 |
 |
| Korean J Intern Med > Volume 38(6); 2023 > Article |
|
Abstract
Background/Aims
We evaluated nailfold capillaroscopy (NFC) of interstitial pneumonia with autoimmune features (IPAF) and compared it with that of patients with connective tissue disease-interstitial lung disease (CTD-ILD) and idiopathic interstitial pneumonia (IIP).
Methods
Patients with newly diagnosed as ILD were evaluated using NFC. Baseline demographic, clinical, serological, and high-resolution CT findings were collected. NFC was semi-quantitatively scored with six domains ranging from 0 to 18. In addition, the overall patterns (scleroderma/non-scleroderma patterns) were determined.
Results
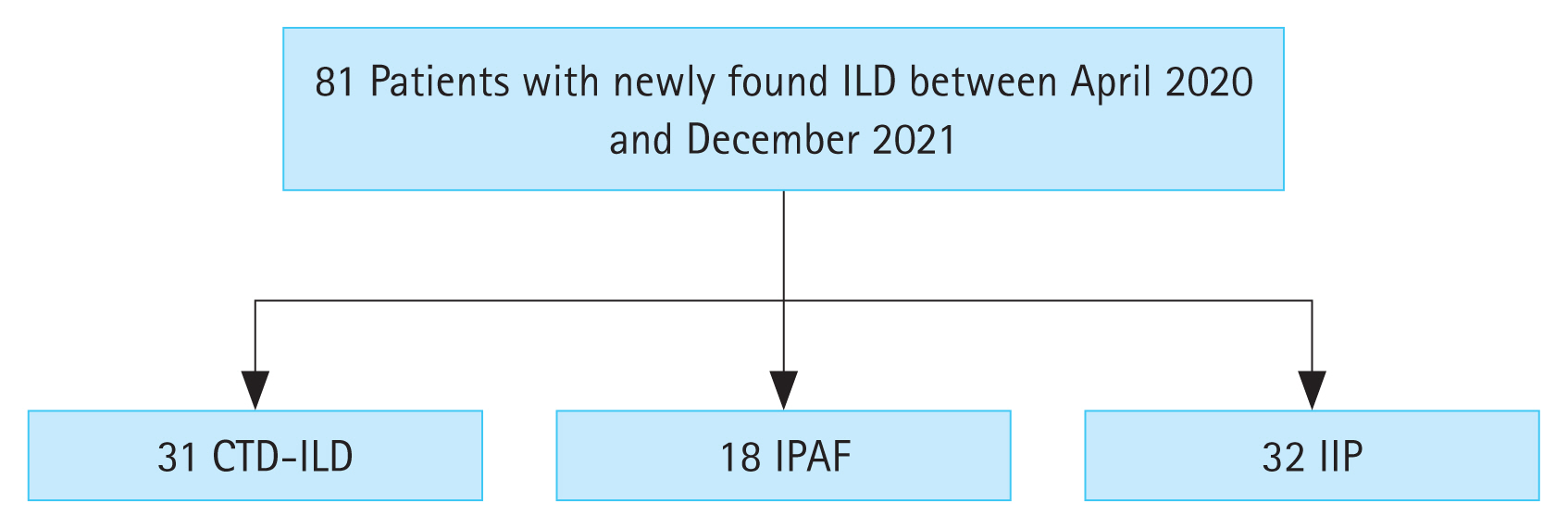
A total of 81 patients (31 with CTD-ILD, 18 with IPAF, and 32 with IIP) were included. The non-specific interstitial pneumonia pattern was the most common ILD pattern in the CTD-ILD and IPAF groups, whereas the usual interstitial pneumonia pattern was the most common in the IIP group. The semi-quantitative score of the CTD-ILD group was higher than that of the IPAF or IIP groups (5.8 vs 4.2 vs 3.0, p < 0.001, respectively). Giant capillaries and haemorrhages were more frequently present in the CTD-ILD and IPAF groups than in the IIP group. A scleroderma pattern was present in 27.8% of the IPAF group, whereas none of the IIP patients showed a scleroderma pattern.
Interstitial lung disease (ILD) is a relatively common pulmonary manifestation in autoimmune diseases [1]. The ILD can be categorized into five subgroups: 1) idiopathic interstitial pneumonia (IIP), 2) connective tissue disease (CTD)-ILD, 3) hypersensitivity pneumonitis, 4) sarcoidosis-related ILD, and 5) other ILD [2]. Treatment options for CTD-ILD differ from those for IIP [3]. The concept of interstitial pneumonia with autoimmune features (IPAF) was introduced by Mosca et al. [4]. Patients with IPAF simultaneously presented with ILD and some autoimmune features but did not fulfil the specific criteria for autoimmune disease. The European Respiratory Society (ERS)/American Thoracic Society (ATS) Task Force on Undifferentiated Forms of Connective Tissue Disease-ILD established classification criteria for IPAF [5]. IPAF is assumed to be a preclinical stage of autoimmune disease that predominantly presents with pulmonary manifestations (ILD) [6].
The classification criteria for systemic sclerosis (SSc) include ILD [7]. Although classification criteria for SSc includes ILD as one item as classification criteria, however, other autoimmune diseases, such as rheumatoid arthritis (RA), inflammatory myositis, primary Sj├ČgrenŌĆÖs syndrome (pSS), and systemic lupus erythematosus (SLE) can also present with ILD [3,8]. Nailfold capillaroscopy (NFC) can detect abnormalities in microcirculation, and abnormal findings of NFC are mainly found in patients with SSc [9]. Semi-quantitative measurement of NFC was introduced by Cutolo, which includes six components (capillary density, dilated capillary, giant capillary, haemorrhage, ramification, and disorganization of the vascular array), and this scoring system was reliable for monitoring microcirculation damage in patients with SSc [9]. Recent classification criteria for SSc suggested abnormal NFC as one of the items in the classification criteria [7], and the European Alliance of Associations for Rheumatology (EULAR) Study Group on Microcirculation in Rheumatic Diseases (EULAR SG MC/RD) established a standardized definition of scleroderma and non-scleroderma patterns of NFC [10]. Patients with RaynaudŌĆÖs phenomenon (RP) who presented with a scleroderma pattern of NFC showed a likelihood of progression to CTD [11], whereas RP patients without scleroderma pattern in the NFC showed a high predictive value for excluding SSc [12]. One prospective multi-centre study revealed that patients with combination of RP, SSc specific autoantibodies, and puffy fingers had higher risk for progression to SSc [13]. The scleroderma pattern of NFC is not only found in patients with SSc but also in other autoimmune diseases, such as inflammatory myositis and mixed connective tissue disease (MCTD) [14]. Although, previous study evaluated NFC finding in patients with IPAF [15], however, semi-quantitative scoring of NFC in IPAF patients was not assessed, yet.
In the present study, we aimed to measure the semi-quantitative scoring of the NFC in IPAF patients and compare this with patients with CTD-ILD and IIP. Furthermore, the frequency of scleroderma patterns according to the EULAR SG MC/RD in each group was evaluated.
Patients with newly found ILD were evaluated with NFC in a single tertiary university-based hospital, Konkuk University Medical Center, from April 2020 to December 2021. The study was conducted by cross-sectional study. The inclusion criteria were as follows: 1) age over 19 years, 2) underwent NFC, and 3) presence of chest computed tomography (CT). The patients who fulfilled specific criteria for autoimmune diseases (SSc, RA, pSS, inflammatory myositis, SLE, MCTD) were grouped as CTD-ILD group [7,16ŌĆō20]. Patients who did not fulfil specific classification criteria for an autoimmune disease but satisfied the 2015 ERS/ATS criteria were classified into the IPAF group [5]. Patients with ILD who did not have other aetiologies of ILD were classified into the IIP group. This study was conducted following the Declaration of Helsinki and Good Clinical Practice guidelines, and was approved by the Institutional Review Board (IRB) of Konkuk University Medical Center (IRB no: 2020-04-017). Written informed consent was obtained from all participants before enrolment.
Patients suspected of having ILD on a plain chest radiograph underwent chest CT to determine the morphologic pattern of ILD and were classified as usual interstitial pneumonia (UIP), nonspecific interstitial pneumonia (NSIP), organizing pneumonia (OP), NSIP + OP, or lymphoid interstitial pneumonia. In the serologic domain, antinuclear antibody (ANA) was evaluated by indirect immunofluorescence staining using the Hep-2 cell line. Rheumatoid factor (RF), anti-citrullinated peptide antibody, anti-double-stranded DNA Ab, anti-Ro/SSA, anti-La/SSB, anti-ribonucleoprotein, anti-Smith, anti-Scl-70, anti-Jo-1, and anti-centromere Abs were measured by ELISA. ANA was considered positive when the ANA titre was Ōēź 1:320 with a diffuse, homogeneous, or speckled pattern, or any titre with a nucleolar/centromere pattern. An RF titre more than twice the upper reference range was considered positive. The clinical domains were checked by a trained rheumatologist and recorded in electronic medical records.
NFC was performed using video capillaroscopy (YP0901, 200├Ś magnification; Starling Force Co., Ltd., Seoul, Korea) in the 2nd to 5th fingers bilaterally of each patient. The semi-quantitative scoring of NFC was calculated by trained a rheumatologist (S.H. Kim) according to CutoloŌĆÖs method, which is composed of six components, each of which can range from 0 to 3 (total score ranged from 0 to 18) [9], and an average of eight digits was recorded. In addition, according to the EULAR SG MC/RD fast-track algorithm, the overall pattern of NFC findings was categorized into scleroderma (early, active, or late) or non-scleroderma (normal or non-specific abnormality) [10].
The previous study showed that abnormal finding of NFC was found in 10% of IPAF patients, whereas 78% of CTD-ILD patients had NFC abnormality [15]. Assuming alpha 5%, and statistical power (1 ŌłÆ beta) 80%, two-sided testing, and a drop-out rate of 10%, the required number of samples was at least 18 for each group.
One-way analysis of variance or Wilcoxon signed-rank test was properly selected for the analysis of continuous variables and then presented as mean ┬▒ standard deviation or median with interquartile range. A posthoc analysis was performed using the Bonferroni method. Categorical variables were compared using the chi-squared test or FisherŌĆÖs exact test. Statistical significance was set at p < 0.05. All tests were performed using the R software (R for Windows 3.3.2; The R Foundation for Statistical Computing, Vienna, Austria).
A total of 81 patients (31 with CTD-ILD, 18 with IPAF, and 32 with IIP) were included in the analysis. The inclusion and exclusion criteria are described in Fig. 1. The CTD-ILD group comprised 16 patients with SSc, five patients with pSS, three patients with RA, three patients with inflammatory myositis, two patients with SLE, and two patients with MCTD. The mean age of the CTD-ILD group was significantly lower than those of the IPAF and IIP groups (p < 0.001). The proportion of female patients was significantly higher in the CTD-ILD group (77.4%) than in the IIP group (34.4%), and the IPAF group had a higher proportion of female patients than the IIP group. The most frequent morphological pattern of ILD was NSIP in both the CTD-ILD and IPAF groups, whereas UIP was the most frequent ILD pattern in the IIP group. Among the clinical domains, RP was more frequently present in the CTD-ILD and IPAF groups, and the presence of RP was significantly higher in these two groups than in the IIP group (p < 0.001). The duration of RP was comparable between three groups: 16.2 ┬▒ 7.1, 16.4 ┬▒ 5.0, and 15.0 months in CTD-ILD, IPAF, and IIP group, respectively (p = 0.980). In addition, inflammatory arthritis/polyarticular morning stiffness and puffy fingers were more frequently observed in the CTD-ILD and IPAF groups than in the IIP group (p = 0.009 and p = 0.020, respectively). In the autoantibody profile, positivity for ANA was the most frequent serologic component in the CTD-ILD and IPAF groups. Furthermore, most of the autoantibodies were more frequently present in the CTD-ILD group. The presence of anti-dsDNA Ab/anti-Ro/SSA/anti-RNP Ab/anti-Scl70 Ab was significantly higher in CTD-ILD than IPAF or IIP groups. Detailed information on the baseline characteristics is summarized in Table 1.
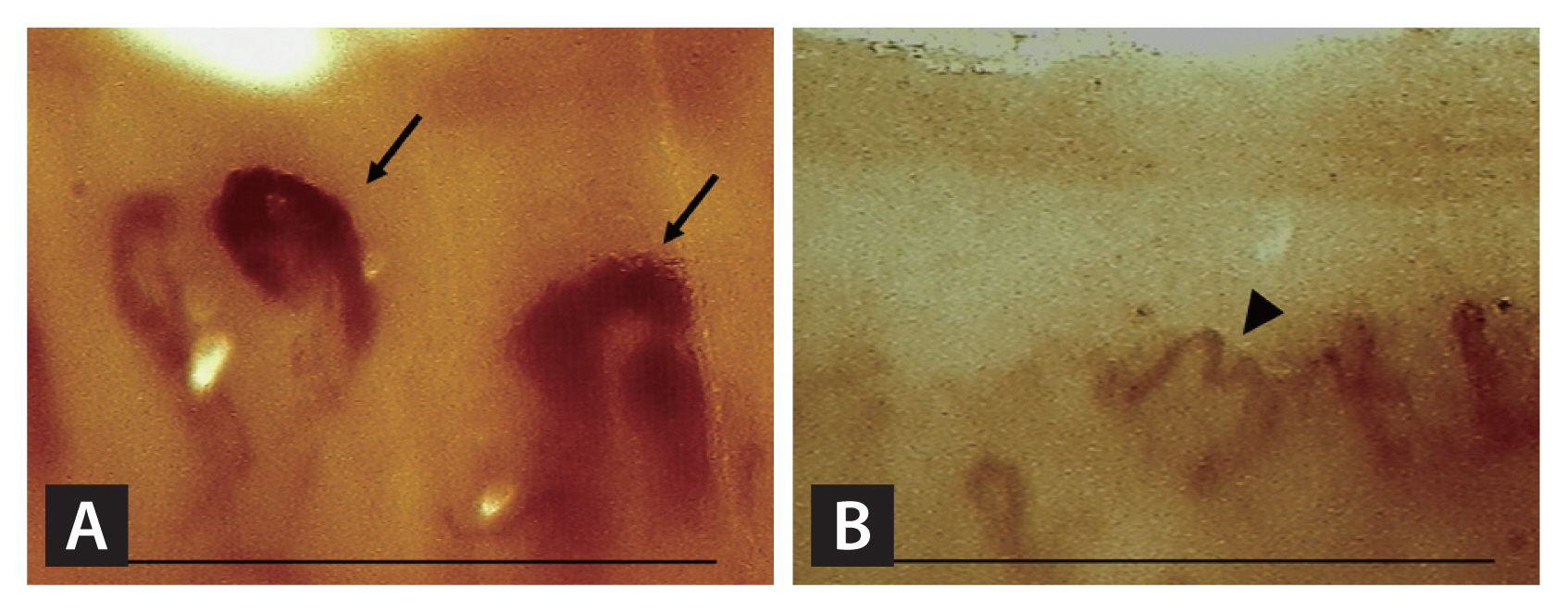
The semiquantitative scores of the NFC in the CTD-ILD, IPAF, and IIP groups were 5.8, 4.2, and 3.0, respectively. The semi-quantitative score of the IPAF group was lower than that of the CTD-ILD group (p = 0.022) but higher than that of the IIP group (p = 0.048). Giant capillaries and haemorrhage of the nailfold showed significant differences between the CTD-ILD, IPAF, and IIP groups (p < 0.001 and p = 0.017, respectively). The giant capillaries were even more abundant in the CTD-ILD group than in the IPAF group (48.4% vs. 16.7%). Giant capillaries and ramifications were not observed in the IIP group. Regarding the EULAR SG MC/RD definition for the overall pattern, 18 patients (58.1%) in the CTD-ILD group and five patients (27.8%) in the IPAF group showed a scleroderma pattern, whereas none of the patients in the IIP group demonstrated a scleroderma pattern. In the IPAF group, two patients (11.1%) showed a UIP pattern in the morphological domain, and these two patients did not show a scleroderma pattern in the NFC. Representative NFC images of the scleroderma pattern in the IPAF group are shown in Fig. 2. All the detailed NFC findings are presented in Table 2. In addition, the clinical, serologic, and morphologic domains of IPAF classification criteria of IPAF patients who had scleroderma pattern in NFC were summarized in Table 3.
In the present study, we demonstrated that NFC findings differed among patients with CTD-ILD, IPAF, and IIP. The semiquantitative score increased in the order of CTD-ILD, IPAF, and IIP. Furthermore, some patients with IPAF showed scleroderma patterns in the NFC.
The NFC finding is one of the classification criteria for SSc [7]. Sulli et al. [9] initially demonstrated the usefulness of NFC in diagnosing SSc and defined six components to quantify NFC abnormalities in patients with SSc. The EULAR SG MC/RD established a fast-track algorithm for discriminating NFC findings for scleroderma and non-scleroderma patterns [10]. The aforementioned algorithm simplifies the definition of the scleroderma pattern of NFC by focusing on the density of capillaries, giant capillaries, and abnormal capillary [10]. The presence of scleroderma pattern in NFC has predictive value in diagnosing CTD, whereas the absence of scleroderma pattern in NFC could exclude SSc [11,12]. In addition, NFC findings can predict SSc progression [21]. The classification criteria for IPAF were recently established by the ERS/ATS research group [5], and IPAF was stated with various terms until the establishment of recent classification criteria for IPAF [6,22]. IPAF has intermediate characteristics of IIP and CTD-ILD, and studies showed that 13.5ŌĆō18.8% of patients with IPAF developed definite CTD [23,24]. Although the follow-up duration was relatively short (mean follow-up duration: 12ŌĆō45 mo) and only about 13.5ŌĆō18.8% developed definite CTD, the study showed that IPAF patients should be considered as having a pre-clinical autoimmune disease and emphasized the need to identify predictors of definite CTD [23,24]. In the present study, the scleroderma pattern was predominantly present in the CTD-ILD group, but a scleroderma pattern was also found in 27.8% of patients in the IPAF group. The subgroup of IPAF patients with a scleroderma pattern in the NFC may develop definite CTD.
The treatment targets of IIP and CTD-ILD differ. The treatment target of CTD-ILD is to reduce inflammatory and autoimmune responses [3,25], whereas IIP (especially idiopathic pulmonary fibrosis) aims to attenuate the fibrosis process [26,27]. In addition, the survival rate was better in the CTD-ILD and IPAF groups than IIP group [28]. Therefore, discriminating between CTD-ILD, IPAF, and IIP is important in patients who are initially diagnosed with ILD. Several studies have shown the predictive role of NFC in differentiating CTD-ILD from IIP [11,12,14], and some recommend performing NFC when ILD is initially diagnosed to diagnose underlying CTD [29]. One study compared NFC findings between CTD-ILD, IPAF, and IIP and reported that reduced capillary density, giant capillaries, and microhaemorrhages were associated with CTD-ILD [30]. But, the study was performed using a smartphone dermatoscope [30], which cannot sufficiently magnify the NFC and is only recommended as a screening test [31,32]. The present study compared semiquantitative scores between the CTD-ILD, IPAF, and IIP groups using video capillaroscopy for the first time. We showed that IPAF patients had higher semiquantitative scores of NFC than the IIP group, but lower scores than CTD-ILD patients, which is similar to the fact that IPAF may be the pre-clinical state of CTD.
The present study has several limitations. First, the number of patients included was relatively small. Second, the study was performed in a cross-sectional manner and included only baseline data. Therefore, the predictive or prognostic role of the NFC in IPAF could not be evaluated in the present study. Third, the morphologic pattern of ILD was only assessed using CT, and none of the enrolled patients underwent biopsy confirmation. Fourth, the UIP pattern was predominant in the IIP group, whereas the NSIP pattern was the most common ILD pattern in the CTD-ILD and IPAF groups. This difference could be due to the intrinsic bias that affected the semi-quantitative score of the NFC in the present study.
In conclusion, the NFC finding of IPAF is intermediate between that of CTD-ILD and IIP. In addition, about one-fourth of IPAF patients showed a scleroderma pattern of NFC, which may be a key clue for predicting CTD development in IPAF patients.
Notes
CRedit authorship contributions
Sang-Heon Lee: conceptualization, data curation, methodology; Hong Ki Min: conceptualization, data curation, formal analysis, methodology, visualization, writing - original draft, writing - review & editing; Se Hee Kim: data curation; Young Whan Kim: data curation; Kwang Ha Yoo: data curation; Hee Joung Kim: conceptualization, data curation; In Ae Kim: data curation; Hae-Rim Kim: conceptualization, data curation, funding acquisition, writing - review & editing
Figure┬Ā1
Flow chart for patient enrolment. CTD, connective tissue disease; ILD, interstitial lung disease; IIP, idiopathic interstitial pneumonia; IPAF, interstitial pneumonia with autoimmune features.
Figure┬Ā2
Representative nailfold capillaroscopy findings of scleroderma patterns according to EULAR Study Group on Microcirculation in Rheumatic Diseases in IPAF group. (A) ŌĆ£ActiveŌĆØ scleroderma pattern (decreased capillary density: 4 capillaries/1 mm, giant capillary: black arrow) (B) ŌĆ£LateŌĆØ scleroderma pattern (decreased capillary density: 3 capillaries/1mm, abnormal morphology: black arrowhead). Scale bar = 1 mm.
Table┬Ā1
Baseline characteristics of enrolled patients
| Variable | CTD-ILD (n = 31) | IPAF (n = 18) | IIP (n = 32) | p value |
|---|---|---|---|---|
| Sex, female | 24 (77.4)a) | 10 (55.6)b) | 11 (34.4)c) | 0.003 |
| Age (yr) | 58.3 ┬▒ 13.1a) | 69.9 ┬▒ 12.3b) | 71.2 ┬▒ 8.6b) | < 0.001 |
| Morphologic domain | ||||
| ŌĆāILD patternd) | < 0.001 | |||
| ŌĆāŌĆāNSIP | 22 (71.0) | 14 (77.7) | 7 (21.9) | |
| ŌĆāŌĆāNSIP + OP | 1 (3.2) | 1 (5.6) | 1 (3.1) | |
| ŌĆāŌĆāOP | 1 (3.2) | 1 (5.6) | 0 (0.0) | |
| ŌĆāŌĆāUIP | 7 (22.6) | 2 (11.1) | 24 (75.0) | |
| ŌĆāŌĆāLIP | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| ŌĆāUnexplained pleural effusion or thickening | 6 (19.4) | 3 (16.7) | 8 (25.0) | 0.755 |
| ŌĆāUnexplained pericardial effusion or thickening | 1 (3.2) | 0 (0.0) | 0 (0.0) | 0.442 |
| ŌĆāUnexplained intrinsic airway disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | > 0.999 |
| ŌĆāUnexplained pulmonary vasculopathy | 0 (0.0) | 0 (0.0) | 0 (0.0) | > 0.999 |
| Clinical domain | ||||
| ŌĆāMechanic hands | 2 (6.5) | 1 (5.6) | 0 (0.0) | 0.357 |
| ŌĆāDistal digital tip ulceration | 0 (0.0) | 1 (5.6) | 0 (0.0) | 0.170 |
| ŌĆāInflammatory arthritis or polyarticular morning stiffness (Ōēź 60 min) | 8 (25.8)a) | 4 (22.2)a) | 0 (0.0)b) | 0.009 |
| ŌĆāPalmar telangiectasia | 1 (3.2) | 0 (0.0) | 0 (0.0) | 0.442 |
| ŌĆāRaynaudŌĆÖs phenomenon | 14 (45.2)a) | 8 (44.4)a) | 1 (3.1)b) | < 0.001 |
| ŌĆāPuffy finger | 7 (22.6)a) | 2 (11.1)a) | 0 (0.0)b) | 0.020 |
| ŌĆāGottronŌĆÖs sign | 2 (6.5) | 1 (5.6) | 0 (0.0) | 0.357 |
| Serologic domain | ||||
| ŌĆāAntinuclear Ab | 29 (93.5)a) | 9 (50.0)b) | 1 (3.1)c) | < 0.001 |
| ŌĆāRheumatoid factor | 7 (22.6) | 4 (22.2) | 1 (3.1) | 0.057 |
| ŌĆāAnti-CCP Ab | 5 (16.1) | 3 (16.7) | 1 (3.1) | 0.181 |
| ŌĆāAnti-dsDNA Ab | 5 (16.1)a) | 0 (0.0)b) | 0 (0.0)b) | 0.014 |
| ŌĆāAnti-Ro/SSA Ab | 16 (51.6)a) | 4 (22.2)b) | 0 (0.0)c) | < 0.001 |
| ŌĆāAnti-La/SSb Ab | 3 (9.7) | 0 (0.0) | 0 (0.0) | 0.081 |
| ŌĆāAnti-RNP Ab | 4 (12.9)a) | 0 (0.0)b) | 0 (0.0)b) | 0.034 |
| ŌĆāAnti-Smith Ab | 2 (6.5) | 0 (0.0) | 0 (0.0) | 0.191 |
| ŌĆāAnti-Scl-70 Ab | 11 (35.5)a) | 2 (11.1)b) | 0 (0.0)c) | 0.001 |
| ŌĆāAnti-centromere Ab | 5 (16.1) | 2 (11.1) | 0 (0.0) | 0.068 |
| ŌĆāAnti-Jo-1 Ab | 2 (6.5) | 1 (5.6) | 0 (0.0) | 0.357 |
Ab, antibody; CTD, connective tissue disease; ILD, interstitial lung disease; IIP, idiopathic interstitial pneumonia; IPAF, interstitial pneumonia with autoimmune features; LIP, lymphoid interstitial pneumonia; NSIP, nonspecific interstitial pneumonia; OP, organizing pneumonia; RNP, ribonucleoprotein; UIP, usual interstitial pneumonia.
Table┬Ā2
Nailfold capillaroscopy findings of enrolled patients
| CTD-ILD (n = 31) | IPAF (n = 18) | IIP (n = 32) | p value | |
|---|---|---|---|---|
| Semiquantitative score of nailfold capillaroscopy (0ŌĆō18) | 5.8 ┬▒ 2.5a) | 4.2 ┬▒ 1.9b) | 3.0 ┬▒ 1.1c) | < 0.001 |
| Decreased density of capillary | 30 (96.8) | 17 (94.4) | 31 (96.9) | 0.895 |
| Presence of dilated capillary (20 ╬╝m Ōēż apical diameter < 50 ╬╝m) | 29 (93.5) | 17 (94.4) | 30 (93.8) | 0.992 |
| Presence of giant capillary (50 ╬╝m Ōēź apical diameter) | 15 (48.4)a) | 3 (16.7)b) | 0 (0.0)c) | < 0.001 |
| Presence of haemorrhage | 14 (45.2)a) | 6 (33.3)a) | 4 (12.5)b) | 0.017 |
| Presence of ramification | 5 (16.1) | 2 (11.1) | 0 (0.0) | 0.068 |
| Disorganisation of the vascular array | 30 (96.8) | 17 (94.4) | 30 (93.8) | 0.850 |
| EULAR Study Group on Microcirculation in Rheumatic Diseases patternd) | <0.001 | |||
| ŌĆāEarly scleroderma pattern | 4 (12.9) | 0 (0.0) | 0 (0.0) | |
| ŌĆāActive scleroderma pattern | 10 (32.3)a) | 4 (22.2)a) | 0 (0.0)b) | |
| ŌĆāLate scleroderma pattern | 4 (12.9) | 1 (5.6) | 0 (0.0) | |
| ŌĆāNonspecific abnormalities | 13 (41.9)a) | 13 (72.2)b) | 26 (81.2)b) | |
| ŌĆāNormal | 0 (0.0)a) | 0 (0.0)a) | 6 (18.8)b) |
CTD, connective tissue disease; ILD, interstitial lung disease; IIP, idiopathic interstitial pneumonia; IPAF, interstitial pneumonia with autoimmune features.
Table┬Ā3
Clinical, serologic, morphologic features of IPAF patients with scleroderma pattern of NFC
REFERENCES
1. de Lauretis A, Veeraraghavan S, Renzoni E. Review series: aspects of interstitial lung disease: connective tissue disease-associated interstitial lung disease: how does it differ from IPF? How should the clinical approach differ? Chron Respir Dis 2011;8:53ŌĆō82.



2. Cottin V, Hirani NA, Hotchkin DL, et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018;27:180076.



3. Gao Y, Moua T. Treatment of the connective tissue disease-related interstitial lung diseases: a narrative review. Mayo Clin Proc 2020;95:554ŌĆō573.


4. Mosca M, Neri R, Bombardieri S. Undifferentiated connective tissue diseases (UCTD): a review of the literature and a proposal for preliminary classification criteria. Clin Exp Rheumatol 1999;17:615ŌĆō620.

5. Fischer A, Antoniou KM, Brown KK, et al. ERS/ATS Task Force on Undifferentiated Forms of CTD-ILD. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 2015;46:976ŌĆō987.


6. Min HK, Kim SH, Lee SH, Kim HR. Recent advances in the diagnosis and management of interstitial pneumonia with autoimmune features: the perspective of rheumatologists. Korean J Intern Med 2021;36:515ŌĆō526.




7. van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747ŌĆō1755.


8. Moon J, Lee JS, Yoon YI, et al. Study Group, Korean Rheumatoid Arthritis Interstitial Lung Disease (KORAIL). Association of serum biomarkers with pulmonary involvement of rheumatoid arthritis interstitial lung disease: from KORAIL cohort baseline data. J Rheum Dis 2021;28:234ŌĆō241.



9. Sulli A, Secchi ME, Pizzorni C, Cutolo M. Scoring the nailfold microvascular changes during the capillaroscopic analysis in systemic sclerosis patients. Ann Rheum Dis 2008;67:885ŌĆō887.


10. Smith V, Vanhaecke A, Herrick AL, et al. EULAR Study Group on Microcirculation in Rheumatic Diseases. Fast track algorithm: how to differentiate a ŌĆ£scleroderma patternŌĆØ from a ŌĆ£non-scleroderma patternŌĆØ. Autoimmun Rev 2019;18:102394.


11. Ingegnoli F, Ughi N, Crotti C, Mosca M, Tani C. Outcomes, rates and predictors of transition of isolated RaynaudŌĆÖs phenomenon: a systematic review and meta-analysis. Swiss Med Wkly 2017;147:w14506.



12. Bissell LA, Abignano G, Emery P, Del Galdo F, Buch MH. Absence of Scleroderma pattern at nail fold capillaroscopy valuable in the exclusion of Scleroderma in unselected patients with RaynaudŌĆÖs Phenomenon. BMC Musculoskelet Disord 2016;17:342.




13. Bellando-Randone S, Del Galdo F, Lepri G, et al. Progression of patients with RaynaudŌĆÖs phenomenon to systemic sclerosis: a five-year analysis of the European Scleroderma Trial and Research group multicentre, longitudinal registry study for Very Early Diagnosis of Systemic Sclerosis (VEDOSS). Lancet Rheumatol 2021;3:e834ŌĆōe843.

14. Bernardino V, Rodrigues A, Llad├│ A, Panarra A. Nailfold capillaroscopy and autoimmune connective tissue diseases in patients from a Portuguese nailfold capillaroscopy clinic. Rheumatol Int 2020;40:295ŌĆō301.



15. Sambataro D, Sambataro G, Libra A, et al. Nailfold videocapillaroscopy is a useful tool to recognize definite forms of systemic sclerosis and idiopathic inflammatory myositis in interstitial lung disease patients. Diagnostics (Basel) 2020;10:253.



16. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569ŌĆō2581.

17. Shiboski CH, Shiboski SC, Seror R, et al. International Sj├ČgrenŌĆÖs Syndrome Criteria Working Group. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sj├ČgrenŌĆÖs syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol 2017;69:35ŌĆō45.




18. Lundberg IE, Tj├żrnlund A, Bottai M, et al. International Myositis Classification Criteria Project consortium, The Euromyositis register and The Juvenile Dermatomyositis Cohort Biomarker Study and Repository (JDRG) (UK and Ireland). 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 2017;76:1955ŌĆō1964.

19. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol 2019;71:1400ŌĆō1412.


20. Tanaka Y, Kuwana M, Fujii T, et al. 2019 Diagnostic criteria for mixed connective tissue disease (MCTD): from the Japan research committee of the ministry of health, labor, and welfare for systemic autoimmune diseases. Mod Rheumatol 2021;31:29ŌĆō33.


21. Paxton D, Pauling JD. Does nailfold capillaroscopy help predict future outcomes in systemic sclerosis? A systematic literature review. Semin Arthritis Rheum 2018;48:482ŌĆō494.


22. Fernandes L, Nasser M, Ahmad K, Cottin V. Interstitial Pneumonia with Autoimmune Features (IPAF). Front Med (Lausanne) 2019;6:209.



23. Sebastiani M, Cassone G, De Pasquale L, et al. Interstitial pneumonia with autoimmune features: a single center prospective follow-up study. Autoimmun Rev 2020;19:102451.


24. Sambataro G, Vancheri A, Torrisi SE, et al. The morphological domain does not affect the rate of progression to defined autoimmune diseases in patients with interstitial pneumonia with autoimmune features. Chest 2020;157:238ŌĆō242.


25. Shao T, Shi X, Yang S, et al. Interstitial lung disease in connective tissue disease: a common lesion with heterogeneous mechanisms and treatment considerations. Front Immunol 2021;12:684699.



26. King TE Jr, Bradford WZ, Castro-Bernardini S, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083ŌĆō2092.


27. Hwang H, Lee JK, Choi SM, et al. Efficacy of lower dose pirfenidone for idiopathic pulmonary fibrosis in real practice: a retrospective cohort study. Korean J Intern Med 2022;37:366ŌĆō376.




28. Lim JU, Gil BM, Kang HS, Oh J, Kim YH, Kwon SS. Interstitial pneumonia with autoimmune features show better survival and less exacerbations compared to idiopathic pulmonary fibrosis. BMC Pulm Med 2019;19:120.




29. Tirelli C, Morandi V, Valentini A, et al. Multidisciplinary approach in the early detection of undiagnosed connective tissue diseases in patients with interstitial lung disease: a retrospective cohort study. Front Med (Lausanne) 2020;7:11.



30. Jee AS, Parker MJS, McGill N, et al. Nailfold capillaroscopy by smartphone-dermatoscope for connective tissue disease diagnosis in interstitial lung disease: a prospective observational study. ERJ Open Res 2021;7:00416ŌĆō2021.



31. Smith V, Herrick AL, Ingegnoli F, et al. EULAR Study Group on Microcirculation in Rheumatic Diseases and the Scleroderma Clinical Trials Consortium Group on Capillaroscopy. Standardisation of nailfold capillaroscopy for the assessment of patients with RaynaudŌĆÖs phenomenon and systemic sclerosis. Autoimmun Rev 2020;19:102458.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



