 |
 |
| Korean J Intern Med > Volume 39(1); 2024 > Article |
|
Abstract
Acute lymphoblastic leukemia (ALL) is one of the most rapidly changing hematological malignancies with advanced understanding of the genetic landscape, detection methods of minimal residual disease (MRD), and the development of immunotherapeutic agents with good clinical outcomes. The annual incidence of adult ALL in Korea is 300–350 patients per year. The WHO classification of ALL was revised in 2022 to reflect the molecular cytogenetic features and suggest new adverse-risk subgroups, such as Ph-like ALL and ETP-ALL. We continue to use traditional adverse-risk features and cytogenetics, with MRD-directed post-remission therapy including allogeneic hematopoietic cell transplantation. However, with the introduction of novel agents, such as ponatinib, blinatumomab, and inotuzumab ozogamicin incorporated into frontline therapy, good MRD responses have been achieved, and overall survival outcomes are improving. Accordingly, some clinical trials have suggested a possible era of chemotherapy-free or transplantation-free approaches in the near future. Nevertheless, relapse of refractory ALL still occurs, and some poor ALL subtypes, such as Ph-like ALL and ETP-ALL, are unsolved problems for which novel agents and treatment strategies are needed. In this review, we summarize the currently applied diagnostic and therapeutic practices in the era of advanced genetic analysis and targeted immunotherapies in United States and Europe and introduce real-world Korean data.
The incidence of acute lymphoblastic leukemia (ALL) is very low with a crude annual incidence of about 1.1/100,000 in the entire population (0.2% of all cancers) and 0.8/100,000 in the adult Korean population (Table 1). In the US, 1.5–1.7/100,000 is the incidence in the entire population (0.3% of all cancers), and the American Cancer Society estimates that 6,540 new ALL cases were reported in 2023.
ALL is a hematological malignant neoplasm characterized by abnormal clones with arrest of differentiation and uncontrolled proliferation in the bone marrow and extramedullary sites [1]. Although a clinically relevant genetic profile of adult ALL has not been fully elucidated, understanding the genetic characteristics has improved with advances in polymerase chain reaction (PCR) and next-generation sequencing (NGS) methods [2–5]. These advancements have improved the universal availability of sensitive minimal residual disease (MRD) detection, for treating patients with MRD-directed strategies, and predicting treatment outcomes [6–8]. Disease subgroups are classified in more detail according to the gene expression profile and detection of specific gene deletions and mutations. New subtypes of ALL, such as Ph-like ALL and early T-cell precursor (ETP)-ALL, have been introduced as poor-risk groups with a high MRD level and poor outcomes compared to other ALL subtypes [9–11].
ALL is a pediatric malignant disease. Treatment outcomes have improved tremendously with a 5-year disease free survival (DFS) rate of 85% [12]. However, the survival outcome is still poor in adult ALL, and hyper-CVAD (hyperfractionated cyclophophamide, vincristine, adriamycin, and dexamethasone) and pediatric-inspired regimens are being modified, and many trials have been incorporating novel agents to improve outcomes. The treatment outcomes of Ph-positive ALL have been improved with the introduction of tyrosine kinase inhibitors (TKIs) in 2000, but the 5-year DFS is 50–60% in Korea although the complete remission (CR) rate is > 90%. However, incorporating ponatinib into frontline therapy results in improved survival outcomes almost similar to those of pediatric ALL and has opened the possibility of chemotherapy-free approaches [13]. Treatment of B-cell precursor ALL (B-ALL) has been accelerating since the 2010s with the introduction of blinatumomab, inotuzumab ozogamicin (INO), and anti-CD19 chimeric antigen receptor T-cell (CAR-T) therapies, and the therapeutic paradigm is largely changing. However, treating relapsed or refractory (R/R) T-cell ALL (T-ALL) has been difficult because no specific agents are available with significantly good treatment outcomes [14,15].
Allogeneic hematopoietic cell transplantation (allo-HCT) is standard post-remission therapy for adult patients with high-risk ALL. We must achieve MRD-negative CR for good survival outcomes after allo-HCT, particularly in high-risk ALL and R/R ALL, and novel frontline approaches may avoid all-HCT in a larger number of patients if a good MRD response is achieved.
In this review, we introduce updated diagnostic and treatment guidelines and summarize recent clinical trial data and real-world experience in Korea.
B-ALL results from arrest of B-cell lymphoid differentiation, which undergoes abnormal proliferation and survival of B-precursor-stage cells. B-ALL was traditionally characterized with several recurrent cytogenetic aberrations, such as hyperdiploidy, hypodiploidy, and specific translocations or rearrangements (Table 2) [16]. Among them, the BCR::ABL1 rearrangement originated from Philadelphia chromosome t(9;22)(q34;q11.2), which is the most frequently observed recurrent cytogenetic aberration in adult B-ALL, and this Ph-positive ALL is regarded as a specific disease entity due to its unique TKI-based treatment strategy. Before TKIs were introduced into the treatment protocol, Ph-positive ALL was considered a poor-risk subtype showing 5-year overall survival (OS) of < 10% and a maximum of 20–30% if treated with allo-HCT [17,18]. With the introduction of imatinib in 2000 (since 2001 in Korea) [19–25], dasatinib in 2006 (since 2008 in Korea) [26–28], and ponatinib in 2010 (since 2016 in Korea) [29–32], the remission rates have improved to > 90% and long-term survival outcomes have improved (Fig. 1) [13].
New chromosomal and gene alterations have been identified with recent advances in molecular cytogenetic studies, including conventional G banding, fluorescence in situ hybridization, PCR, and NGS methods. Based on cytogenetic studies and gene expression profile analysis, Mullighan et al. [10] discovered a disease subgroup with a similar gene expression profile to Ph-positive ALL without the BCR::ABL1 rearrangement. They named the subgroup BCR::ABL1-like or Ph-like ALL. This subgroup was identified as a high-risk B-ALL subtype with a poor survival outcome and a high MRD level after chemotherapy [10,11]. This new category has a heterogeneous genetic background, and the major genetic characteristics include CRLF2 rearrangements and mutations, ABL-class rearrangements, JAK2 or EPOR rearrangements, mutations activating JAK-STAT signaling and RAS signaling, and uncommon kinase alterations [33–36]. Some genetic abnormalities are therapeutic targets of TKIs, such as dasatinib or ruxolitinib [36–38].
The Catholic Hematology Hospital in Korea reported that Ph-like ALL was observed in 16.6% of 344 patients with B-ALL, who had a higher 5-year OS (60.6 vs. 27.1%; p = 0.008) than the poor-risk Ph-negative ALL subgroup, while no difference was observed with the standard-risk ALL subgroup when patients were treated with hyper-CVAD based remission induction therapy followed by allo-HCT based post-remission therapy (Fig. 2A) [39].
T-ALL is an aggressive acute leukemia derived from T-lymphocyte progenitors which accounts for 15–25% of all cases of ALL [40–44]. Although the epidemiology and characteristics of T-ALL are not well known in adults due to low incidence, it usually presents with a high white blood cell (WBC) count, mediastinal mass, and frequent extramedullary involvement, including central nervous system (CNS) leukemia [44,45]. Among T-ALL cases, Coustan-Smith et al. discovered ETP-ALL as a distinct biologic subtype of T-ALL that comprises up to 15% of children and young adults with T-ALL and is associated with poor clinical outcomes [46–48]. This subtype is characterized by the absence of CD1a/CD8, weak CD5 expression, and the presence of one or more stem cell or myeloid markers [46]. Recent data from a larger cohort of children treated with MRD response-based strategies suggests that the survival outcome of ETP-ALL is not significantly inferior to that of non-ETP-ALL [49], but conflicting data have been reported in adults [9,50,51].
Recent Korean data from the Catholic Hematology Hospital revealed that the frequency of ETP-ALL is 36.2% in 58 adults with T-ALL, which may be a relatively larger proportion compared to previous pediatric data or Western adult data [9,46]. The Korean data showed similar remission and allo-HCT proceeding rates for ETP-ALL compared to non-ETP-ALL, although many patients relapsed before allo-HCT. Subsequent 5-year OS was poor in ETP-ALL (33.3%) and non-ETP-ALL (29.5%), but patients who were treated with allo-HCT showed acceptable 5-year OS (ETP-ALL, 53.8% vs. non-ETP-ALL, 55.4% in Fig. 2B). This report suggested poor outcomes of both T-ALL subgroups when treated with myeloid-specific hyper-CVAD, which consisted of mitoxantrone plus high dose cytarabine for even cycles [28,52], but patients treated with allo-HCT had acceptable treatment outcomes. Thus, the Korean data recommend modified hyper-CVAD combined with novel agents or asparaginase-containing pediatric-inspired regimens, and allo-HCT has a role for long-term remission and good survival outcomes in cases of T-ALL [53].
ALL is classified according to the molecular cytogenetic profile at diagnosis. B-ALL is divided into three subgroups of Ph-positive ALL, Ph-like ALL, and other Ph-negative ALL. T-ALL is divided into two subgroups of ETP-ALL and non-ETP-ALL, which has various cytogenetic abnormalities with different prognostic values. Adverse-risk cytogenetic features have been suggested in various guidelines for other Ph-negative ALL and non-ETP-ALL, and some were regarding transplantation outcomes. A complex karyotype with three or more karyotype abnormalities, low hypodiploidy, and an MLL rearrangement, particularly t(4;11), monosomy 7, or TCF3::HLF with t(17;19) are adverse-risk cytogenetics [54]. The Catholic Hematology Hospital in Korea presented data in International Congress of the Korean Society of Hematology (ICKSH) 2022 and ICKSH2023 suggesting that the complex karyotype (≥ 3) and low hypodiploidy are related with monosomal karyotype, t(4;11), and chromosome 7 abnormalities (−7, del 7) are adverse-risk groups with poor survival outcomes even after allo-HCT (Fig. 2A). Thus, Ph-positive ALL, Ph-like ALL, ETP-ALL, and other ALL with adverse-risk cytogenetics are a high-risk group. Therefore, allo-HCT is the standard treatment for post-remission therapy and better long-term survival outcomes. The transplantation-related mortality rate has decreased and improved survival outcomes have been observed with advances in several HCT techniques and novel prophylactic agents (Fig. 2C, D). Ph-positive ALL was diagnosed in 35–45% of cases, Ph-like ALL in 10–15%, Ph-negative ALL in 30–35%, ETP-ALL in 5%, and non-ETP-ALL in 10% at the Catholic Hematology Hospital in Korea.
Recent advances in NGS methods allowing for whole genome sequencing and whole exome sequencing have provided more comprehensive gene mutation information related to the development and resistance mechanism of ALL characterized by the sequential acquisition of genetic aberrations that drive initiation and maintenance of the leukemic clone [2]. Several genetic changes involved in ALL pathogenesis have been identified, including those involved in lymphoid differentiation (PAX5, IKZF1, EBF1, and LEF1), cell cycle regulation (CDKN2A, CDKN2B, RB1, and TP53), proliferation and cell survival (KRAS, NRAS, JAK2, FLT3, and CRLF2), lymphoid signaling (BTLA and CD200 TOX), transcriptional regulation (TAL1, RUNX1, HEB, GATA3, and E2A), and coactivation (TBL1XR1 and ERG), as well as regulators of chromatin structure and epigenetic regulators (CTCF and CREBBP) [3,4]. Among these abnormalities, deletions in the IKZF1 gene, which encodes the lymphoid transcription factor Ikaros, have received the most attention due to their frequency in B-ALL, particularly in Ph-positive ALL [55].
The Catholic Hematology Hospital in Korea analyzed deletions in the IKZF1 gene using multiplex ligation-dependent probe amplification, which revealed the IKZF1 deletion in 78.8% of 118 patients with Ph-positive ALL. These data indicate a higher relapse rate in Ph-positive ALL with the biallelic IKZF1 deletion [56]. It is now known that a concurrent deletion in IKZF1 and at least one additional deletion in the CDKN2A, CDKN2B, PAX5, and PAR1 region in the absence of the ERG deletion is the IKZF1-plus group which is more significantly related with poor survival outcomes in Ph-positive and Ph-negative ALL [57]. As an independent prognostic genetic marker, CDKN2A and CDKN2B deletions are a poor-risk factor for survival outcome in adult and pediatric ALL [58]. By combination of copy number alterations and gene mutation, recent Korean data from the Catholic Hematology Hospital showed that if the IKZF1, CDKN2A or CDKN2B deletion, or the TP53 mutation are combined, significantly high relapse rates and poor survival outcomes are observed in Ph-positive and Ph-negative ALL (Fig. 3) [59].
MRD is the most relevant prognostic marker for clinical outcomes in patients with hematological malignancy and ALL is a representative disease that has been the focus of the MRD-based treatment approach [8,60–62]. Several PCR-based and multiparametric flow cytometric (MFC) methods have been developed to detect residual malignancies in the past 30 years [63]. The BCR::ABL1 transcript was used for MRD monitoring of real-time quantitative PCR in patients with Ph-positive ALL, and the MRD response showed its efficacy to predict survival outcomes [64]. Trials have been conducted to treat patients without allo-HCT if the patients achieved a negative MRD result after initial chemotherapy, and some trials have indicated the possibility of a transplantation-free approach for patients with Ph-positive ALL [65,66].
Several Korean data from the Catholic Hematology Hospital have also revealed the role of MRD in Ph-positive ALL, but most of these data were associated with patients treated with allo-HCT. A poor MRD response was observed in 18.9% of cases after the imatinib trial and in 22.4% after the dasatinib trial before transplantation, revealing significantly higher relapse rates and poorer survival outcomes (Fig. 4A) [21–23,28]. Detecting MRD has become more important to reduce post-remission therapy toxicity and predict survival outcome with the introduction of next-generation TKIs and blinatumomab [67,68]. Foà et al. [68] reported that dasatinib plus a steroid followed by blinatumomab provided long-term disease control in patients achieving a complete molecular response (CMR). Up to 50% of patients were treated with allo-HCT in that study [68,69]. The combination of ponatinib and blinatumomab was evaluated in a recent clinical trial at MD Anderson Cancer Center (MDACC). Frontline ponatinib plus blinatumomab showed 87% CMR and a 96% 3-year OS for newly diagnosed patients with Ph-positive ALL (Fig. 1A). Only 1 of 40 enrolled patients underwent allo-HCT in this trial [70].
In cases other than ALL with specific fusion genes such as Ph-positive ALL, MRD monitoring has been used to detect tumor-specific immunoglobulin or T-cell receptor gene rearrangement since the 1980s [71–73] and the target variability and sensitivity were improved by PCR techniques [74–78]. At present, PCR-based high throughput sequencing (HTS) provides up to 10−6 sensitivity depending on the amount of DNA and target variability higher than 95% [6–8]. In parallel to the PCR-based method, flow cytometry was developed as a less labor-intensive, rapid multicolored approach [79–82]. With advances in flow cytometry and a more than 8-color antibody panel, MFC analysis provides up to 10−5 sensitivity [83]. Thus, a considerable proportion of MRD-positive patients by the HTS method might be MRD negative according to the MFC method [84]. MRD-based treatment guidelines are more precisely suggested in Ph-negative ALL, which recommends repeated multiagent chemotherapy without allo-HCT if a patient achieves negative MRD after remission and has no high-risk features at diagnosis [85].
The MRD time points and the cut-off for MRD negativity according to the method remain controversial. Because there are several protocols for inducing remission, the time points can vary according to the chemotherapy schedule. MDACC data show that a post 2 or 3 cycles of chemotherapy (10-week) MRD level > 10−4 is associated with a higher relapse rate [86], and Italian and German groups showed that the MRD level > 10−4 after 16–22 weeks was associated with a poor DFS [65,87]. A Spanish group used a cut-off of > 5 × 10−4 at 16 weeks after treatment [88]. In Korea, NGS-based MRD detection became available in 2021 and MFC-based MRD detection in 2022 (Table 3). Recent NGS-MRD data from the Catholic Hematology Hospital showed that the first MRD check point was 6–8 weeks and a MRD level > 10−3 was observed in 42.9% of cases. The second check point was 10–12 weeks and a MRD level > 10−3 was observed in 18.4% of cases indicating a higher relapse rate and poorer OS after allo-HCT (Fig. 4B).
Several modifications of the hyper-CVAD based regimen, the mainstream of frontline therapy for adult patients with ALL, and pediatric-inspired regimens have been attempted by many cancer centers in many countries. Hyper-CVAD was introduced in 1992 by MDACC which showed a 91% CR rate, 6% NRM rate, and a 39% 5-year OS, significant improvements compared to that of the previous VAD regimen [89–91]. Several drugs were added to augmented hyper-CVAD to achieve better clinical outcomes for particular ALL subgroups with specific features. Nelarabine was added for T-ALL [92,93], rituximab or ofatumumab was added for Burkitt-type with high expression of CD20 [94–96], and TKIs were added to Ph-positive ALL as discussed above [19–30,70], all of which significantly improved the survival rate for over 40 years.
Pediatric-inspired regimen was first spotlighted by Cancer and Leukemia Group B, which analyzed the outcomes of adolescents aged 16–20 years old who were treated with either an adult regimen or a pediatric-inspired augmented BFM regimen by the Children’s Cancer Group [97]. The adolescent and young adult group revealed transitional characteristics with a sudden decrease in survival rate, which made researchers wonder whether a chemotherapy regimen might benefit this age group [98]. The augmented BFM regimen and other pediatric-inspired regimens are characterized by a less myelotoxic nature reinforced with higher doses of steroid, vincristine, and L-asparaginase, which showed significantly better survival outcomes in adolescents and young adults [99–102].
The two major types of regimens have been used in Korea with several modifications. The Catholic Hematology Hospital uses the modified hyper-CVAD, which is a myeloid-specific modified type consisting of mitoxantrone plus high dose cytarabine for even cycles [28,52]. As a pediatric-inspired regimen, the Korean ALL Working Group conducted the KALLA1406/1407 study, which reported a significantly better treatment outcome in patients < 40 years old [103]. Recent data suggest that rituximab has a significant beneficial effect in patients with high CD20 expression when treated with the pediatric-inspired regimen described above [104]. For T-ALL, myeloid-specific hyper-CVAD showed poor induction of remission and maintenance even in cases of ETP-ALL, suggesting that the pediatric-inspired regimen might be better for T-ALL [39,53]. Regardless of the induction regimen, imatinib has been the only available TKI in Korea for Ph-positive ALL. Dasatinib and ponatinib are available when a patient becomes resistant or cannot tolerate imatinib-based therapy. Dasatinib plus modified hyper-CVAD [28] and nilotinib plus pediatric-inspired regimen [105] have been used for clinical trials in Korea, but the results showed no significant benefit compared to previous imatinib data.
Recent clinical trials have focused on reducing toxicity or chemo-free remission induction therapy by incorporating effective TKIs and/or monoclonal antibodies that have a proven significant effect for R/R ALL. The most focused agents are blinatumomab and INO for patients with B-ALL, and dasatinib or ponatinib for patients with Ph-positive ALL. Incorporating these drugs into traditional chemotherapy protocols made it possible to reduce the potential myelotoxic adverse events.
Reduced toxicity mini-hyper-CVD combined with INO was first tried for elderly patients with Ph-negative ALL in 2011 [106], which is now followed by sequential blinatumomab consolidation in MDACC trials [107–109]. Early elderly data indicate no early deaths within 4 weeks, and the overall response was 98% with 96% MRD-negativity. All of the TKIs including ponatinib showed poor survival outcomes after relapse of Ph-positive ALL [110,111]. Novel TKIs have been tried in frontline therapy and ponatinib was combined with hyper-CVAD, which showed significantly good MRD response and improved long-term survival outcomes. It also showed the possibility of a transplantation-free approach in MRD responders with good survival outcomes, but ponatinib-related toxicity was addressed in that study [30–32]. Accordingly, subsequent trials used a 30 mg dose of ponatinib for frontline therapy [70,112–114]. The first phase 3 trial, which compared the outcomes of low-dose chemotherapy with 600 mg imatinib or 30 mg ponatinib had significantly higher MRD-negative CR rate (34.4 vs. 16.7%) in the ponatinib group [112]. Subsequent clinical trials used lower dose chemotherapy plus 30 mg ponatinib followed by allo-HCT or blinatumomab consolidation, and both showed CMR of > 70% and good long-term OS [113,114].
A chemotherapy-free regimen was first tried with dasatinib plus steroid for Ph-positive ALL [67], which was followed by blinatumomab consolidation in the D-ALBA trial by the GIMEMA group [68,69]. Since then, the same group tried ponatinib plus steroids; toxicity was detected in the 45 mg ponatinib group of elderly patients with comorbidities [29]. The MDACC recently reported a chemotherapy-free regimen consisting of ponatinib and blinatumomab. The data showed a CMR of 87% and 1-year OS of 95% (Fig. 1A) [70].
Frontline therapies with the novel agents discussed above are not widely available in Korea. However, since 2023, 45 mg ponatinib became available for use during 14 days of the first cycle of hyper-CVAD followed by 30 mg or 15 mg ponatinib (if CMR) for a total of 7 additional cycles of alter-native HDMTX/ARA and hyper-CVAD if patients can afford to without reimbursement from national insurance [30–32].
The standard post-remission therapy for adult patients with high-risk ALL is allo-HCT. The high-risk features of ALL have been suggested as various disease-related and patient-specific factors. Traditionally, old age, hyperleukocytosis, CNS involvement, T-ALL, Ph-positive ALL, and poor response to initial induction chemotherapy are important adverse-risk features. Among the Ph-negative population, MRC UKALL/ ECOG suggested that patients could be categorized as low risk (no risk factors based on age or WBC count), intermediate risk (age > 35 yr or elevated WBC count), or high risk (both age > 35 yr and elevated WBC count). In addition, as discussed above, there are several poor cytogenetic abnormalities such as complex karyotype, monosomal karyotype, hypodiploidy, t(4;11) translocation, 7 abnormalities, and t(8;14) translocation. Among them, Burkitt leukemia with a t(8;14) translocation is now successfully treated with rituximab combined with the hyper-CVAD and HDMTX/ ARA alternative regimens [94–96]. With the exception of Burkitt leukemia, allo-HCT should still be considered for post-remission therapy of other poor cytogenetic abnormalities, particularly in cases of a positive MRD level > 0.01%. Even in standard-risk ALL, if the MRD level at 10–16 weeks post-induction is > 0.01%, allo-HCT is the standard treatment of choice [8,86]. In contrast, the outcome of chemotherapy alone for patients with negative MRD was shown in a recent prospective randomized clinical trial comparing the outcomes between conventional chemotherapy and blinatumomab combined consolidation. Almost 50% of MRD-negative ALL patients relapsed or died after conventional chemotherapy, while a significantly lower proportion of patients had events during blinatumomab combined consolidation [115].
In Korea, several transplantation outcomes have been reported for Ph-positive ALL and Ph-negative ALL, according to the pre-HCT MRD response, ALL subtypes, donor types, and intensity of the preconditioning regimen. Overall, an early MRD response was the most predictive factor for a low relapse rate and a better survival outcome (Fig. 4A, B) [21–23,28]; conditioning intensity was not a significant factor for survival outcome as we conducted a risk-adapted approach for the reduced toxicity conditioning regimen candidates [52,116,117]. The poor prognosis of Ph-like ALL and ETP-ALL could be overcome by allo-HCT base post-remission therapy (Fig. 2A, B), and the pediatric-inspired regimen may have a beneficial effect on remission of T-ALL rather than hyper-CVAD based chemotherapy [39,53]. Total body irradiation is considered beneficial as a preconditioning regimen for patients with T-ALL and high-risk pediatric ALL [118,119]. Among the donor types of allo-HCT, cord blood transplantation (CBT) exhibits superior graft-versus-host disease and relapse free survival (GRFS) compared to conventional donor types (Fig. 4C) [120], but Ph-positive ALL with pre-HCT MRD > 0.1% had a significantly poorer outcome after CBT [121]. As an alternative donor, we recently compared CBT and haploidentical donor transplantation (HIDT) which revealed high early non-relapse mortality after CBT and a high relapse rate after HIDT. Overall, OS and DFS between alternative donor types were not significantly different (Fig. 4D) [122].
As discussed earlier, MRD is the most important prognostic marker for treatment outcome in ALL, and detection methods are well established compared to other hematological malignancies. Thus, we reduce the intensity of post-remission therapy for the MRD-negative subgroup, but must be vigilant in choosing the post-remission therapy, including allo-HCT and post-HCT strategies, for the MRD-positive subgroup. At present, blinatumomab is the only approved agent for Ph-positive and Ph-negative B-ALL with positive MRD (> 0.1%) in the BLAST trial, which evaluated 116 patients with MRD-positive CR and 88 (78%) converted to MRD-negative CR after one cycle of blinatumomab. Among the 107 evaluable patients with Ph-negative ALL, 84 (78.5%) developed MRD-negative CR and 61 underwent allo-HCT with inestimable median OS [123–125]. More favorable results were observed in a MDACC trial conducted in 37 patients with MRD-positive CR (> 0.01%), which showed MRD-negative conversion in 84% of Ph-negative ALL cases and 61% of Ph-positive ALL with an estimated 3-year OS of 67% [126]. Fractionated low-dose INO was evaluated in ALL patients with MRD-positive CR after inducing remission or post-transplantation, which also showed MRD-negative conversion in 67% [127] and good survival outcome after allo-HCT [128]. The standard therapy for this group of patients remains allo-HCT, but the possibility of cure without allo-HCT is expected when more cycles of blinatumomab become available [125,126].
Blinatumomab was approved in Korea in June 2023 for patients with MRD > 0.1% as evaluated by PCR, NGS, or MFC methods at any time after inducing remission.
In adult patients with B-ALL, CR rates are generally > 90% but long-term survival ranges from 30 to 60% if treated with standard chemotherapy with or without allo-HCT. Thus, relapses are frequently observed in cases of adult ALL. The Catholic Hematology Hospital showed refractoriness to modified hyper-CVAD protocol in 7.7% of Ph-negative ALL and 6.7% of Ph-positive ALL patients, and relapse during chemotherapy was observed in 13.7% of Ph-negative ALL and 15.4% of Ph-positive ALL patients. Overall, about 20% of cases relapsed or were refractory to chemotherapy and 25–30% of patients treated with allo-HCT subsequently relapsed [39,53,116,117]. Clinical outcome after relapse was dismal in the era without novel agents with < 50% remission rate and < 20% long-term survival with a median OS of 3–6 months; long-term OS was observed in 40% of patients treated with allo-HCT in remission [129–132]. Several immune-based treatments have been used for safe salvage and bridge therapy to allo-HCT, such as bi-specific T-cell engager [133,134], CAR-T therapy [135], and the antibody-drug conjugate platform [136]. Thus, recently introduced novel agents such as ponatinib, blinatumomab, INO, and CAR-T therapy were first used to treat R/R ALL and showed efficacy in terms of higher complete response rate, allo-HCT proceeding rate, and improved OS compared with the traditional standard of care.
Blinatumomab monotherapy for R/R Ph-negative ALL was evaluated in a phase 2 study in January 2012 and in the phase 3 TOWER study started in July 2014, but accelerated FDA approval in December 2014 was based on comparison of the outcome of blinatumomab with historical standard therapy in a propensity-score matched analysis [137–139]. A blinatumomab phase 3 trial showed significantly higher response rate (43.9 vs. 24.6%) with more MRD-negativity (76 vs. 48%) compared to standard chemotherapy. However, the median survival was 7.7 months, and long-term OS was < 25% [137]. Blinatumomab monotherapy was also evaluated in the phase 2 ALCANTARA study of R/R Ph-positive ALL patients, who showed an overall response rate of 35.6% with MRD-negativity in 88% and the median OS of the responders was 23 months [140,141]. MDACC treated R/R Ph-positive ALL using blinatumomab and ponatinib with an overall response of 92.0% with 79.0% MRD-negativity and a 2-year OS of 64% [70,142].
INO monotherapy for R/R ALL was also evaluated in phase 1 and 2 trials in 2010 and the phase 3 INO-VATE study in October 2012, and FDA approval was granted in August 2017 [143–146]. Similar to blinatumomab, the INO phase 3 trial indicated a significantly higher response rate (80.7 vs. 29.4%) with more MRD-negativity (92 vs. 19%) compared to standard chemotherapy. However, the median survival was 7.7 months, and 3-year OS was 20.3%. Better survival outcomes were observed in patients treated with allo-HCT (39.4% 2-year OS, median OS of 12.6 mo) although a high incidence (up to 22%) of hepatic sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD) was a significant adverse event particularly if patients were treated with dual-alkylator preconditioning or had a history of hepatic dysfunction [147,148]. In 2013, INO was used in combination with mini-hyper-CVD in R/R Ph-negative ALL patients [149] and blinatumomab was added for consolidation in 2018 [150,151], resulting in an overall response of 83% (CR in 63%, MRD-negativity in 84%), and 3-year OS of 40% (34% without blinatumomab and 54% with blinatumomab). They also reported that amending the regimen with low fractionated dose of INO (0.6 and 0.3 mg/BSA during course 1 and 0.3 and 0.3 mg/BSA during subsequent courses) reduced the incidence of SOS/VOD from 13% to 2% in this trial [151].
Blinatumomab became available in 2016 in Korea. Single arm studies showed an overall response of 44.9% but a higher response rate (68.8%) was observed when patients were treated in first-line salvage [152,153]. A more recent matched cohort comparative study reported a very good overall response rate (80.8 vs. 53.8%) with lower regimen-related death rate (1.9 vs. 40.4%) compared to historical standard chemotherapy such as the MEC regimen [154]. Comparative data revealed a 3-year OS of 33.2% with a median OS of 26.3 months, which was significantly superior compared to the historical control (Fig. 5A), but the long-term relapse and disease-free survival rates were similar between the two groups of patients in CR (24.7 vs. 21.4%, p = 0.140). Korean data indicate that early relapse with a short CR duration of < 12 months, combined extramedullary relapse (EMR), and high peripheral blood blast count are associated with poor clinical outcomes [153], but isolated EMR after allo-HCT responded well to blinatumomab monotherapy [155,156]. The INO data has not been published yet, but recent real-world data were reported as an abstract at the ICKSH in 2022. The overall response rate was 61.3% with a best MRD response of 81.2%, but early mortality was observed in 12.0% and 2-year OS was 19.3% (44.5% after allo-HCT). which was comparable to the INOVATE trial (Fig. 5B). SOS/VOD was observed in 10%, and all cases were severe to very severe grade.
As blinatumomab and INO are being incorporated into frontline combination therapy, novel strategies, such as CAR-T cell therapy and other investigational drug therapies, are needed for R/R ALL. Two commercial anti-CD19 CAR-T products have been approved in the USA; the 4-1BB costimulatory construct tisagenlecleucel (Tisa-cel) is now available for patients < 26 years old and was evaluated in the ELIANA trial. The study showed an overall response rate of 82% (MRD-negativity in 98%) and a recently reported long-term 5-year OS of 55% [157,158]. Grade 3–4 cytokine release syndrome (CRS) and neurotoxicity was reported in 48.1 and 12.7%, but CIBMTR real-world data reported 16.1 and 9.0%, respectively [159]. The other is the CD28 costimulatory construct brexucaptagene autoleucel (Brexu-cel), which is approved for patients ≥ 18 years and was evaluated in the ZUMA-3 trial. That study had an overall response rate of 67% in patients < 26 years old and 72% in patients ≥ 26 years. The median OS durations were 28.6 months and 34.1 months, respectively [160–162]. The ZUMA-3 data also revealed a benefit of Brexu-cel regardless of age, number of previous therapies, previous blinatumomab exposure, and subsequent allo-HCT status. Grade 3–4 CRS and neurotoxicity was reported in 24% and 26% of cases, respectively. Other than FDA-approved products, obecaptagene autoleucel (Obe-cel) was specifically developed to reduce toxicity and was evaluated in the phase 1b/2 FELIX study for R/R ALL (NCT04404660). That study showed an overall response rate of 76% (MRD-negativity in 97%), and grade 3–4 CRS and neurotoxicity was observed in 3.2 and 7.4%, respectively [163].
Tisa-cel has been available in Korea for adults < 26 years old since 2021 and a few adult cases have been treated with no significant complications [164]. A clinical trial for anbalcaptagene autoleucel (Anbal-cel), developed by a Korean company, has been ongoing since 2023 in adult patients with R/R ALL. Anbal-cel is a novel anti-CD19 CAR-T characterized by short hairpin RNA knockdown of PD-1 and TIGIT, and was first evaluated in a phase 1/2 study of R/R large B-cell lymphoma (NCT04836507) [165].
The Catholic Hematology Hospital showed refractoriness to modified hyper-CVAD protocol in 22.6% of cases and relapse during chemotherapy was observed in 12.7% of T-ALL cases. R/R T-ALL is an unexplored territory compared to R/R B-ALL, for which various classes of drugs and biological agents have been used but no specific therapy is significantly effective [14,15]. Nelarabine was approved in 2005 for effective induction of remission and a safe bridge to allo-HCT and has been the most widely applied drug as a monotherapy or combined therapy for R/R T-ALL [166–170]. Nelarabine is also used in frontline combination therapy. Although most reports include pediatric data, recent adult data have revealed that nelarabine monotherapy has a CR rate of 61% [171], and nelarabine-etoposide-cyclophosphamide combination therapy had a CR rate of 62% and the majority of responding patients successfully underwent allo-HCT at a median OS of 12.8 months. Neurotoxicity was observed in 14% in monotherapy and 27% in combination therapy, but all were less than grade 4 and recovered [169,171]. Among the various approaches, BH3 mimetics (BCL2 family inhibitors), such as venetoclax and navitoclax, have become of interest, as T-ALL and ETP-ALL are dependent on the BCL-XL and BCL2 signal pathways, respectively [172–174]. Thus, various venetoclax combination salvage therapies for R/R T-ALL has been tested and may play a role in the induction of remission and a safe bridge to allo-HCT [175–181]. Other novel approaches include inhibiting the LCK pathway with dasatinib [182], CD38 targeted therapy using daratumumab, inhibiting mTOR with everolimus, inhibition of NOTCH1, BET, JAK, PI3K, MEL, and CDK, and P53 reactivators for better treatment outcomes in T-ALL patients.
Nelarabine combination therapy is available in Korea, but no data have been published on treatment outcomes of R/R T-ALL.
Leukemic involvement of the CNS is not frequently observed at diagnosis, but the incidence of CNS relapse increases with the incidence of BM relapse. The incidence of CNS relapse in adult ALL is about 5% under current CNS prophylaxis strategies [183,184]. Prophylaxis against CNS disease includes intrathecal (IT) chemotherapy and radiotherapy (RT), although prophylactic RT is not frequently used. CNS relapse may occur together with BM relapse or other EMR lesions (up to 75%), or in isolated form (~25%), which is more frequently observed after allo-HCT [185]. Regardless of the relapse type, patients with a CNS relapse have poor survival outcomes of < 1 year [186]. CNS relapse is mostly treated with local IT chemotherapy and/or craniospinal RT first, followed by systemic therapy using CNS-active agents and allo-HCT [184]. As novel immunotherapeutic agents have been approved for R/R ALL, their specific use for CNS relapse is an area of great interest because of potential efficacy and concerns of CNS toxicity [187–190].
The Catholic Hematology Hospital in Korea recently analyzed a large cohort data of adult ALL (n = 1,043) for CNS leukemia, which showed 3 (0.3%) patients with significant CNS disease at diagnosis, 12 (1.2%) with CNS relapse during hyper-CVAD based chemotherapy, and post allo-HCT CNS relapse in 34 (4.5%) of 755 patients treated with allo-HCT. These data specifically show that 29 of 34 (85.3%) patients with post-HCT CNS relapse were Ph-positive ALL, and most were positive MRD before allo-HCT and exhibited hyperleukocytosis at diagnosis [191].
ALL is now divided into several subtypes, including Ph-positive ALL, Ph-like ALL, Ph-negative B-ALL, T-ALL with standard-risk or adverse-risk cytogenetics, and ETP-ALL. We suggest that low hypodiploidy, a monosomal karyotype, MLL rearrangement with t(4;11), complex karyotype with three or more aberrations, and chromosome 7 abnormalities (−7, del 7) are adverse-risk karyotypes. More recent findings suggest that deleting IKZF1-plus particularly by CD-KN2A/B deletion and/or TP53 mutation are very poor genetic abnormalities in Ph-positive and Ph-negative ALL. Most of all, post-remission MRD status is now well evaluated and applied for the post-remission treatment strategy, and the poor prognostic impact of MRD-positivity is clear.
For patients with these high-risk features, induction of remission and post-remission therapy including allo-HCT are so important that many clinical trials are incorporating novel agents into frontline, post-remission, and post-transplantation therapy. Among them, ponatinib-incorporated frontline therapy including a chemotherapy-free regimen has been changing the paradigm of Ph-positive ALL treatment with a good MRD response and favorable long-term survival outcomes. Blinatumomab has been approved in patients with MRD-positive CR. Blinatumomab and INO have also been used as frontline therapy with lower toxicity, improved survival outcomes, and a good MRD response. All of these agents have proven effective in R/R ALL, but long-term survival remains poor. Precisely modified frontline therapies using novel agents are the key to curing ALL. Therefore, cellular immunotherapy, such as CAR-T and their advanced products, will be further introduced for R/R ALL in the near future. However, we are still experiencing patients with R/R T-ALL or patients that relapse after use of those novel agents for whom no standard salvage therapy exists.
In conclusion, adult ALL may be a favorable disease entity like pediatric ALL or chronic myeloid leukemia with novel therapeutic agents. Thus, current intensive therapy will be substituted with less intensive chemotherapy- or transplantation-free approaches. However, there is always a proportion of R/R ALL for which the development of new drugs and strategies will be important.
Notes
Figure 1
Survival outcomes of Ph-positive ALL. (A) Data from the MD Anderson Cancer Center (USA) (frontline ponatinib incorporated in hyper-CVAD since 2011 and chemotherapy-free ponatinib plus blinatumomab since 2018). (B) Data from the Catholic Hematology Hospital in Korea (ponatinib became available in 2016 only for R/R Ph-positive ALL patients). ALL, acute lymphoblastic leukemia; OS, overall survival; BLINA, blinatumomab; PONAT, ponatinib; HCVAD, hyper-CVAD; DASAT, dasatinib; IMAT, imatinib; TKI, tyrosine kinase inhibitor; R/R, relapsed or refractory. Figure 1A adapted from Jabbour et al. J Hematol Oncol 2023;16:22 [13].
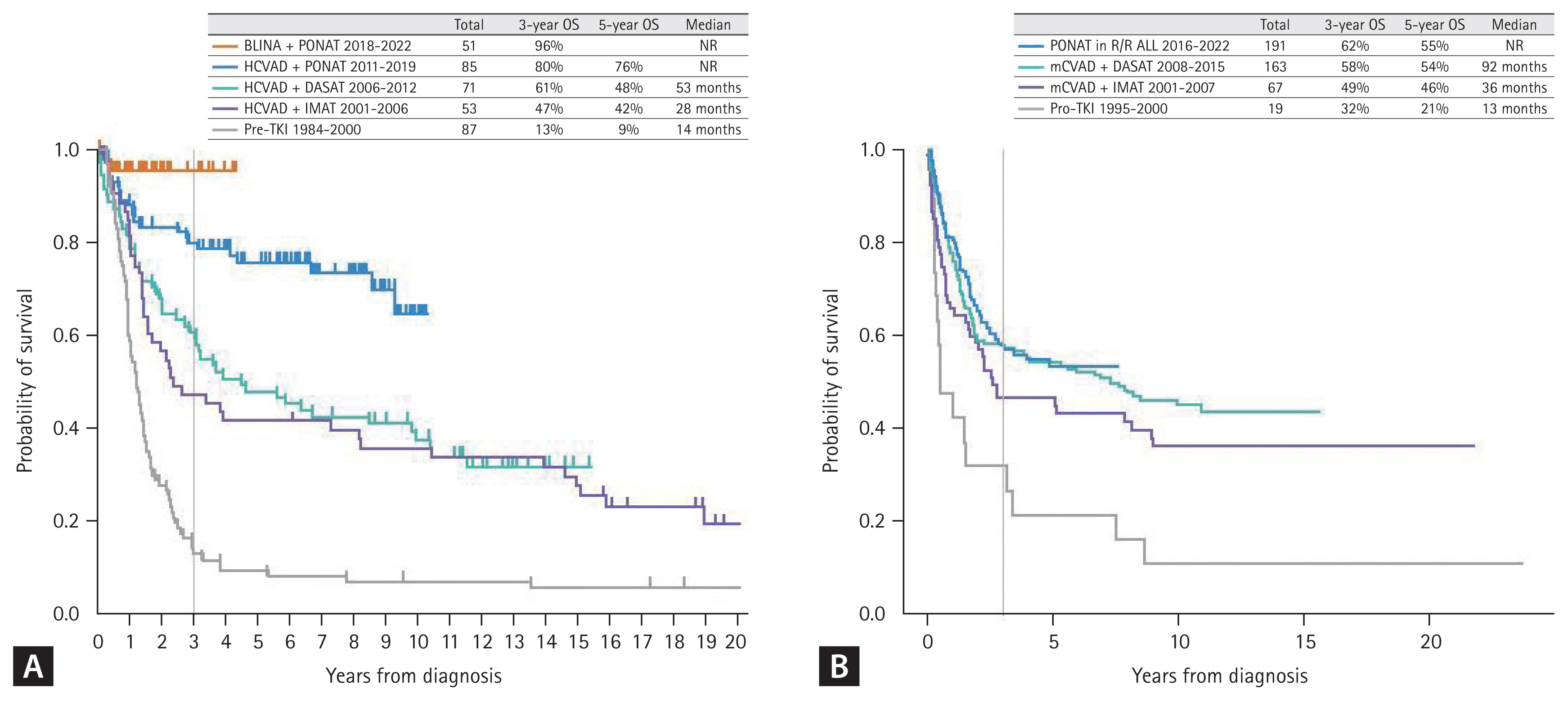
Figure 2
Transplantation outcomes of adult patients with ALL according to subtype and treatment era from data of the Catholic Hematology Hospital in Korea. (A) B-ALL subtype. (B) T-ALL subtype. (C) Ph-negative ALL according to the treatment era. (D) T-ALL according to the treatment era. ALL, acute lymphoblastic leukemia; ETP, Early T-cell precursor; allo-HCT; allogeneic hematopoietic cell transplantation; OS, overall survival; NR, not reached. Figure 2A adapted from Cho et al. Bone Marrow Transplant 2021;56:1953–1963 [39]. Figure 2B adapted from Yoon et al. Eur J Haematol 2023;110:137–148 [53].

Figure 3
Post-transplantation relapse according to alterations in gene copy number and mutations in ALL. (A) Ph-positive ALL (no TP53 mutation observed). (B) Ph-negative ALL (among IKZF1, CDKN2A/2B, and TP53). ALL, acute lymphoblastic leukemia. Adapted from Yoon et al. Blood 2023;142(Suppl 1):4342.
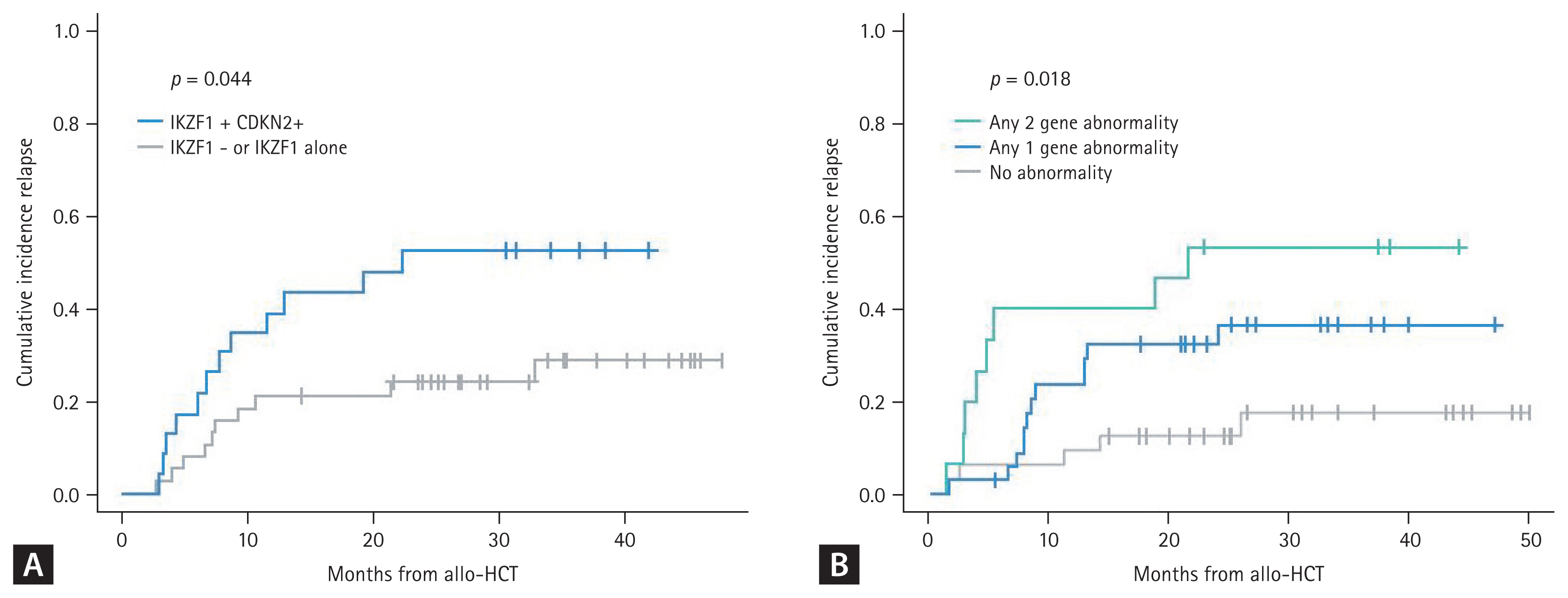
Figure 4
ALL transplantation outcome according to the pre-HCT MRD response and donor type. (A) Transplantation outcome of Ph-positive ALL according to the BCR::ABL1 RQ-PCR MRD response before allo-HCT. (B) Transplantation outcome of Ph-negative ALL according to the IGH/IGK-NGS MRD response at 10–12 weeks. (C) GRFS according to the donor type. (D) OS after the alternative donor types. ALL, acute lymphoblastic leukemia; PMR, poor molecular responder; IMR, intermediate molecular responder; LMR, late molecular responder; EMR, early molecular responder; CMR, complete molecular responder; MMR, major molecular responder; CB, cord blood; MMUD, mismatched unrelated donor; MSD, matched sibling donor; MUD, matched unrelated donor; CBT, cord blood transplantation; HIDT, haploidentical donor transplantation; GRFS, GVHD and relapse free survival; OS, overall survival. Figure 4A, C, and D adapted from [23], [120], and [122], respectively.
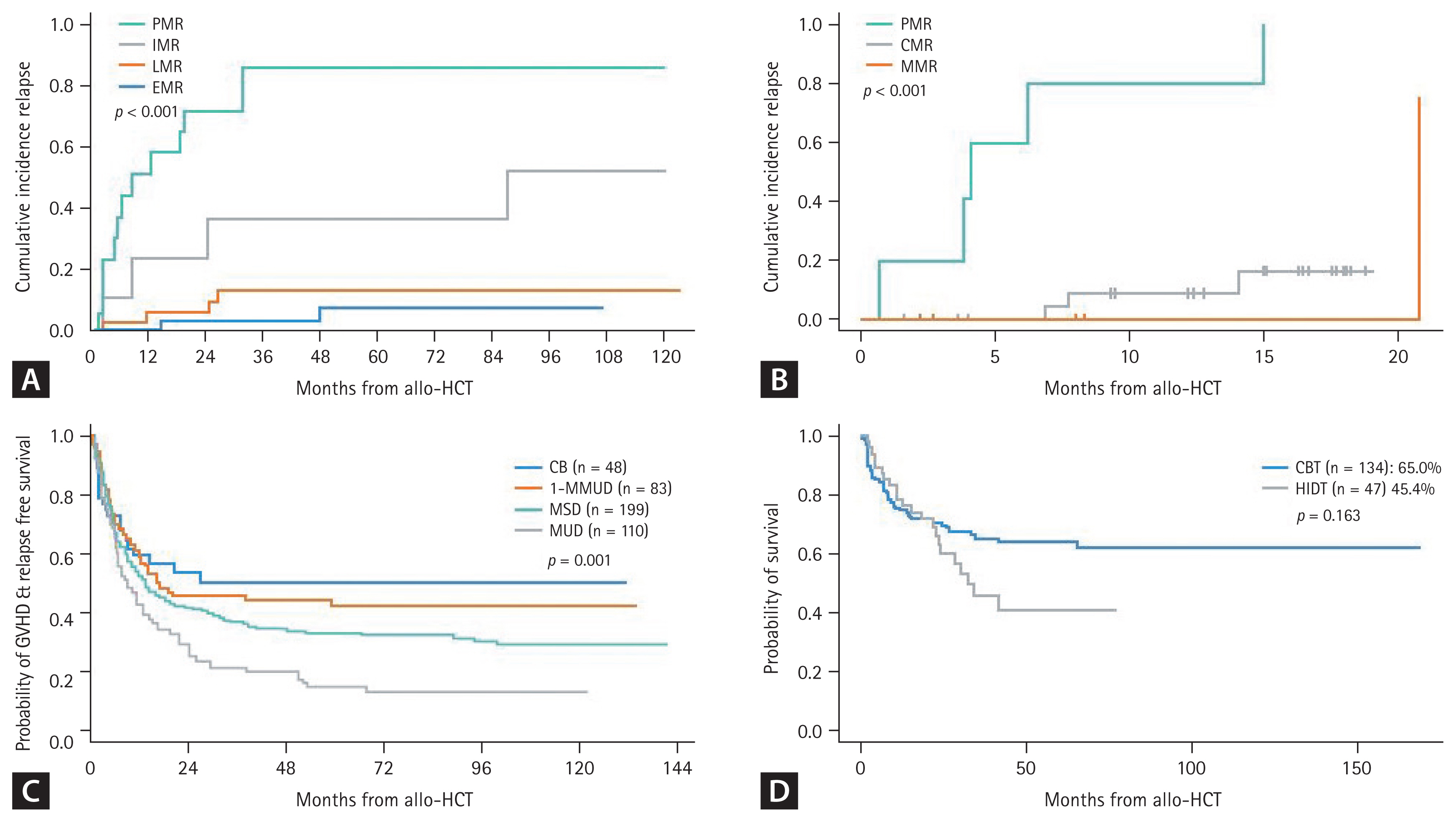
Figure 5
Treatment outcome of R/R ALL according to salvage treatment. (A) Blinatumomab vs. historical conventional chemotherapy (matched cohort analysis). (B) INO. ALL, acute lymphoblastic leukemia; Allo-HCT, allogeneic hematopoietic cell transplantation; INO, inotuzumab ozogamicin; CR, complete response; OS overall survival. Figure 5A adapted from Yoon et al. Ther Adv Hematol 2023;14:20406207231154713.
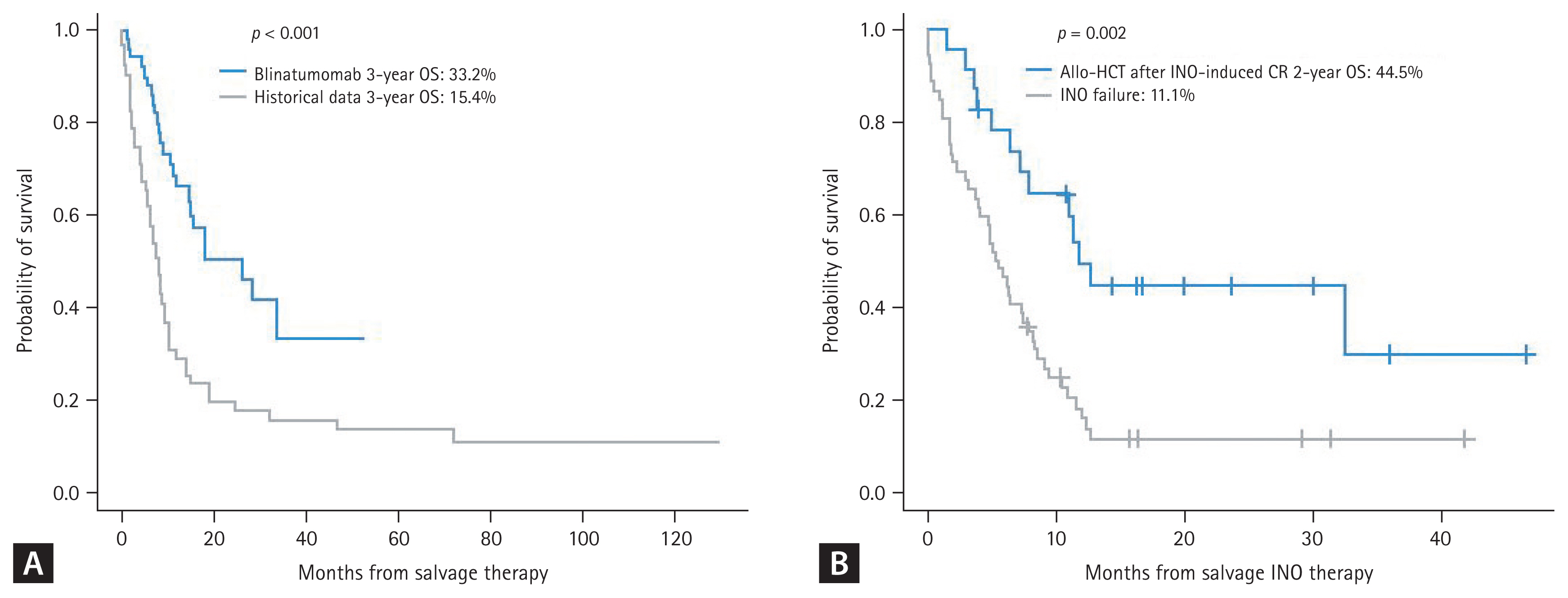
Table 1
Annual incidence of ALL in Korea
Table 2
ALL WHO classification revised in 2022
Adapted from Alaggio et al. Leukemia 2022;36:1720–1748 [16].
Table 3
MRD response at each time point after inducing remission
REFERENCES
2. Moorman AV. The clinical relevance of chromosomal and genomic abnormalities in B-cell precursor acute lymphoblastic leukaemia. Blood Rev 2012;26:123–135.


3. Moorman AV. New and emerging prognostic and predictive genetic biomarkers in B-cell precursor acute lymphoblastic leukemia. Haematologica 2016;101:407–416.

4. Schwab CJ, Chilton L, Morrison H, et al. Genes commonly deleted in childhood B-cell precursor acute lymphoblastic leukemia: association with cytogenetics and clinical features. Haematologica 2013;98:1081–1088.



5. Liu Y, Easton J, Shao Y, et al. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet 2017;49:1211–1218.




6. Ladetto M, Brüggemann M, Monitillo L, et al. Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders. Leukemia 2014;28:1299–1307.



7. Logan AC, Vashi N, Faham M, et al. Immunoglobulin and T cell receptor gene high-throughput sequencing quantifies minimal residual disease in acute lymphoblastic leukemia and predicts post-transplantation relapse and survival. Biol Blood Marrow Transplant 2014;20:1307–1313.



8. van Dongen JJ, van der Velden VH, Brüggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood 2015;125:3996–4009.




9. Jain N, Lamb AV, O’Brien S, et al. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood 2016;127:1863–1869.




10. Mullighan CG, Miller CB, Radtke I, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature 2008;453:110–114.



11. Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol 2009;10:125–134.



12. Lee JW, Kim S, Jang PS, Chung NG, Cho B. Differing outcomes of patients with high hyperdiploidy and ETV6-RUNX1 rearrangement in Korean pediatric precursor B cell acute lymphoblastic leukemia. Cancer Res Treat 2021;53:567–575.




13. Jabbour E, Short NJ, Jain N, et al. The evolution of acute lymphoblastic leukemia research and therapy at MD Anderson over four decades. J Hematol Oncol 2023;16:22.




14. Baek DW, Lee JM, Kim J, Cho HJ, Moon JH, Sohn SK. Therapeutic strategies, including allogeneic stem cell transplantation, to overcome relapsed/refractory adult T-cell acute lymphoblastic leukemia. Expert Rev Hematol 2021;14:765–775.


15. Cordo’ V, van der Zwet JCG, Canté-Barrett K, Pieters R, Meijerink JPP. T-cell acute lymphoblastic leukemia: a roadmap to targeted therapies. Blood Cancer Discov 2020;2:19–31.




16. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia 2022;36:1720–1748.


17. Thomas X, Thiebaut A, Olteanu N, et al. Philadelphia chromosome positive adult acute lymphoblastic leukemia: characteristics, prognostic factors and treatment outcome. Hematol Cell Ther 1998;40:119–128.

18. Dombret H, Gabert J, Boiron JM, et al.; GET-LALA Group. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia--results of the prospective multicenter LALA-94 trial. Blood 2002;100:2357–2366.


19. Daver N, Thomas D, Ravandi F, et al. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the frontline treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica 2015;100:653–661.



20. Lee KH, Lee JH, Choi SJ, et al. Clinical effect of imatinib added to intensive combination chemotherapy for newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia 2005;19:1509–1516.



21. Lee S, Kim YJ, Min CK, et al. The effect of first-line imatinib interim therapy on the outcome of allogeneic stem cell transplantation in adults with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 2005;105:3449–3457.



22. Lee S, Kim YJ, Chung NG, et al. The extent of minimal residual disease reduction after the first 4-week imatinib therapy determines outcome of allogeneic stem cell transplantation in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer 2009;115:561–570.


23. Lee S, Kim DW, Cho BS, et al. Impact of minimal residual disease kinetics during imatinib-based treatment on transplantation outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia 2012;26:2367–2374.



24. Yanada M, Takeuchi J, Sugiura I, et al.; Japan Adult Leukemia Study Group. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol 2006;24:460–466.


25. Bassan R, Rossi G, Pogliani EM, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol 2010;28:3644–3652.


26. Ravandi F, O’Brien SM, Cortes JE, et al. Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer 2015;121:4158–4164.



27. Ravandi F, Othus M, O’Brien SM, et al. US intergroup study of chemotherapy plus dasatinib and allogeneic stem cell transplant in philadelphia chromosome positive ALL. Blood Adv 2016;1:250–259.


28. Yoon JH, Yhim HY, Kwak JY, et al. Minimal residual disease-based effect and long-term outcome of first-line dasatinib combined with chemotherapy for adult Philadelphia chromosome-positive acute lymphoblastic leukemia. Ann Oncol 2016;27:1081–1088.


29. Martinelli G, Papayannidis C, Piciocchi A, et al. INCB84344-201: Ponatinib and steroids in frontline therapy for unfit patients with Ph+ acute lymphoblastic leukemia. Blood Adv 2022;6:1742–1753.


30. Kantarjian H, Short NJ, Jain N, et al. Frontline combination of ponatinib and hyper-CVAD in Philadelphia chromosome-positive acute lymphoblastic leukemia: 80-months follow-up results. Am J Hematol 2023;98:493–501.


31. Sasaki K, Jabbour EJ, Ravandi F, et al. Hyper-CVAD plus ponatinib versus hyper-CVAD plus dasatinib as frontline therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: a propensity score analysis. Cancer 2016;122:3650–3656.




32. Jabbour E, Short NJ, Ravandi F, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: long-term follow-up of a single-centre, phase 2 study. Lancet Haematol 2018;5:e618–e627.



33. Roberts KG, Morin RD, Zhang J, et al. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell 2012;22:153–166.



34. Russell LJ, Capasso M, Vater I, et al. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood 2009;114:2688–2698.

35. Palmi C, Vendramini E, Silvestri D, et al. Poor prognosis for P2RY8-CRLF2 fusion but not for CRLF2 over-expression in children with intermediate risk B-cell precursor acute lymphoblastic leukemia. Leukemia 2012;26:2245–2253.


36. Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med 2014;371:1005–1015.


37. Lengline E, Beldjord K, Dombret H, Soulier J, Boissel N, Clappier E. Successful tyrosine kinase inhibitor therapy in a refractory B-cell precursor acute lymphoblastic leukemia with EBF1-PDGFRB fusion. Haematologica 2013;98:e146–e148.



38. Weston BW, Hayden MA, Roberts KG, et al. Tyrosine kinase inhibitor therapy induces remission in a patient with refractory EBF1-PDGFRB-positive acute lymphoblastic leukemia. J Clin Oncol 2013;31:e413–e416.


39. Cho H, Kim Y, Yoon JH, et al. Non-inferior long-term outcomes of adults with Philadelphia chromosome-like acute lymphoblastic leukemia. Bone Marrow Transplant 2021;56:1953–1963.


40. Guru Murthy GS, Pondaiah SK, Abedin S, Atallah E. Incidence and survival of T-cell acute lymphoblastic leukemia in the United States. Leuk Lymphoma 2019;60:1171–1178.


41. Litzow MR, Ferrando AA. How I treat T-cell acute lymphoblastic leukemia in adults. Blood 2015;126:833–841.



42. Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood 2012;119:34–43.




43. Rowe JM, Buck G, Burnett AK, et al.; ECOG; MRC/NCRI Adult Leukemia Working Party. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood 2005;106:3760–3767.


44. Marks DI, Paietta EM, Moorman AV, et al. T-cell acute lymphoblastic leukemia in adults: clinical features, immunophenotype, cytogenetics, and outcome from the large randomized prospective trial (UKALL XII/ECOG 2993). Blood 2009;114:5136–5145.




45. Lazarus HM, Richards SM, Chopra R, et al.; Medical Research Council (MRC)/National Cancer Research Institute (NCRI) Adult Leukaemia Working Party of the United Kingdom and the Eastern Cooperative Oncology Group. Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis: results from the international ALL trial MRC UKALL XII/ECOG E2993. Blood 2006;108:465–472.

46. Coustan-Smith E, Mullighan CG, Onciu M, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol 2009;10:147–156.



47. Inukai T, Kiyokawa N, Campana D, et al. Clinical significance of early T-cell precursor acute lymphoblastic leukaemia: results of the Tokyo Children’s Cancer Study Group Study L99-15. Br J Haematol 2012;156:358–365.


48. Ma M, Wang X, Tang J, et al. Early T-cell precursor leukemia: a subtype of high risk childhood acute lymphoblastic leukemia. Front Med 2012;6:416–420.



49. Patrick K, Wade R, Goulden N, et al. Outcome for children and young people with Early T-cell precursor acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol 2014;166:421–424.



50. Genescà E, Morgades M, Montesinos P, et al. Unique clinico-biological, genetic and prognostic features of adult early T-cell precursor acute lymphoblastic leukemia. Haematologica 2020;105:e294–e297.



51. Bond J, Graux C, Lhermitte L, et al. Early response-based therapy stratification improves survival in adult early thymic precursor acute lymphoblastic leukemia: a group for research on adult acute lymphoblastic leukemia study. J Clin Oncol 2017;35:2683–2691.


52. Yoon JH, Min GJ, Park SS, et al. Minimal residual disease-based long-term efficacy of reduced-intensity conditioning versus myeloablative conditioning for adult Philadelphia-positive acute lymphoblastic leukemia. Cancer 2019;125:873–883.



53. Yoon JH, Kim HS, Min GJ, et al. Cytogenetic and molecular characteristics and outcomes of adult patients with early T-cell precursor acute lymphoblastic leukemia. Eur J Haematol 2023;110:137–148.



54. Lazaryan A, Dolan M, Zhang MJ, et al.; Acute Leukemia Committee of the CIBMTR. Impact of cytogenetic abnormalities on outcomes of adult Philadelphia-negative acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation: a study by the Acute Leukemia Working Committee of the Center for International Blood and Marrow Transplant Research. Haematologica 2020;105:1329–1338.


55. Mullighan CG, Su X, Zhang J, et al.; Children’s Oncology Group. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med 2009;360:470–480.


56. Kim M, Park J, Kim DW, et al. Impact of IKZF1 deletions on long-term outcomes of allo-SCT following imatinib-based chemotherapy in adult Philadelphia chromosome-positive ALL. Bone Marrow Transplant 2015;50:354–362.



57. Stanulla M, Dagdan E, Zaliova M, et al.; TRANSCALL Consortium; International BFM Study Group. IKZF1plus Defines a new minimal residual disease-dependent very-poor prognostic profile in pediatric B-cell precursor acute lymphoblastic leukemia. J Clin Oncol 2018;36:1240–1249.


58. Zhang W, Kuang P, Liu T. Prognostic significance of CDKN2A/ B deletions in acute lymphoblastic leukaemia: a meta-analysis. Ann Med 2019;51:28–40.



59. Yoon JH, Kwag D, Min GJ, et al. Adverse prognostic role of copy number alterations and mutations in adults with philadelphia chromosome-negative acute lymphoblastic leukemia. Blood 2023;142(Suppl 1):4342.


60. Berry DA, Zhou S, Higley H, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol 2017;3:e170580.



61. Ribera JM, Morgades M, Ciudad J, et al. Chemotherapy or allogeneic transplantation in high-risk Philadelphia chromosome-negative adult lymphoblastic leukemia. Blood 2021;137:1879–1894.

62. Yilmaz M, Kantarjian H, Wang X, et al. The early achievement of measurable residual disease negativity in the treatment of adults with Philadelphia-negative B-cell acute lymphoblastic leukemia is a strong predictor for survival. Am J Hematol 2020;95:144–150.



63. Short NJ, Jabbour E, Albitar M, et al. Recommendations for the assessment and management of measurable residual disease in adults with acute lymphoblastic leukemia: a consensus of North American experts. Am J Hematol 2019;94:257–265.




64. Chong SL, Asnawi AWA, Leong TS, et al. Impact of timely BCR-ABL1 monitoring before allogeneic stem cell transplantation among patients with BCR-ABL1-positive B-acute lymphoblastic leukemia. Blood Res 2021;56:175–183.



65. Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood 2009;113:4153–4162.



66. Bassan R, Pavoni C, Intermesoli T, et al. Updated risk-oriented strategy for acute lymphoblastic leukemia in adult patients 18–65 years: NILG ALL 10/07. Blood Cancer J 2020;10:119.




67. Chiaretti S, Ansuinelli M, Vitale A, et al. A multicenter total therapy strategy for de novo adult Philadelphia chromosome positive acute lymphoblastic leukemia patients: final results of the GIMEMA LAL1509 protocol. Haematologica 2021;106:1828–1838.


68. Foà R, Bassan R, Vitale A, et al.; GIMEMA Investigators. Dasatinib-blinatumomab for Ph-positive acute lymphoblastic leukemia in adults. N Engl J Med 2020;383:1613–1623.


69. Chiaretti S, Bassan R, Vitale A, et al. P353: forty months update of the GIMEMA LAL2116 (D-ALBA) protocol and ancillary LAL2217 study for newly diagnosed adult PH+ ALL. HemaSphere 2022;6:253–254.

70. Jabbour E, Short NJ, Jain N, et al. Ponatinib and blinatumomab for Philadelphia chromosome-positive acute lymphoblastic leukaemia: a US, single-centre, single-arm, phase 2 trial. Lancet Haematol 2023;10:e24–e34.


71. Breit TM, Wolvers-Tettero IL, Hählen K, van Wering ER, van Dongen JJ. Extensive junctional diversity of gamma delta T-cell receptors expressed by T-cell acute lymphoblastic leukemias: implications for the detection of minimal residual disease. Leukemia 1991;5:1076–1086.

72. d’Auriol L, Macintyre E, Galibert F, Sigaux F. In vitro amplification of T cell gamma gene rearrangements: a new tool for the assessment of minimal residual disease in acute lymphoblastic leukemias. Leukemia 1989;3:155–158.

73. Yamada M, Hudson S, Tournay O, et al. Detection of minimal disease in hematopoietic malignancies of the B-cell lineage by using third-complementarity-determining region (CDR-III)-specific probes. Proc Natl Acad Sci U S A 1989;86:5123–5127.



74. Beishuizen A, de Bruijn MA, Pongers-Willemse MJ, et al. Heterogeneity in junctional regions of immunoglobulin kappa deleting element rearrangements in B cell leukemias: a new molecular target for detection of minimal residual disease. Leukemia 1997;11:2200–2207.



75. Szczepański T, Willemse MJ, Brinkhof B, van Wering ER, van der Burg M, van Dongen JJ. Comparative analysis of Ig and TCR gene rearrangements at diagnosis and at relapse of childhood precursor-B-ALL provides improved strategies for selection of stable PCR targets for monitoring of minimal residual disease. Blood 2002;99:2315–2323.



76. van der Velden VH, Szczepanski T, Wijkhuijs JM, et al. Age-related patterns of immunoglobulin and T-cell receptor gene rearrangements in precursor-B-ALL: implications for detection of minimal residual disease. Leukemia 2003;17:1834–1844.


77. van Dongen JJ, Langerak AW, Brüggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003;17:2257–2317.



78. Hein K, Short N, Jabbour E, Yilmaz M. Clinical value of measurable residual disease in acute lymphoblastic leukemia. Blood Lymphat Cancer 2022;12:7–16.




79. Porwit-MacDonald A, Björklund E, Lucio P, et al. BIOMED-1 concerted action report: flow cytometric characterization of CD7+ cell subsets in normal bone marrow as a basis for the diagnosis and follow-up of T cell acute lymphoblastic leukemia (T-ALL). Leukemia 2000;14:816–825.



80. Coustan-Smith E, Sancho J, Hancock ML, et al. Clinical importance of minimal residual disease in childhood acute lymphoblastic leukemia. Blood 2000;96:2691–2696.


81. Lúcio P, Parreira A, van den Beemd MW, et al. Flow cytometric analysis of normal B cell differentiation: a frame of reference for the detection of minimal residual disease in precursor-B-ALL. Leukemia 1999;13:419–427.


82. Dworzak MN, Fröschl G, Printz D, et al.; Austrian Berlin-Frankfurt-Münster Study Group. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. Blood 2002;99:1952–1958.


83. Theunissen P, Mejstrikova E, Sedek L, et al.; EuroFlow Consortium. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood 2017;129:347–357.


84. Wu D, Emerson RO, Sherwood A, et al. Detection of minimal residual disease in B lymphoblastic leukemia by high-throughput sequencing of IGH. Clin Cancer Res 2014;20:4540–4548.


85. Brown PA, Shah B, Advani A, et al. Acute Lymphoblastic Leukemia, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:1079–1109.

86. Ravandi F, Jorgensen JL, Thomas DA, et al. Detection of MRD may predict the outcome of patients with Philadelphia chromosome-positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood 2013;122:1214–1221.


87. Gökbuget N, Kneba M, Raff T, et al.; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood 2012;120:1868–1876.



88. Ribera JM, Oriol A, Morgades M, et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol 2014;32:1595–1604.

89. Kantarjian HM, Walters RS, Keating MJ, et al. Results of the vincristine, doxorubicin, and dexamethasone regimen in adults with standard- and high-risk acute lymphocytic leukemia. J Clin Oncol 1990;8:994–1004.

90. Kantarjian HM, O’Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol 2000;18:547–561.


91. Kantarjian H, Thomas D, O’Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer 2004;101:2788–2801.


92. Abaza Y, M Kantarjian H, Faderl S, et al. Hyper-CVAD plus nelarabine in newly diagnosed adult T-cell acute lymphoblastic leukemia and T-lymphoblastic lymphoma. Am J Hematol 2018;93:91–99.



93. Dunsmore KP, Winter SS, Devidas M, et al. Children’s Oncology Group AALL0434: A phase III randomized clinical trial testing nelarabine in newly diagnosed T-cell acute lymphoblastic leukemia. J Clin Oncol 2020;38:3282–3293.



94. Thomas DA, O’Brien S, Faderl S, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol 2010;28:3880–3889.



95. Jabbour E, Richard-Carpentier G, Sasaki Y, et al. Hyper-CVAD regimen in combination with ofatumumab as frontline therapy for adults with Philadelphia chromosome-negative B-cell acute lymphoblastic leukaemia: a single-arm, phase 2 trial. Lancet Haematol 2020;7:e523–e533.


96. Sasaki K, Kantarjian HM, Morita K, et al. Hyper-CVAD plus ofatumumab versus hyper-CVAD plus rituximab as frontline therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: a propensity score analysis. Cancer 2021;127:3381–3389.



97. Stock W, La M, Sanford B, et al.; Children’s Cancer Group; Cancer and Leukemia Group B studies. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood 2008;112:1646–1654.




98. Siegel SE, Stock W, Johnson RH, et al. Pediatric-inspired treatment regimens for adolescents and young adults with philadelphia chromosome-negative acute lymphoblastic leukemia: a review. JAMA Oncol 2018;4:725–734.



99. Boissel N, Auclerc MF, Lhéritier V, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol 2003;21:774–780.


100. de Bont JM, Holt Bv, Dekker AW, et al. Significant difference in outcome for adolescents with acute lymphoblastic leukemia treated on pediatric vs adult protocols in the Netherlands. Leukemia 2004;18:2032–2035.



101. Hallböök H, Gustafsson G, Smedmyr B, Söderhäll S, Heyman M, Swedish Adult Acute Lymphocytic Leukemia Group; Swedish Childhood Leukemia Group. Treatment outcome in young adults and children >10 years of age with acute lymphoblastic leukemia in Sweden: a comparison between a pediatric protocol and an adult protocol. Cancer 2006;107:1551–1561.


102. Rytting ME, Thomas DA, O’Brien SM, et al. Augmented Berlin-Frankfurt-Münster therapy in adolescents and young adults (AYAs) with acute lymphoblastic leukemia (ALL). Cancer 2014;120:3660–3668.




103. Baek DW, Kim DY, Sohn SK, et al. Pediatric-inspired regimen with late intensification and increased dose of L-asparaginase for adult acute lymphoblastic leukemia: the KALLA 1406/1407 study. Korean J Intern Med 2021;36:1471–1485.




104. Baek DW, Park HS, Sohn SK, et al.; Adult Acute Lymphoblastic Leukemia Working Party, the Korean Society of Hematology. Rituximab plus multiagent chemotherapy for newly diagnosed CD20-positive acute lymphoblastic leukemia: a prospective phase II study. Korean J Intern Med 2023;38:734–746.




105. Kim DY, Joo YD, Lim SN, et al.; Adult Acute Lymphoblastic Leukemia Working Party of the Korean Society of Hematology. Nilotinib combined with multiagent chemotherapy for newly diagnosed Philadelphia-positive acute lymphoblastic leukemia. Blood 2015;126:746–756.



106. Kantarjian H, Ravandi F, Short NJ, et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: a single-arm, phase 2 study. Lancet Oncol 2018;19:240–248.


107. Haddad F, Jabbour E, Nasnas C, et al. P373: updates from a phase ii trial of mini-hyper-cvd-inotuzumab with or without blinatumomab in older patients with newly diagnosed Philadelphia chromosome (PH)-negative acute lymphoblastic leukemia. Hemasphere 2023;7(Suppl):e3563066.


108. Short N, Jabbour E, Jain N, et al. P358: hyper-CVAD with blinatumomab and inotuzumab ozogamicin for patients with newly diagnosed Philadelphia chromosome-negative B-cell acute lymphoblastic leukemia: a phase II study. Hemasphere 2023;7(Suppl):e67564ca.


109. Jabbour E, Short NJ, Senapati J, et al. Mini-hyper-CVD plus inotuzumab ozogamicin, with or without blinatumomab, in the subgroup of older patients with newly diagnosed Philadelphia chromosome-negative B-cell acute lymphocytic leukaemia: long-term results of an open-label phase 2 trial. Lancet Haematol 2023;10:e433–e444.


110. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood 2018;132:393–404.




111. Liam CCK, Boo YL, Chong SL, Sathar J, Ong TC, Tan SM. Philadelphia-positive (PH+) acute lymphoblastic leukemia (ALL): developing strategies for curing this stubborn disease. Blood Res 2022;57:158–161.



112. Jabbour E, Kantarjian HM, Aldoss I, et al. First report of PhALLCON: A phase 3 study comparing ponatinib (pon) vs imatinib (im) in newly diagnosed patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL). J Clin Oncol 2023;41(36 Suppl):398868–398868.

113. Ribera JM, García-Calduch O, Ribera J, et al. Ponatinib, chemotherapy, and transplant in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Adv 2022;6:5395–5402.




114. Nguyen D, Jabbour E, Short N, et al. A phase II study of the sequential combination of low-intensity chemotherapy (minihyper-CVD) and ponatinib followed by blinatumomab and ponatinib in patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). Blood 2022;140(Supplement 1):6127–6129.


115. Litzow MR, Sun Z, Paietta E, et al. Consolidation therapy with blinatumomab improves overall survival in newly diagnosed adult patients with B-lineage acute lymphoblastic leukemia in measurable residual disease negative remission: results from the ECOG-ACRIN E1910 randomized phase III National Co-operative Clinical Trials Network Trial. Blood 2022;140(Supplement 2):LBA–1.
116. Eom KS, Shin SH, Yoon JH, et al. Comparable long-term outcomes after reduced-intensity conditioning versus myeloablative conditioning allogeneic stem cell transplantation for adult high-risk acute lymphoblastic leukemia in complete remission. Am J Hematol 2013;88:634–641.


117. Cho BS, Lee S, Kim YJ, et al. Reduced-intensity conditioning allogeneic stem cell transplantation is a potential therapeutic approach for adults with high-risk acute lymphoblastic leukemia in remission: results of a prospective phase 2 study. Leukemia 2009;23:1763–1770.



118. Jang W, Jo S, Yoo JW, et al. Prognostic impact of total body irradiation dose in pediatric acute lymphoblastic leukemia patients treated with allogeneic hematopoietic stem cell transplantation in second complete remission. Blood Res 2022;57:256–263.

119. Cahu X, Labopin M, Giebel S, et al.; Acute Leukemia Working Party of EBMT. Impact of conditioning with TBI in adult patients with T-cell ALL who receive a myeloablative allogeneic stem cell transplantation: a report from the acute leukemia working party of EBMT. Bone Marrow Transplant 2016;51:351–357.


120. Yoon JH, Min GJ, Park SS, et al. Impact of donor type on long-term graft-versus-host disease-free/relapse-free survival for adult acute lymphoblastic leukemia in first remission. Bone Marrow Transplant 2021;56:828–840.


121. Yoon JH, Min GJ, Park SS, et al. Durable outcomes of double cord blood transplantation in adults with acute lymphoblastic leukemia: high-risk features for early and long-term mortality. Ther Adv Hematol 2022;13:20406207221076762.




122. Lee S, Yoon JH, Lee S. Selecting the right alternative donor: comparison of outcomes of HLA-mismatched alternative donor hematopoietic cell transplantations in adult patients with acute lymphoblastic leukemia regarding KIR-ligand mismatch. Blood 2023;142(Supplement 1):2247.



123. Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 2018;131:1522–1531.




124. Goekbuget N, Dombret H, Zugmaier G, et al. Blinatumomab for minimal residual disease (MRD) in adults with B-cell precursor acute lymphoblastic leukemia (BCP-ALL): median overall survival (OS) is not reached in complete MRD responders at a median follow-up of 53.1 months. Blood 2018;132(Supplement 1):554.


125. Gökbuget N, Zugmaier G, Dombret H, et al. Curative outcomes following blinatumomab in adults with minimal residual disease B-cell precursor acute lymphoblastic leukemia. Leuk Lymphoma 2020;61:2665–2673.


126. Jabbour EJ, Short NJ, Jain N, et al. Blinatumomab is associated with favorable outcomes in patients with B-cell lineage acute lymphoblastic leukemia and positive measurable residual disease at a threshold of 10–4 and higher. Am J Hematol 2022;97:1135–1141.



127. Nasr LF, Short NJ, Senapati J, et al. Inotuzumab ozogamicin (INO) for the treatment of measurable residual disease (MRD) in patients with B-cell acute lymphoblastic leukemia (B-ALL): results from a phase II study. J Clin Oncol 2023;41(16 Suppl):e19008–e19008.

128. Metheny L, Sobecks RM, Cho C, et al. Multicenter phase I study of post-transplant low-dose inotuzumab ozogamicin to prevent relapse of acute lymphoblastic leukemia. Blood 2022;140(Supplement 1):1887–1889.


129. Gökbuget N, Stanze D, Beck J, et al.; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 2012;120:2032–2041.



130. Fielding AK, Richards SM, Chopra R, et al.; Medical Research Council of the United Kingdom Adult ALL Working Party; Eastern Cooperative Oncology Group. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007;109:944–950.



131. Tavernier E, Boiron JM, Huguet F, et al.; GET-LALA Group; Swiss Group for Clinical Cancer Research SAKK; Australasian Leukaemia and Lymphoma Group. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia 2007;21:1907–1914.



132. Gökbuget N, Dombret H, Ribera JM, et al. International reference analysis of outcomes in adults with B-precursor Ph-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica 2016;101:1524–1533.



133. Klinger M, Brandl C, Zugmaier G, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/ CD3-bispecific BiTE antibody blinatumomab. Blood 2012;119:6226–6233.



134. Hoffmann P, Hofmeister R, Brischwein K, et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/ CD3-bispecific single-chain antibody construct. Int J Cancer 2005;115:98–104.


135. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–1517.



136. Advani A, Coiffier B, Czuczman MS, et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin’s lymphoma: results of a phase I study. J Clin Oncol 2010;28:2085–2093.

137. Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 2017;376:836–847.



138. Gökbuget N, Kelsh M, Chia V, et al. Blinatumomab vs historical standard therapy of adult relapsed/refractory acute lymphoblastic leukemia. Blood Cancer J 2016;6:e473.




139. Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015;16:57–66.


140. Martinelli G, Boissel N, Chevallier P, et al. Complete hematologic and molecular response in adult patients with relapsed/ refractory Philadelphia chromosome–positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study. J Clin Oncol 2017;35:1795–1802.


141. Martinelli G, Boissel N, Chevallier P, et al. Long-term follow-up of blinatumomab in patients with relapsed/refractory Philadelphia chromosome-positive B-cell precursor acute lymphoblastic leukaemia: final analysis of ALCANTARA study. Eur J Cancer 2021;146:107–114.


142. Haddad F, Jabbour E, Zoghbi M, et al. P379: ponatinib and blinatumomab in relapsed/refractory Philadelphia-positive acute lymphoblastic leukemia or chronic myeloid leukemia in lymphoid blast phase: subgroup analysis from a phase II trial. Hemasphere 2023;7(Suppl):e6913408.


143. Kantarjian H, Thomas D, Jorgensen J, et al. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer 2013;119:2728–2736.



144. Jabbour E, O’Brien S, Huang X, et al. Prognostic factors for outcome in patients with refractory and relapsed acute lymphocytic leukemia treated with inotuzumab ozogamicin, a CD22 monoclonal antibody. Am J Hematol 2015;90:193–196.


145. Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med 2016;375:740–753.



146. Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer 2019;125:2474–2487.




147. Kantarjian HM, DeAngelo DJ, Advani AS, et al. Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: results from the open-label, randomised, phase 3 INOVATE study. Lancet Haematol 2017;4:e387–e398.


148. Marks DI, Kebriaei P, Stelljes M, et al. Outcomes of allogeneic stem cell transplantation after inotuzumab ozogamicin treatment for relapsed or refractory acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2019;25:1720–1729.


149. Jabbour E, Ravandi F, Kebriaei P, et al. Salvage chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD for patients with relapsed or refractory Philadelphia chromosome-negative acute lymphoblastic leukemia: a phase 2 clinical trial. JAMA Oncol 2018;4:230–234.



150. Jabbour E, Sasaki K, Short NJ, et al. Long-term follow-up of salvage therapy using a combination of inotuzumab ozogamicin and mini-hyper-CVD with or without blinatumomab in relapsed/refractory Philadelphia chromosome-negative acute lymphoblastic leukemia. Cancer 2021;127:2025–2038.


151. Kantarjian H, Haddad FG, Jain N, et al. Results of salvage therapy with mini-hyper-CVD and inotuzumab ozogamicin with or without blinatumomab in pre-B acute lymphoblastic leukemia. J Hematol Oncol 2023;16:44.


152. Jung SH, Lee SR, Yang DH, et al. Efficacy and safety of blinatumomab treatment in adult Korean patients with relapsed/ refractory acute lymphoblastic leukemia on behalf of the Korean Society of Hematology ALL Working Party. Ann Hematol 2019;98:151–158.


153. Yoon JH, Min GJ, Park SS, et al. Feasible outcome of blinatumomab followed by allogeneic hematopoietic cell transplantation for adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage. Cancer Med 2019;8:7650–7659.




154. Yoon JH, Kwag D, Lee JH, et al. Superior survival outcome of blinatumomab compared with conventional chemotherapy for adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia: a propensity scorematched cohort analysis. Ther Adv Hematol 2023;14:20406207231154713.




155. Lee SH, Yoon JH, Min GJ, et al. Response to blinatumomab or inotuzumab ozogamicin for isolated extramedullary relapse of adult acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation: a case study. Int J Hematol 2022;115:135–139.



156. Yoon SY, Yoon JH, Min GJ, et al. Experience of blinatumomab salvage for patients with acute lymphoblastic leukemia presenting with isolated extramedullary relapse after previous allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2020;55:1469–1472.



157. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–448.


158. Rives S, Maude SL, Hiramatsu H, et al. S112: tisagenlecleucel in pediatric and young adult patients (PTS) WITH relapsed/ refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL): final analyses from the Eliana study. HemaSphere 2022;6:13–14.

159. Pasquini MC, Hu ZH, Curran K, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv 2020;4:5414–5424.


160. Shah BD, Cassaday RD, Park JH, et al. Impact of age, prior therapies, and subsequent transplant on long-term outcomes of adults with relapsed or refractory B-cell acute lymphoblastic leukemia (R/R B-ALL) treated with brexucabtagene autoleucel (brexu-cel) in ZUMA-3. J Clin Oncol 2023;41(16 Suppl):7023–7023.

161. Shah BD, Bishop MR, Oluwole OO, et al. KTE-X19 anti-CD19 CAR T-cell therapy in adult relapsed/refractory acute lymphoblastic leukemia: ZUMA-3 phase 1 results. Blood 2021;138:11–22.




162. Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet 2021;398:491–502.


163. Roddie C, Sandhu KS, Tholouli E, et al. Safety and efficacy of obecabtagene autoleucel (obe-cel, AUTO1), a fast-off rate CD19 CAR, in relapsed/refractory adult B-cell acute lymphoblastic leukemia (r/r B-ALL): top line results of the pivotal FELIX study. J Clin Oncol 2023;41(16 Suppl):7000–7000.

164. Yoo JW. Management of adverse events in young adults and children with acute B-cell lymphoblastic leukemia receiving anti-CD19 chimeric antigen receptor (CAR) T-cell therapy. Blood Res 2023;58(S1):S20–S28.



165. Kim WS, Kim SJ, Yoon S, Kim JR. Phase 1/2 study of anbalcabtagene autoleucel, novel anti-CD19 CAR-T cell therapy with dual silencing of PD-1 and TIGIT in relapsed or refractory large B-cell lymphoma. J Clin Oncol 2022;40(16 Suppl):7522–7522.

166. Berg SL, Blaney SM, Devidas M, et al.; Children’s Oncology Group. Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children’s Oncology Group. J Clin Oncol 2005;23:3376–3382.


167. Cohen MH, Johnson JR, Massie T, et al. Approval summary: nelarabine for the treatment of T-cell lymphoblastic leukemia/ lymphoma. Clin Cancer Res 2006;12:5329–5335.



168. Commander LA, Seif AE, Insogna IG, Rheingold SR. Salvage therapy with nelarabine, etoposide, and cyclophosphamide in relapsed/refractory paediatric T-cell lymphoblastic leukaemia and lymphoma. Br J Haematol 2010;150:345–351.


169. Shimony S, Liu Y, Valtis YK, et al. Nelarabine combination therapy for relapsed or refractory T-cell acute lymphoblastic lymphoma/leukemia. Blood Adv 2023;7:1092–1102.


170. Gökbuget N, Basara N, Baurmann H, et al. High single-drug activity of nelarabine in relapsed T-lymphoblastic leukemia/ lymphoma offers curative option with subsequent stem cell transplantation. Blood 2011;118:3504–3511.



171. Candoni A, Lazzarotto D, Ferrara F, et al. Nelarabine as salvage therapy and bridge to allogeneic stem cell transplant in 118 adult patients with relapsed/refractory T-cell acute lymphoblastic leukemia/lymphoma. A CAMPUS ALL study. Am J Hematol 2020;95:1466–1472.



172. Chonghaile TN, Roderick JE, Glenfield C, et al. Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer Discov 2014;4:1074–1087.




173. Shah K, Al Ashiri L, Nasimian A, Ahmed M, Kazi JU. Venetoclax-resistant T-ALL cells display distinct cancer stem cell signatures and enrichment of cytokine signaling. Int J Mol Sci 2023;24:5004.



174. Aumann S, Shaulov A, Haran A, Gross Even-Zohar N, Vainstein V, Nachmias B. The emerging role of venetoclax-based treatments in acute lymphoblastic leukemia. Int J Mol Sci 2022;23:10957.



175. Pullarkat VA, Lacayo NJ, Jabbour E, et al. Venetoclax and navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov 2021;11:1440–1453.


176. Richard-Carpentier G, Jabbour E, Short NJ, et al. Clinical experience with venetoclax combined with chemotherapy for relapsed or refractory T-cell acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk 2020;20:212–218.


177. Samra B, Alotaibi AS, Short NJ, et al. Outcome of adults with relapsed/refractory T-cell acute lymphoblastic leukemia or lymphoblastic lymphoma. Am J Hematol 2020;95:E245–E247.



178. Zappone E, Cencini E, Defina M, et al. Venetoclax in association with decitabine as effective bridge to transplant in a case of relapsed early T-cell lymphoblastic leukemia. Clin Case Rep 2020;8:2000–2002.




179. Yue X, Chen Q, He J. Combination strategies to overcome resistance to the BCL2 inhibitor venetoclax in hematologic malignancies. Cancer Cell Int 2020;20:524.




180. Jain N, Stevenson KE, Winer ES, et al. A multicenter phase I study combining venetoclax with mini-hyper-CVD in older adults with untreated and relapsed/refractory acute lymphoblastic leukemia. Blood 2019;134(Supplement 1):3867.


181. Venugopal S, Kantarjian H, Short NJ, et al. A phase II study of mini-hyper-CVD plus venetoclax in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia. Blood 2021;138(Supplement 1):1239.


182. Gocho Y, Liu J, Hu J, et al. Network-based systems pharmacology reveals heterogeneity in LCK and BCL2 signaling and therapeutic sensitivity of T-cell acute lymphoblastic leukemia. Nat Cancer 2021;2:284–299.




183. Larson RA. Managing CNS disease in adults with acute lymphoblastic leukemia. Leuk Lymphoma 2018;59:3–13.


184. Kopmar NE, Cassaday RD. How I prevent and treat central nervous system disease in adults with acute lymphoblastic leukemia. Blood 2023;141:1379–1388.



185. Reman O, Pigneux A, Huguet F, et al.; GET-LALA group. Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis and/or at first relapse: results from the GET-LALA group. Leuk Res 2008;32:1741–1750.


186. Sancho JM, Ribera JM, Oriol A, et al.; Programa para el Estudio y Tratamiento de Hemopatias Malignas Group. Central nervous system recurrence in adult patients with acute lymphoblastic leukemia: frequency and prognosis in 467 patients without cranial irradiation for prophylaxis. Cancer 2006;106:2540–2546.


187. Alfayez M, Kantarjian HM, Short NJ, et al. Safety and efficacy of blinatumomab in patients with Central Nervous System (CNS) disease: a single institution experience. Blood 2018;132(Supplement 1):2702.


188. Leahy AB, Newman H, Li Y, et al. CD19-targeted chimeric antigen receptor T-cell therapy for CNS relapsed or refractory acute lymphocytic leukaemia: a post-hoc analysis of pooled data from five clinical trials. Lancet Haematol 2021;8:e711–e722.



189. Jacoby E, Ghorashian S, Vormoor B, et al. CD19 CAR T-cells for pediatric relapsed acute lymphoblastic leukemia with active CNS involvement: a retrospective international study. Leukemia 2022;36:1525–1532.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



