Abstract
Background/Aims
Systemic lupus erythematosus (SLE) responder index (SRI)-4 response has been achieved with belimumab treatment in patients with moderate disease activity in cornerstone clinical trials and following studies. However, most studies involved patients treated with a mean prednisolone-equivalent dose of approximately 10 mg/d and focused on the steroid-sparing effect of belimumab. We aimed to identify the effect of belimumab in patients with mild-to-moderate SLE who were treated with low-dose or no corticosteroids.
Methods
We retrospectively reviewed the electronic medical records of patients treated with belimumab for at least 6 months between May 2021 and June 2022. The primary endpoint was SRI-4 response at 6 months.
Results
Thirty-one patients were included (13 low dose- and 18 steroid non-users). The mean age was 39.2 ± 11.4 years, and 90.3% of patients were female. The baseline Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) score was 6.0 (4.0–9.0). The primary endpoint was achieved in 32.3% (10/31) of patients. Significant improvements in anemia, C4 levels, and SELENA-SLEDAI score were observed during treatment. Univariate analysis showed that the baseline SELENA-SLEDAI and arthritis were significantly associated with SRI-4 response at 6 months, and only the SELENA-SLEDAI remained significant (p = 0.014) in multivariate analysis.
Conclusions
This cohort study is the first to report the efficacy of belimumab after minimizing the effect of corticosteroids. Belimumab showed efficacy in improving the SELENA-SLEDAI score, anemia, and low C4 in patients who did not receive corticosteroids or received only low doses.
Keywords: Biologic; Systemic lupus erythematosus; Corticosteroid
INTRODUCTION
Belimumab is the first biologic agent approved for the treatment of systemic lupus erythematosus (SLE) by the U.S. Food and Drug Administration (March 9, 2011) [
1]. B cells play an important role in SLE pathogenesis, and B-cell activating factor, also called B-lymphocyte stimulator (BLyS), is one of the mechanisms underlying the increase in B cells. Belimumab is a monoclonal antibody that binds to soluble B-cell activating factor and inhibits its activity [
2]. The Belimumab in Subjects With Systemic Lupus Erythematosus (BLISS)-52 trial showed significant differences between the placebo and belimumab groups in the reduction of the Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) score by ≥ 4 points after 52 weeks (58% in the belimumab [10 mg/kg] group vs. 46% in the placebo group;
p = 0.0024) [
3]. Real- world studies consistently demonstrated a systemic lupus erythematosus responder index (SRI)-4 in patients with moderate disease activity [
4,
5]. In these studies, SRI-4 was significantly associated with baseline features including high SELENA-SLEDAI score, corticosteroid dose, smoking status, BlyS level, and combining polyarthritis [
4,
5]. However, most of the previous studies have been conducted in patients with moderate-to-severe SLE activity treated with a mean prednisolone- equivalent dose of 10–20 mg/d [
3–
6]. The effects of belimumab in patients with SLE who are not undergoing corticosteroid treatment have not been well elucidated. Furthermore, studies have shown that high baseline corticosteroid dosage or prednisolone ≥ 7.5 mg/d were independent predictors of an SRI-4 response [
4,
5]. Therefore, in order to examine the effect of belimumab while minimizing the corticosteroid effect, we reviewed the medical records of SLE patients who either did not receive corticosteroids or received low doses of corticosteroids.
METHODS
Patient and data collection
The present study retrospectively analyzed the data of patients with SLE aged ≥ 18 years and treated with belimumab at a single tertiary center in Seoul, South Korea. As belimumab has been available in South Korea since 2021, patients in the belimumab group first received the drug between May 2021 and June 2022. Patients who experienced adverse effects from corticosteroids or were at risk of developing them were given belimumab instead. If the patient did not meet the national health insurance criteria, belimumab was administered without reimbursement. SLE was diagnosed according to the 2012 Systemic Lupus International Collaborating Clinics Criteria [
7] and 2019 European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) Classification Criteria [
8]. Lupus nephritis (LN) was diagnosed and classified according to the International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 classification system [
9]. This study was performed in accordance with the Declaration of Helsinki and its later amendments. The Institutional Review Board of Asan Medical Center approved this study (IRB No. 2023-0317). Because of the retrospective nature of the study, the requirement of informed consent was waived. Patient information was pseudonymized to reduce the risk of data subject identification according to the Institutional Review Board of Asan Medical Center. After deleting the identification information of research subjects, random research subject numbers were assigned and managed, and personal identification information will not be used even when future research results are published. The following patient data were collected from the electronic medical records: age, sex, time from SLE and LN diagnosis to belimumab administration, type of immunosuppressant (IS), concomitant corticosteroid dose, histological features of kidney biopsy, and laboratory data, including complete blood count (CBC), creatinine level, estimated glomerular filtration rate (eGFR), urine protein/creatinine ratio, complement level, anti-double- stranded DNA (anti-dsDNA) level, and extractable nuclear antigen antibody (ENA). Laboratory data of CBC, creatinine level, eGFR, urine protein/creatinine ratio, complement level, and anti-dsDNA level at 3 and 6 months were also reviewed.
Study variables and outcomes
Belimumab was administered at a dose of 10 mg/kg. Concomitant IS referred to co-administration for at least 1 month. SLE disease activity was measured using the SELENA-SLEDAI score at baseline and at 3 and 6 months after belimumab treatment initiation. Clinical manifestations at baseline indicated whether there were positive items based on the SELENA-SLEDAI. The primary outcome was SRI-4 response at 6 months, and other clinical outcomes including mean SELENA-SLEDAI score, laboratory data including CBC, complement proteins, and anti-ds DNA at 6 months were measured and compared to those at baseline. Leukopenia was defined as white blood cell (WBC) count < 4,000/μL, anemia as hemoglobin < 12.0 mg/dL, and thrombocytopenia as a platelet count < 150 × 103/μL. We also tried to identify the factors associated with the SRI-4 response.
Statistical analysis
Categorical variables are described as frequencies and percentages (n, %), and continuous variables are described as means (standard deviations [SDs]) or medians (interquartile ranges [IQRs]). Parametric and non-parametric data between the two groups were compared using the independent t-test and Mann–Whitney U test, respectively. Categorical variables were compared using chi-square test or Fisher’s exact test. Repeated-measures analysis of variance was performed to compare data at each time point. The p value of post-hoc analysis was corrected using the Bonferroni test. Logistic regression was performed to identify factors associated with the SRI-4 response. The results are described as odds ratios (ORs) and 95% confidence intervals. All statistical analyses were conducted using SPSS (version 21.0; IBM Corp., Armonk, NY, USA). A p value lower than 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
During the study period, 49 patients were treated with belimumab (
Fig. 1). After excluding patients who stopped belimumab treatment before 6 months (n = 2), 31 of the 47 patients who used less than 5 mg of prednisolone or an equivalent dose were included. All patients were followed-up for 6 months after belimumab administration. Of them, 18 were steroid non-users and 13 were steroid users. Steroid non-users were off corticosteroid treatment for a median duration of 476 days (276.8–842.3 d). The baseline characteristics of patients with SLE are shown in
Table 1. The mean age was 39.2 ± 11.4 years, and 28 (90.3%) patients were female. The baseline SELENA-SLEDAI score was 6.0 (4.0–9.0). Sixteen patients (51.6%) were diagnosed with LN confirmed on biopsy before administration of belimumab. According to the ISN/RPS classification system, 9 (56.3%) patients were class III or III + V and 7 (43.8%) were IV or IV + V. At the initiation of administration of belimumab, only 8 showed renal manifestation according to SELENA-SLEDAI (hematuria, proteinuria, or pyuria) (
Table 1). All patients had low complement levels and high anti-ds DNA levels at baseline. We divided the patients into two groups according to their use of corticosteroids: the steroid non-user group (group 1) and the low-dose steroid user group (group 2), the latter of which used less than 5 mg of prednisolone or an equivalent dose. When analyzed according to the use of corticosteroids, no statistically significant differences were found between the two groups.
Changes in clinical parameters in patients administered belimumab
We divided patients into those with and without cytopenia and evaluated improvement over time (
Table 2). Patients with anemia showed a significant increase in hemoglobin levels during belimumab treatment. Patients with leukopenia and thrombocytopenia showed a trend of increasing cell count over time, although this was not significant. Serologically, complement levels increased and anti-dsDNA levels decreased over time, although the only significant finding was the increase in C4 levels. SELENA-SLEDAI decreased significantly after the administration of belimumab (
p = 0.016).
Factors associated with SRI-4 response
Among 31 patients, 10 patients (32.3%) achieved an SRI-4 response at 6 months. Factors associated with the SRI-4 response were then identified using logistic regression analysis (
Table 3). Baseline serology, laboratory findings, presence of LN, concomitant ISs, and ENA positivity did not show a significant association with the SRI-4 response. Baseline SELENA-SLEDAI and the presence of arthritis were significantly associated with the SRI-4 response in univariate analysis, although only SELENA-SLEDAI remained significant in multivariate analysis.
Adverse effects of patients administered belimumab
The adverse effects of belimumab administration are described in
Table 4. Seven patients experienced adverse effects. Of them, one patient recovered after outpatient treatment due to acute gastroenteritis and one patient had an infusion reaction that was resolved with pre-medication of pheniramine before belimumab infusion; all other cases of adverse effects were resolved spontaneously.
DISCUSSION
The steroid-sparing effect of belimumab was previously demonstrated [
3] and is consistent with other studies that 70–90% of patients in the trials were on 10–30 mg/d mean dose of prednisolone-equivalent [
4–
6,
10–
13]. In the present study, we evaluated patients who did not use corticosteroids or who were treated with low-doses. Nonetheless, patients with cytopenia showed a trend of improving cell counts, and serologically, a trend of increasing complement and decreasing anti-ds DNA was observed. Additionally, SELENA-SLEDAI decreased significantly over 6 months.
In a previous study on cytopenia, patients with leukopenia, anemia, and thrombocytopenia all showed significant improvement at the 24-month follow-up after the administration of belimumab [
10]. Regarding the serologic effects of belimumab, a significant increase in C3 and C4 and a significant decrease in anti-ds DNA have been reported after 52 weeks of belimumab administration [
14]. Our results also support these results showing significant changes in hemoglobin levels and C4 levels and changing trends in WBC counts, platelet counts, and anti-ds DNA levels. Since the effect of belimumab on serologic activity has been demonstrated in larger studies [
4,
14], we should consider the possibility that significant effects may not have been detected in the present study because of the small number of patients.
The SRI-4 response rate of our study was lower than that in previous studies, which may be a result of the shorter follow-up period of our study. However, it is lower than that in other studies that had similar follow-up durations. An Italian multicenter cohort study of 446 subjects with a mean SLEDAI-2K score of 9.3 ± 3.3 of whom 39.4% had a SLEDAI-2K ≥ 10 reported an SRI-4 response rate of 49.2% at 6 months [
15]. We hypothesized that the underlying disease activity in the patients in this study was mild-to-moderate and that most patients scored four points because they had a positive SELENA-SLEDAI serology item; therefore, improvement in 6 months may be difficult to achieve. A previous study showed that in 35.7% of patients, the median time to anti-ds DNA seroconversion was 6.6 months, and in 38.7% of patients, C3 and C4 normalized in a median time of 7 months [
5].
There has been extensive research on the predictors of SRI-4 response [
4,
5,
15]. Known predictors included initial SLEDAI-2K ≥ 10, polyarthritis, PD ≥ 7.5 mg/d, high baseline prednisone equivalent dosages, and high baseline BlyS level [
4,
5,
15]. Current smoking status was reported as a negative predictor of the SRI-4 response rate [
5]. Consistent with previous findings, arthritis and baseline SELENA-SLEDAI also showed predictive value in our univariate analysis, and SELENA-SLEDAI remained significant in multivariate analysis.
The present study has several limitations. First, the study was conducted in a single center and involved only Asian patients. Second, belimumab was only recently introduced in South Korea. Therefore, patient groups were small, and long-time follow-up results could not be presented. Longterm follow-up studies with more patients are needed.
In summary, belimumab was shown to be effective in improving SELENA-SLEDAI, anemia, and low C4 in patients treated with no or low doses of corticosteroids. Therefore, belimumab may be beneficial for patients with mild-to-moderate SLE activity, including those who were not undergoing corticosteroid treatment.
KEY MESSAGE
1. Among SLE patients who did not receive corticosteroids or received low doses, 32.3% achieved SRI-4 response within 6 months of belimumab treatment.
2. SLE patients with low-dose or no corticosteroids showed improvement in anemia, complement levels, and SELENA-SLEDAI after treatment with belimumab.
3. Baseline SELENA-SLEDAI was significantly associated with SRI-4 response in patients with minimal use of corticosteroids.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author, YG Kim. The data are not publicly available due to ethical review board restrictions as it contains information that could compromise the privacy of research participants.
Figure 1
Patient selection flowchart.
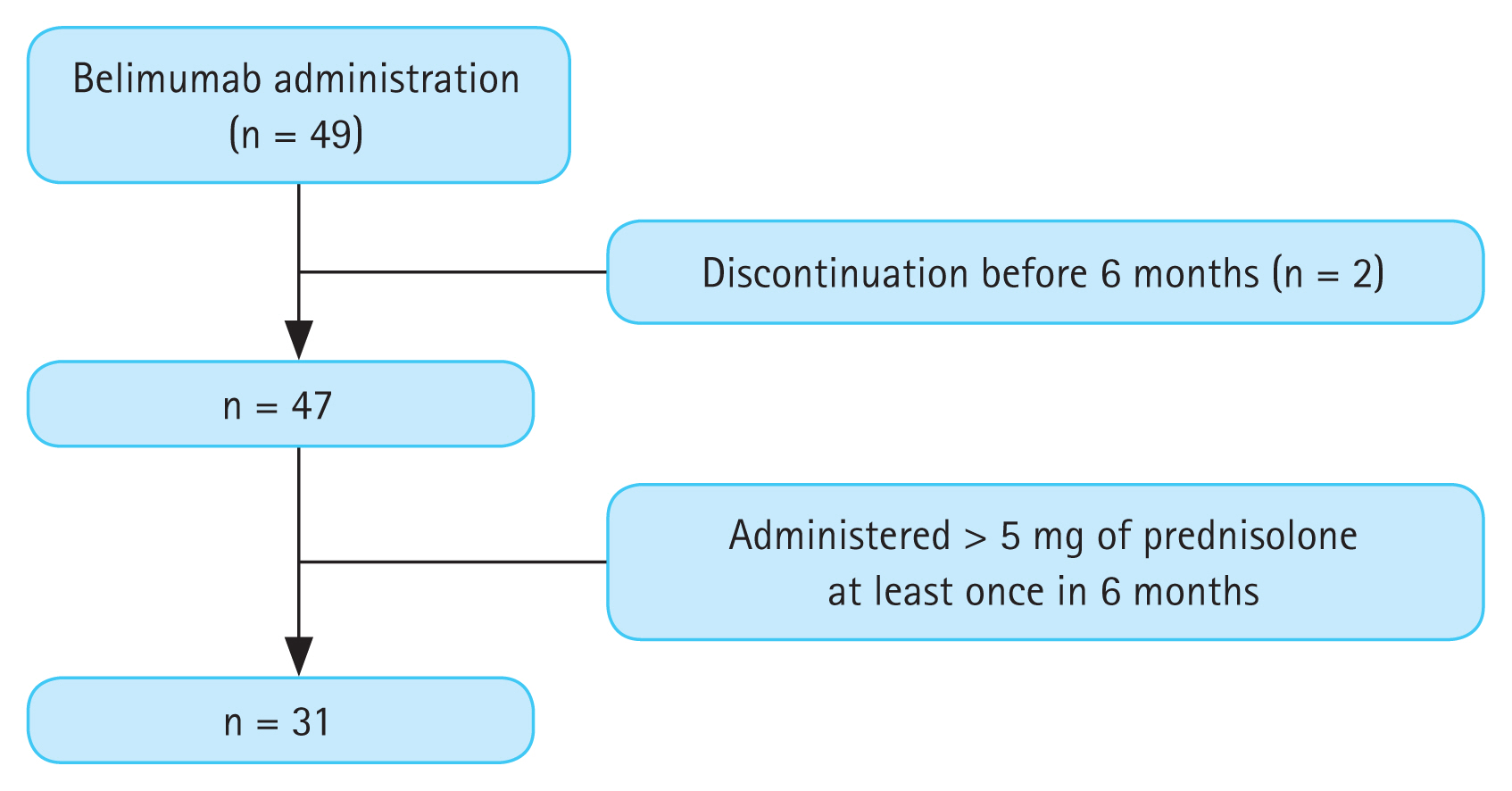
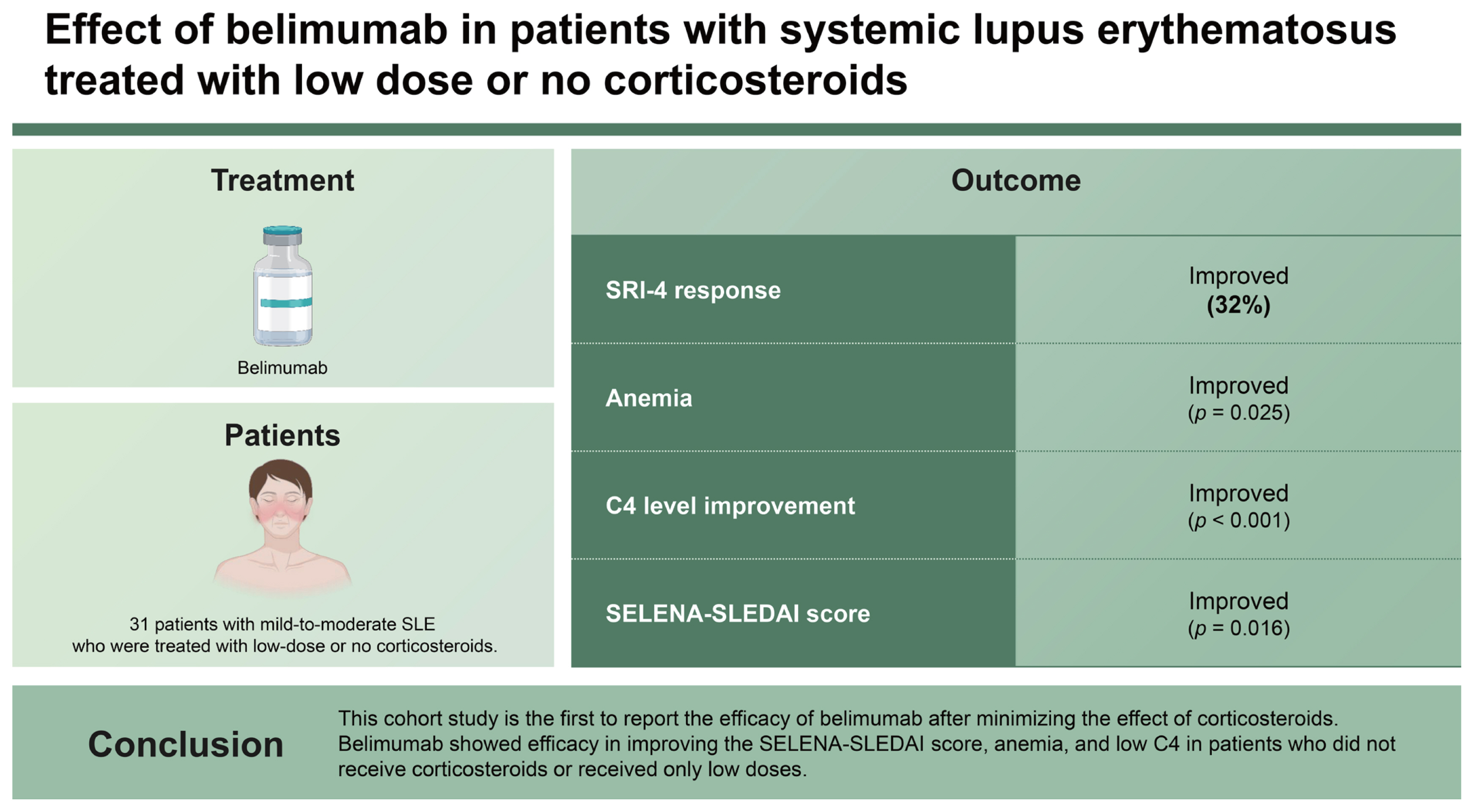
Table 1
Baseline characteristics of patients with SLE treated with belimumab and low dose or no corticosteroids
|
Variable |
All (n = 31) |
No corticosteroid (n = 18) |
Low dose corticosteroid (n = 13) |
p value |
|
Age, yr |
39.2 ± 11.4 |
39.1 ± 9.7 |
39.2 ± 13.9 |
0.978 |
|
Sex, female |
28 (90.3) |
16 (88.9) |
12 (92.3) |
0.755 |
|
Duration since SLE diagnosis, mo |
169.8 ± 96.8 |
188.1 ± 98.5 |
144.6 ± 92.2 |
0.223 |
|
Duration since LN diagnosis, mo |
119.4 ± 70.4 |
118.0 ± 75.3 |
123.8 ± 62.9 |
0.893 |
|
SELENA-SLEDAI |
6.0 (4.0–9.0) |
5.5 (4.0–10.5) |
6.0 (4.0–8.5) |
0.919 |
|
LN |
16 (51.6) |
12 (66.7) |
4 (30.8) |
0.052 |
|
Corticosteroid dose, mg/d |
|
|
5.0 (2.5–5.0) |
|
|
Clinical manifestations |
|
Renal |
8 (25.8) |
4 (22.2) |
4 (30.8) |
0.598 |
|
Skin |
7 (22.6) |
2 (11.1) |
5 (38.5) |
0.077 |
|
Arthritis |
6 (19.4) |
2 (11.1) |
4 (30.8) |
0.179 |
|
Hematologic |
12 (38.7) |
9 (50.0) |
3 (23.1) |
0.135 |
|
Laboratory findings |
|
WBC, /μL |
4,077.4 ± 1,618.8 |
3,944.4 ± 1,878.7 |
4,261.5 ± 1,219.7 |
0.599 |
|
Hb, mg/dL |
11.45 ± 1.57 |
11.18 ± 1.66 |
11.81 ± 1.42 |
0.283 |
|
Platelet, × 103/μL |
187.7 ± 73.44 |
180.4 ± 81.61 |
197.8 ± 62.09 |
0.525 |
|
ESR, mm/h |
32.9 ± 27.5 |
33.6 ± 28.0 |
32.0 ± 27.9 |
0.882 |
|
CRP, mg/dL |
0.10 (0.10–0.18) |
0.14 (0.10–0.20) |
0.10 (0.10–0.10) |
0.049 |
|
Creatinine, mg/dL |
0.79 ± 0.22 |
0.84 ± 0.25 |
0.73 ± 0.16 |
0.146 |
|
eGFR, mL/min/1.73 m2
|
80.7 ± 13.7 |
77.9 ± 15.4 |
84.5 ± 10.3 |
0.190 |
|
UPCR, mg/g |
162.7 (112.8–280.6) |
171.8 (109.1–406.3) |
146.8 (111.4–176.7) |
0.311 |
|
ENA positivity |
|
Anti-RNP, U/mL |
20 (64.5) |
14 (77.8) |
6 (46.2) |
0.074 |
|
Anti-Sm, U/mL |
16 (51.6) |
10 (55.6) |
6 (46.2) |
0.611 |
|
Anti-Ro, U/mL |
22 (71.0) |
15 (83.3) |
7 (53.8) |
0.079 |
|
Anti-La, U/mL |
12 (38.7) |
8 (44.4) |
4 (30.8) |
0.448 |
|
Anti-Scl70, U/mL |
1 (3.2) |
0 (0.0) |
1 (7.7) |
0.253 |
|
C3, mg/dL |
64.6 (46.9–72.7) |
61.6 (45.0–69.8) |
68.3 (51.5–74.4) |
0.357 |
|
C4, mg/dL |
10.25 ± 5.14 |
9.51 ± 5.76 |
11.27 ± 4.16 |
0.357 |
|
Anti-dsDNA, IU/mL |
86.6 (32.8–158.0) |
86.9 (34.4–228.0) |
80.6 (30.3–132.5) |
0.484 |
|
Concomitant IS (0–6 mo) |
|
Hydroxychloroquine |
31 (100.0) |
18 (100.0) |
13 (100.0) |
> 0.999 |
|
Mycophenolate mofetil |
17 (54.8) |
11 (61.1) |
6 (46.2) |
0.417 |
|
Tacrolimus |
8 (25.8) |
6 (33.3) |
2 (15.4) |
0.268 |
|
Methotrexate |
5 (16.1) |
3 (16.7) |
2 (15.4) |
0.925 |
|
Azathioprine |
4 (12.9) |
0 (0.0) |
4 (30.8) |
0.013 |
|
Previous treatment |
|
Hydroxychloroquine |
31 (100.0) |
18 (100.0) |
13 (100.0) |
> 0.999 |
|
Azathioprine |
22 (71.0) |
13 (72.2) |
9 (69.2) |
0.859 |
|
Mycophenolate mofetil |
21 (67.7) |
14 (77.8) |
7 (53.8) |
0.166 |
|
Methotrexate |
16 (51.6) |
8 (44.4) |
8 (61.5) |
0.355 |
|
Tacrolimus |
12 (38.7) |
9 (50.0) |
3 (23.1) |
0.135 |
|
Cyclophosphamide |
11 (35.5) |
9 (50.0) |
2 (15.4) |
0.051 |
|
Rituximab |
6 (19.4) |
5 (27.8) |
1 (7.7) |
0.169 |
Table 2
Changes in clinical parameters in patients with SLE treated with belimumab and no or low dose of corticosteroids (n = 31)
|
Variable |
Baseline |
Duration of 0–3 months |
Duration of 0–6 months |
p value |
Post-hoc test |
|
p valuea)
|
p valueb)
|
p valuec)
|
|
CBC |
|
WBC, /μL |
|
Leukopenia (+) |
2,713.3 ± 597.5 |
246.7 ± 639.0 |
333.3 ± 882.1 |
0.143 |
0.246 |
> 0.999 |
0.430 |
|
Leukopenia (−) |
5,356.3 ± 1,141.3 |
−387.5 ± 831.8 |
−368.8 ± 955.5 |
0.165 |
0.471 |
> 0.999 |
0.496 |
|
Hb, mg/dL |
|
Anemia (+) |
10.56 ± 1.13 |
0.17 ± 0.87 |
0.40 ± 0.73 |
0.025 |
1.000 |
0.424 |
0.074 |
|
Anemia (−) |
13.06 ± 0.72 |
−0.37 ± 0.87 |
−0.22 ± 1.15 |
0.543 |
0.557 |
> 0.999 |
> 0.999 |
|
Platelet, × 103/μL |
|
Thrombocytopenia (+) |
106.0 ± 30.4 |
24.0 ± 29.8 |
21.5 ± 49.4 |
0.153 |
0.240 |
1.000 |
0.459 |
|
Thrombocytopenia (−) |
232.6 ± 45.2 |
9.6 ± 46.1 |
8.9 ± 34.0 |
0.301 |
> 0.999 |
0.899 |
0.902 |
|
Serology |
|
C3, mg/dL |
59.13 ± 15.46 |
1.16 ± 9.95 |
6.24 ± 21.51 |
0.117 |
> 0.999 |
0.505 |
0.351 |
|
C4, mg/dL |
10.25 ± 5.14 |
2.15 ± 2.56 |
3.21 ± 4.57 |
< 0.001 |
< 0.001 |
0.278 |
0.001 |
|
Anti-dsDNA, IU/mL |
138.41 ± 159.56 |
−45.76 ± 104.46 |
−36.70 ± 174.88 |
0.252 |
0.063 |
> 0.999 |
0.755 |
|
Renal profile |
|
Creatinine, mg/dL |
0.79 ± 0.22 |
0.04 ± 0.12 |
0.02 ± 0.09 |
0.168 |
0.243 |
> 0.999 |
0.503 |
|
eGFR, mL/min/1.73m2
|
80.71 ± 13.67 |
−1.90 ± 6.10 |
−2.13 ± 6.44 |
0.076 |
0.277 |
> 0.999 |
0.227 |
|
UPCR, mg/g |
418.10 ± 777.02 |
−108.02 ± 350.99 |
−100.15 ± 413.98 |
0.306 |
0.423 |
> 0.999 |
0.917 |
|
SELENA-SLEDAI |
7.58 ± 4.23 |
−2.00 ± 3.25 |
−1.87 ± 4.08 |
0.016 |
0.005 |
> 0.999 |
0.048 |
Table 3
Univariate and multivariate analysis of factors associated with SRI-4 response (n = 31)
|
Variable |
Unadjusted OR (95% CI) |
p value |
Adjusted OR (95% CI) |
p value |
|
Age |
1.043 (0.973–1.117) |
0.237 |
|
|
|
Duration from SLE diagnosis to treatment |
1.002 (0.994–1.010) |
0.649 |
|
|
|
Duration from LN diagnosis to treatment |
0.982 (0.956–1.009) |
0.200 |
|
|
|
Baseline SELENA-SLEDAI |
2.109 (1.230–3.618) |
0.007 |
1.972 (1.145–3.394) |
0.014 |
|
Baseline C3 |
1.018 (0.967–1.073) |
0.493 |
|
|
|
Baseline C4 |
1.023 (0.882–1.186) |
0.767 |
|
|
|
Baseline anti-dsDNA |
1.002 (0.998–1.007) |
0.355 |
|
|
|
Baseline UPCR |
1.001 (1.000–1.002) |
0.177 |
|
|
|
Baseline creatinine |
0.058 (0.001–4.760) |
0.206 |
|
|
|
Baseline eGFR |
1.033 (0.964–1.107) |
0.363 |
|
|
|
Baseline CRP |
14.912 (0.029–7,564.754) |
0.395 |
|
|
|
Baseline ESR |
0.998 (0.970–1.027) |
0.882 |
|
|
|
LN |
0.264 (0.053–1.325) |
0.106 |
|
|
|
Clinical manifestation |
|
Renal |
2.833 (0.534–15.042) |
0.221 |
|
|
|
Skin |
1.821 (0.321–10.342) |
0.499 |
|
|
|
Arthritis |
20.000 (1.888–211.842) |
0.013 |
4.282 (0.254–72.082) |
0.313 |
|
Hematologic |
3.750 (0.772–18.209) |
0.101 |
|
|
|
Concomitant IS (0–6 mo) |
|
MMF |
0.214 (0.042–1.092) |
0.064 |
|
|
|
Azathioprine |
2.375 (0.283–19.924) |
0.425 |
|
|
|
Tacrolimus |
0.222 (0.023–2.122) |
0.191 |
|
|
|
Methotrexate |
4.071 (0.558–29.725) |
0.166 |
|
|
|
ENA positivity |
|
Anti-RNP, U/mL |
0.400 (0.084–1.903) |
0.250 |
|
|
|
Anti-Sm, U/mL |
0.500 (0.108–2.314) |
0.375 |
|
|
|
Anti-Ro, U/mL |
5.538 (0.586–52.331) |
0.135 |
|
|
|
Anti-La, U/mL |
3.750 (0.772–18.209) |
0.101 |
|
|
Table 4
Adverse effects in patients treated with belimumab (n = 31)
|
Age, yr |
Sex |
Adverse effect |
Management |
|
1 |
59 |
F |
Skin rash |
Resolved by pre-medication |
|
2 |
34 |
F |
Lightheadedness |
Resolved without treatment |
|
3 |
48 |
F |
Abdominal pain |
Resolved without treatment |
|
4 |
38 |
F |
Dizziness |
Resolved without treatment |
|
5 |
44 |
F |
Skin reaction |
Resolved without treatment |
|
6 |
27 |
F |
Skin reaction |
Resolved without treatment |
|
7 |
34 |
M |
Acute gastroenteritis (outpatient) |
Resolved by medication |
REFERENCES
2. Hahn BH. Belimumab for systemic lupus erythematosus. N Engl J Med 2013;368:1528–1535.


3. Navarra SV, Guzmán RM, Gallacher AE, et al.; BLISS-52 Study Group. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:721–731.


4. Iaccarino L, Andreoli L, Bocci EB, et al. Clinical predictors of response and discontinuation of belimumab in patients with systemic lupus erythematosus in real life setting. Results of a large, multicentric, nationwide study. J Autoimmun 2018;86:1–8.


5. Parodis I, Sjöwall C, Jönsen A, et al. Smoking and pre-existing organ damage reduce the efficacy of belimumab in systemic lupus erythematosus. Autoimmun Rev 2017;16:343–351.


7. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–2686.


8. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol 2019;71:1400–1412.


9. Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 2004;15:241–250.









 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



