of atrophy. A previous study has shown that chronic atrophic gastritis in patients with no history of H. pylori active infection or antimicrobial therapy may most likely be attributed to unintentional eradication of H. pylori after antibiotic treatment for another infectious disease, unreported successful eradication, or H. pylori that spontaneously disappeared [23]. Therefore, in our H. pylori-negative group, although we tried to exclude past infection based on patient history, it seems that patients with past infection were not completely excluded. As shown in Supplementary Table 2, the group without atrophy had endoscopic findings similar to those of treatment-naïve patients, and the group with atrophy was similar to patients with past infection [7]. Accordingly, further studies are needed to distinguish between patients with past infection and naïve patients by performing pepsinogen tests.
Our scoring system is based on the presence of RAC, diffuse or spotty redness, and nodularity. In a previous study on the diagnosis of H. pylori gastritis, endoscopists with less than 2 years of experience were able to properly diagnose H. pylori gastritis based on the presence of RAC [11]. In some cases, since diffuse redness and spotty redness can be difficult to distinguish endoscopically, we addressed this ambiguity by combining them into one factor. Previous studies have shown good intra-observer variability when using nodularity and redness to diagnose H. pylori gastritis [13,14]. As we used endoscopic features in our scoring system, which are relatively easy to evaluate, we believe that our system can be easily used by inexperienced endoscopists.
In our scoring system, the risk estimates for patients with total scores of 2 and 3 were 0.963 and 0.998, respectively. Although the increase in risk estimates from 0.963 to 0.998 for risk scores 2 and 3 may seem like a small difference in absolute numbers, it may be a meaningful increase in the diagnosis of H. pylori gastritis. However, since the patients in our study were screened for definite H. pylori positivity and negativity, the result may be attributed to the small sample size of the patients. Therefore, external validation of the scoring system with a larger population is needed in the future.
Previously, Kato et al. [24] reported lower-grade diffuse redness among patients with successful eradication treatment. Some studies suggest that hydrophobic mucosal layer interactions and biofilm formation due to H. pylori infection may interfere with eradication treatment. For example, H. pylori localization and colonization occur beneath the mucosal layer, potentially reducing therapeutic effects and removing drugs [25,26]. In our study, diffuse redness or sticky mucus was significantly more common in the eradication failure group than in the eradication success group. This outcome may be attributed to sticky mucus preventing antibiotics from being directly delivered to H. pylori. Therefore, if diffuse redness or sticky mucus is evident, a culture study may be recommended so that antibiotic susceptibility results may be considered during treatment.
Our study has several limitations. First, there may be inter-observer variability as two endoscopists (JYA and JYS) evaluated the endoscopic findings. However, the endoscopists had more than 15 years of experience with 10 or more daily upper endoscopies and were well-trained to describe H. pylori gastritis findings objectively. Second, the initial eradication treatment was heterogeneous based on antibiotic susceptibility and resistance results. Third, as a pepsinogen test to distinguish between treatment-naïve patients and patients with past infection was not performed, H. pylori-negative patients were presumed to be a mix of treatment-naïve patients and patients with past infection. Fourth, fewer H. pylori-negative patients than H. pylori-positive patients were enrolled. Nevertheless, we selected H. pylori-positive and -negative patients definitively by multiple modalities. Fifth, because we recruited patients who underwent infection testing, there may be a possibility of selection bias. Patients who underwent H. pylori testing might have certain characteristics or symptoms that led to the test, which could influence the endoscopic features observed. Therefore, to strengthen the validity of the scoring system, it would be beneficial to conduct prospective studies involving a broader and more diverse patient population with external validation.
In conclusion, we developed a scoring system based on endoscopic morphologic features to predict H. pylori infection, which performed well in the internal validation test. Considering that diffuse redness and sticky mucus might be associated with initial eradication treatment failure, performing H. pylori culture with antibiotic susceptibility testing may be more efficient. No endoscopic features were found to affect culture growth. H. pylori testing should be performed when spotty redness, diffuse redness, nodularity, and RAC are notably absent from endoscopic findings.
Notes
CRedit authorship contributions
Jun-young Seo: investigation, data curation, formal analysis, writing - original draft, writing - review & editing; Ji Yong Ahn: conceptualization, methodology, writing - review & editing, supervision; Seonok Kim: formal analysis; Hee Kyong Na: writing - review & editing; Jeong Hoon Lee: writing - review & editing; Kee Wook Jung: supervision; Do Hoon Kim: supervision; Kee Don Choi: supervision; Ho June Song: supervision; Gin Hyug Lee: supervision; Hwoon-Yong Jung: supervision
Figure 1
Flowchart of patients tested for H. pylori infection. H. pylori, Helicobacter pylori; MALToma, mucosa-associated lymphoid
Figure 2
Endoscopic images showing the presence of (A) regular arrangement of collecting venules, (B) spotty redness, (C) diffuse redness, and (D) nodularity.
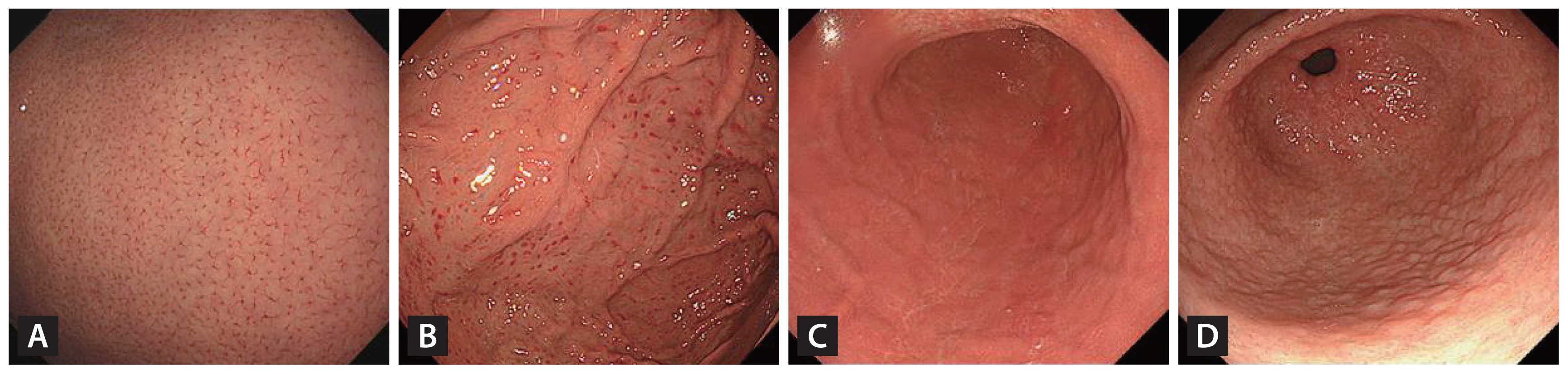
Table 1
Endoscopic features in H. pylori infection diagnosed from the rapid urease test, urea breath test, or culture
Table 2
H. pylori infection risk estimates according to the development and validation set scores
REFERENCES
1. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection--the Maastricht IV/Florence Consensus Report. Gut 2012;61:646–664.


2. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 2017;153:420–429.


3. Uemura N, Okamoto S, Yamamoto S, et al.
Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001;345:784–789.


4. Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology 1997;113:1983–1991.


5. Kamada T, Hata J, Sugiu K, et al. Clinical features of gastric cancer discovered after successful eradication of Helicobacter pylori: results from a 9-year prospective follow-up study in Japan. Aliment Pharmacol Ther 2005;21:1121–1126.


6. Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 2008;372:392–397.


7. Haruma K, Kato M, Inoue K, Murakami K, Kamada T. Kyoto classification of gastritis. Tokyo: Nihon Medical Center, 2017.
8. Kim DB, Chung WC. Accuracy of endoscopic diagnosis of mild atrophic gastritis with Helicobacter pylori infection. Clin Endosc 2018;51:310–312.




9. Alaboudy A, Elbahrawy A, Matsumoto S, Galal GM, Chiba T. Regular arrangement of collecting venules: does patient age affect its accuracy? World J Gastrointest Endosc 2011;3:118–123.



10. Garcés-Durán R, García-Rodríguez A, Córdova H, et al. Association between a regular arrangement of collecting venules and absence of Helicobacter pylori infection in a European population. Gastrointest Endosc 2019;90:461–466.


11. Watanabe K, Nagata N, Shimbo T, et al. Accuracy of endoscopic diagnosis of Helicobacter pylori infection according to level of endoscopic experience and the effect of training. BMC Gastroenterol 2013;13:128.




12. Garcés-Durán R, Galdín-Ferreyra M, Delgado-Guillena PG, et al. Diagnosis of Helicobacter pylori infection by the arrangement of collecting venules using white light endoscopy: evaluation of interobserver agreement. Dig Dis 2022;40:376–384.



13. Chen MJ, Wang TE, Chang WH, Liao TC, Lin CC, Shih SC. Nodular gastritis: an endoscopic indicator of Helicobacter pylori infection. Dig Dis Sci 2007;52:2662–2666.



14. Kim OZ, Rhee KH, Oh H, et al. Prediction of Helicobacter pylori infection by endoscopic severity of erythematous/exudative gastritis in asymptomatic adults. Korean J Gastroenterol 2022;80:135–141Korean.
15. Hojo M, Nagahara A, Kudo T, et al. Endoscopic findings of Helicobacter pylori gastritis in children and young adults based on the Kyoto classification of gastritis and age-associated changes. JGH Open 2021;5:1197–1202.




16. Yuan C, Lin XM, Ou Y, et al. Association between regular arrangement of collecting venules and Helicobacter pylori status in routine endoscopy. BMC Gastroenterol 2021;21:389.




17. Appelman HD. Gastritis: terminology, etiology, and clinicopathological correlations: another biased view. Hum Pathol 1994;25:1006–1019.


18. Kaiserling E, Heinle H, Itabe H, Takano T, Remmele W. Lipid islands in human gastric mucosa: morphological and immunohistochemical findings. Gastroenterology 1996;110:369–374.


19. Kitamura S, Muguruma N, Okamoto K, et al. Clinicopathological assessment of gastric xanthoma as potential predictive marker of gastric cancer. Digestion 2017;96:199–206.



20. Isomoto H, Mizuta Y, Inoue K, et al. A close relationship between Helicobacter pylori infection and gastric xanthoma. Scand J Gastroenterol 1999;34:346–352.


21. Yi SY. Dyslipidemia and H pylori in gastric xanthomatosis. World J Gastroenterol 2007;13:4598–4601.



22. Toyoshima O, Nishizawa T, Arita M, et al.
Helicobacter pylori infection in subjects negative for high titer serum antibody. World J Gastroenterol 2018;24:1419–1428.



23. Kishikawa H, Ojiro K, Nakamura K, et al. Previous Helicobacter pylori infection-induced atrophic gastritis: a distinct disease entity in an understudied population without a history of eradication. Helicobacter 2020;25:e12669.




24. Kato T, Yagi N, Kamada T, Shimbo T, Watanabe H, Ida K. Diagnosis of Helicobacter pylori infection in gastric mucosa by endoscopic features: a multicenter prospective study. Dig Endosc 2013;25:508–518.

- TOOLS
-
METRICS

- Related articles
-
Can Helicobacter pylori eradication affect long-term mortality?2021 May;36(3)





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print


