INTRODUCTION
Systemic sclerosis (SSc) is an autoimmune systemic connective tissue disease that can induce progressive fibrosis and vasculopathy affecting not only the skin but also major internal organs, leading to interstitial lung disease (ILD), cardiac diseases, renal crisis, gastrointestinal diseases, systemic calcinosis, digital vasculopathy, and pulmonary arterial hypertension [
1]. Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a small vessel vasculitis that primarily affects capillaries, arterioles, and venules, and may occasionally involve medium arteries. AAV is categorized into three subtypes according to clinical, laboratory, radiological, and histological features: microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA) [
2,
3].
Several previous studies support the presence of overlap syndrome (OS) of SSc and AAV (SSc-AAV-OS) in SSc patients. First, ANCA-associated glomerulonephritis was found in some SSc patients with renal crisis [
4,
5]. Second, ANCA positivity was found to be more closely related to ILD in SSc patients, regardless of AAV classification [
6]. Third, proteinase 3 (PR3)-ANCA was shown to be associated with neuropathy in SSc patients, although it was not classified as having SSc-AAV-OS [
7]. In addition, our previous study on ANCA positivity in Korean patients with SSc revealed that the detection rate of ANCA was 20.3%, and 1.7% of study subjects could finally be reclassified as having SSc-AAV-OS [
8]. In the previous study, AAV was classified according to the algorithm for AAV and polyarteritis proposed by the European Medicines Agency in 2007 (the 2007 EMA algorithm) and the 2012 revised International Chapel Hill consensus conference nomenclature of vasculitides (the 2012 CHCC definitions) [
2,
3].
Recently, a joint group of the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) proposed new classification criteria for AAV (the 2022 ACR/EULAR criteria or the new criteria) for MPA, GPA, and EGPA. These criteria can be applied only to patients with evidence of small- or medium-vessel vasculitis, which is one of two mandatory requirements [
9ŌĆō
11]. Therefore, it may be clinically significant to investigate the frequency of SSc-AAV-OS according to these new criteria; however, no study thereof has been reported to date. Hence, in this study, we selected SSc patients who exhibited signs suggestive of small- or medium-vessel vasculitis at SSc diagnosis from among the study subjects in our previous study [
8]. We applied the 2022 ACR/EULAR criteria for AAV to selected SSc patients, investigated the frequency of SSc-AAV-OS, and compared it between the new and previous criteria.
DISCUSSION
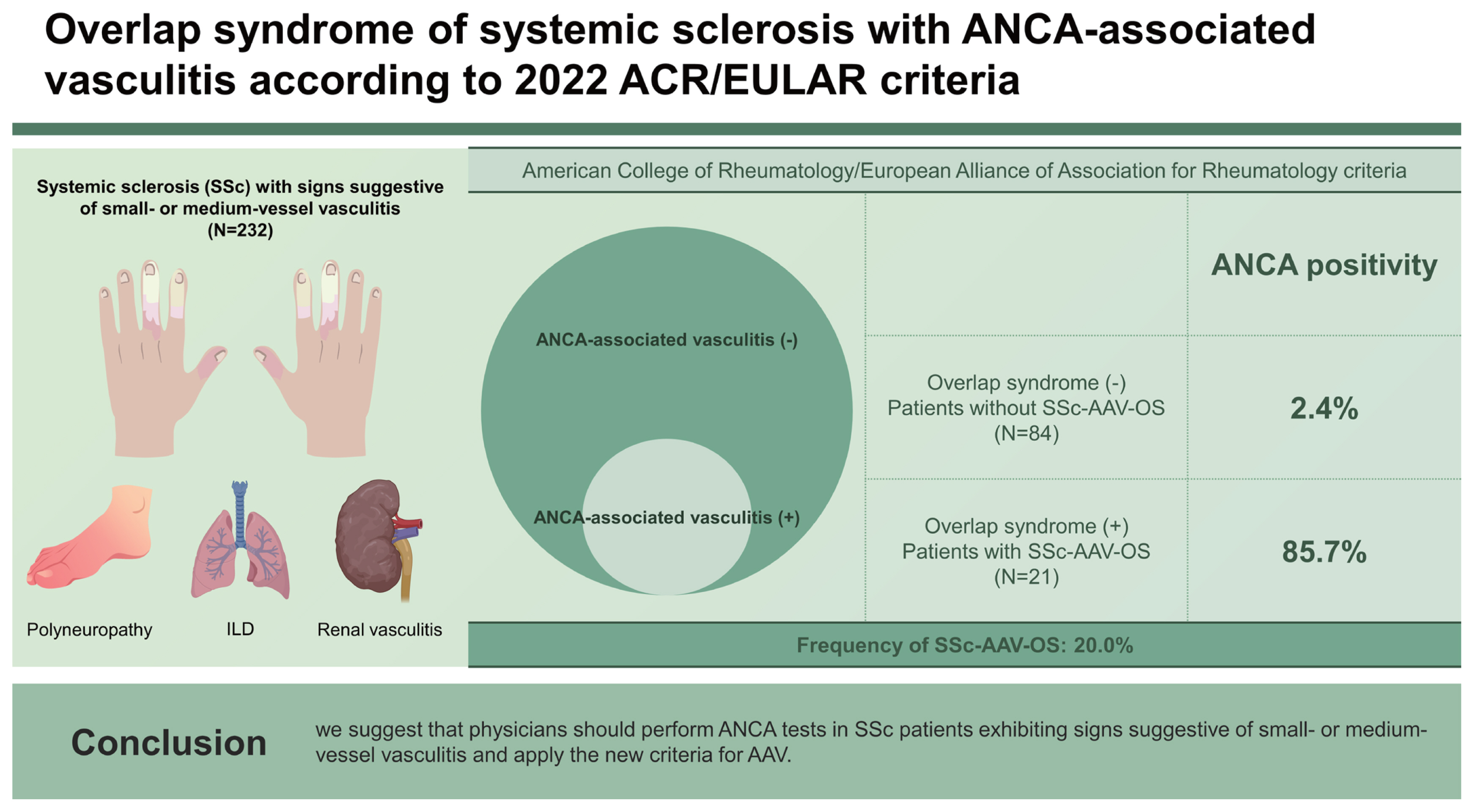
This study investigated the frequency of patients who could be reclassified as having SSc-AAV-OS according to the 2022 ACR/EULAR criteria for AAV among SSc patients with signs suggestive of small- or medium-vessel vasculitis at diagnosis. Among the 105 SSc patients, 14 (13.3%), 5 (4.7%), and 2 (1.9%) were reclassified as having SSc-MPA-OS, SSc-GPA-OS, and SSc-EGPA-OS, respectively. Therefore, we revealed that the overall frequency of SSc-AAV-OS was 20.0%, which was much higher than 1.7% reported for previous criteria for AAV among the SSc patients included in our previous study [
8]. In addition, we found that ANCA positivity contributed to the reclassification of SSc-AAV-OS more than ANCA negativity in SSc patients with signs suggestive of small- or medium-vessel vasculitis. Therefore, we concluded that at the time of SSc diagnosis, approximately a fifth of SSc patients exhibiting ILD, peripheral neuropathy, or suspected renal vasculitis could be reclassified as having SSc-AAV-OS, and MPA was the most common subtype of AAV.
Regarding the mechanism of SSc-AAV-OS among SSc patients, we propose two hypotheses. One possible mechanism is the occurrence of ANCA-induced SSc-AAV-OS. It can be assumed that the inflammatory process of SSc may prime neutrophils and produce circulating pathogenic ANCA, which may activate primed neutrophils and develop AAV [
19,
20]. A previous study reporting that ANCA was detected in up to 12% of SSc patients can support our hypothesis [
21]. The other possible mechanism is vasculopathy-induced SSc-AAV-OS occurrence, regardless of ANCA positivity, because endothelial dysregulation and its-related vasculopathy play important roles in the pathogenesis of both SSc and AAV. As a result, SSc and AAV may share various clinical manifestations, such as ILD, digital vasculitis, or kidney involvement [
1]. It can be challenging to distinguish obstructive vasculopathy, a prominent feature of SSc, from necrotizing vasculitis, a feature of AAV, especially in early stages, and these pathological findings can coexist, leading to SSc-AAV-OS [
20,
22]. This study included only SSc patients with signs suggestive of small- and medium-vessel vasculitis at diagnosis, which may explain the relatively high frequency of SSc-AAV-OS, compared to previous studies. This finding may also emphasize the necessity of ANCA testing in patients with signs suggestive of vasculitis.
ILD, peripheral neuropathy, and suspected renal vasculitis were defined as signs suggestive of small- or medium-vessel vasculitis in this study. ILD can occur in patients with SSc and in patients with AAV. Both usual interstitial pneumonia and nonspecific interstitial pneumonia patterns are observed in AAV-related ILD, and similar patterns are also observed in SSc-related ILD [
23,
24]. The differentiation between SSc-related ILD and AAV-related ILD is difficult by imaging alone. Differentiation between SSc renal crisis and ANCA-associated glomerulonephritis can be challenging in patients with progressive acute renal failure [
5]. In a retrospective study, it is reasonable to confirm the involvement of the lungs, kidneys, and peripheral nervous system to find a sign of vasculitis.
In this study, the frequency of SSc-AAV-OS was 20.0%, which is significantly higher than the 1.7% frequency reported in our previous study. There are two possible explanations for this finding. First, the patient population in this study was more narrowly defined. In the previous study, all patients with SSc who underwent ANCA tests were included, while in this study, only patients with SSc who had vasculitis signs in the lungs, kidneys, and peripheral nervous system were included. This narrower patient population may have resulted in a higher proportion of patients classified as overlapping. Second, the diagnostic criteria for AAV were different in the two studies. In the previous study, AAV was classified using the 2007 EMA algorithm, while in this study, AAV was classified using the 2022 ACR/EULAR criteria. The 2022 ACR/EULAR criteria for AAV assign a higher score to ANCA positivity, resulting in an increased share of ANCA positive patients being diagnosed with AAV. In a previous study, 3 of 36 (8.3%) ANCA positive patients with SSc were classified as SSc-AAV-OS. In this study, 18 out of 20 (90.0%) ANCA positive patients with SSc and vasculitis features were classified as SSc-AAV-OS. Additionally, the inclusion of ILD in the 2022 ACR/EULAR criteria may have resulted in a higher number of patients classified as overlapping SD of SSc and AAV.
In this study, 105 SSc patients with the signs suggestive of small- or medium-vessel vasculitis were included. Five patients had all three signs, and 26 patients had two or more signs (
Supplementary Fig. 1). Of the 5 patients with all three signs, two (40.0%) were reclassified as having SSc-AAV-OS, and six (23.1%) of 26 patients with two or more signs were reclassified as having SSc-AAV-OS. Among the 79 patients with only one sign, 15 (19.0%) were reclassified as having SSc-AAV-OS. Although the proportion of ANCA positivity appeared to provide a greater contribution to the reclassification, as the number of signs suggestive of small- or medium-vessel vasculitis in SSc patients increased, the rate of classification as AAV tended to increase. However, statistical significance was difficult to confirm because of the small number of patients.
In this study, two patients with ANCA positivity could not be reclassified as having SSc-AAV-OS. The presence of serum eosinophilia precluded classification as MPA or GPA. Furthermore, the absence of other characteristic features of EGPA, such as obstructive airway disease and nasal polyps, hindered its classification as EGPA (
Supplementary Table 1). Conversely, three patients without ANCA could be reclassified as having SSc-AAV-OS. Patient 3 exhibited pulmonary lesions indicative of GPA, confirmed granuloma on biopsy, and paranasal sinusitis on imaging, despite testing negative for ANCA. Thus, this patient could be appropriately classified as GPA. Patient 4 presented with obstructive airway disease and serum eosinophilia, aligning with the characteristic features of EGPA. Therefore, this patient could be classified as EGPA. Patient 5 displayed mononeuritis multiplex, serum eosinophilia, and biopsy findings showing extravascular eosinophilic-predominant inflammation. These clinical manifestations support the classification of this patient as EGPA (
Supplementary Table 2).
At the start of this study, three patients who were previously diagnosed with SSc-AAV-OS according to the 2007 EMA algorithm and the 2012 CHCC definitions were excluded from this study. We wondered which subtype of AAV they would be reclassified as according to the new criteria for AAV. All three patients presented with ILD or glomerulonephritis, indicating the presence of small- or medium-vessel vasculitis features. When the 2022 ACR/EULAR criteria for MPA were applied [
9], patient A received +6, +3, and +3 points for the items of MPO-ANCA (or P-ANCA) positivity, ILD on imaging, and pauci-immune glomerulonephritis on biopsy, respectively. Patient A achieved a total score of 12, and thus, could be reclassified as having SSc-MPA-OS. On the other hand, patient B received +6, and +3 points for the items of MPO-ANCA (or P-ANCA) positivity and ILD on imaging. Patient B achieved the total score of 9 and thus could also be reclassified as having SSc-MPA-OS. When the 2022 ACR/EULAR criteria for GPA were applied [
10], patient C received +5, +2, +2, and +1 points based on the items of PR3-ANCA (or C-ANCA) positivity, pulmonary lesions suggesting GPA, granuloma on biopsy, and paranasal sinusitis; however, patient C received ŌłÆ4 points for the item of serum eosinophilia. Finally, patient C achieved a total score of 6, and thus could also be reclassified as having SSc-GPA-OS. In contrast, when the 2022 ACR/EULAR criteria for EGPA were applied [
11], none met the criteria (
Supplementary Table 3).
Interestingly, 21 SSc patients who had not been classified as having SSc-AAV-OS according to the 2007 EMA algorithm modified by the 2012 CHCC definitions in our previous study [
2,
3,
8] met the 2022 ACR/EULAR criteria for AAV and could be reclassified as having SSc-AAV-OS. We analyzed how they were newly reclassified according to AAV subtypes and signs suggestive of small- or medium-vessel vasculitis. Among the 14 patients newly diagnosed with SSc-MPA-OS, 13 patients achieved a total score of 9 points due to MPO-ANCA (or P-ANCA) positivity (+3) and ILD (+3). One patient with mononeuritis multiplex was classified as having SSc-AAV-OS by the item of MPO-ANCA (or P-ANCA) positivity alone. According to the 2007 EMA algorithm modified by the 2012 CHCC definitions, 13 patients with ILD and one with mononeuritis multiplex could not be reclassified as having MPA.
Among the five patients newly diagnosed with SSc-GPA-OS, four patients with ILD achieved the total score of 5 points for the item of PR3-ANCA (or C-ANCA) positivity alone. Whereas, one patient, who exhibited ILD but did not have PR3-ANCA (or C-ANCA), could be reclassified as having SSc-GPA-OS by granuloma (+2), paranasal sinusitis (+1), and lung nodule (+2). In particular, according to the 2007 EMA algorithm modified by the 2012 CHCC definitions, the last patient could not be reclassified as having GPA. This was because previous studies have delineated the histological features of GPA as necrotizing granulomatous inflammation, necrotizing vasculitis, and necrotizing glomerulonephritis, but not as granuloma alone. Furthermore, the patient showed a GPA surrogate marker of lung nodules but did not show ANCA positivity. Among the two patients newly diagnosed with SSc-EGPA-OS, one patient achieved a total score of +8 points with the items of asthma (+3) and serum eosinophilia (+5), and the other patient achieved the total score of +6 points with the items of serum eosinophilia (+5) and mononeuritis multiplex (+1). Therefore, they could be reclassified as having EGPA. However, they did not satisfy the 2007 EMA algorithm modified by the 2012 CHCC definitions for EGPA. Therefore, it was concluded that the 2022 ACR/EULAR criteria are more sensitive than previous criteria in discovering AAV in SSc patients (
Supplementary Table 4).
Although SSc-AAV-OS is a rare condition, its diagnosis is crucial due to potential variations in patient treatment. For instance, a patient presenting with both SSc and progressive acute kidney injury may be due to SSc renal crisis, but if it is ANCA-associated glomerulonephritis, the treatment approach would differ. Additionally, if healthcare providers do not suspect AAV in such cases, there is a high likelihood that they may overlook additional tests like ANCA testing and renal biopsy, leading to challenges in early diagnosis and treatment. Our study found no statistically significant difference between SSc-AAV-OS and non-AAV groups. However, upon examining the cumulative steroid dosage administered during the 1-year period before the last visit, we observed that the SSc-AAV-OS group utilized 1,650.0 mg of prednisolone, which is approximately 1.96 times higher than the 840.0 mg used by the non-AAV group. Hence, further research with a larger patient cohort is necessary to confirm the clinical distinctions of the SSc-AAV-OS group.
This study has several advantages, given that the OS of AAV and connective tissue diseases may occur at a low frequency. First, this study applied the 2022 ACR/EULAR criteria for AAV to SSc patients and investigated the frequency of SSc-AAV-OS for the first time. Second, this study strictly included only patients carrying signs suggestive of small- or medium-vessel vasculitis. Third, this study performed subgroup analyses according to each AAV subtype using the new criteria. Fourth, this study compared the sensitivity of the new and previous criteria to discover SSc-AAV-OS. We believe that this study provides new insights into SSc-AAV-OS. Meanwhile, this study also has several limitations. The number of patients in a single-center study was not large enough to generalize the results and apply them to SSc patients immediately. The retrospective study design could not rule out the possibility of missing data when collecting information which could hinder accurately predicting poor prognosis or medication history. Since this was a retrospective study, there was no opportunity to differentiate ILD or renal vasculitis histologically. The potential for the inclusion of patients with SSc who possessed ANCA test results makes it challenging to rule out the possibility of selection bias in this study. Moreover, owing to these limitations, this study could not suggest management strategies for patients with SSc-AAV-OS. A prospective future study with more SSc patients with signs suggestive of vasculitis will provide more reliable information on SSc-AAV-OS and will help to establish management strategies for patients with SSc-AAV-OS.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement figure 1
Supplement figure 1 Print
Print



