 |
 |
| Korean J Intern Med > Volume 38(4); 2023 > Article |
|
Abstract
Background/Aims
Methods
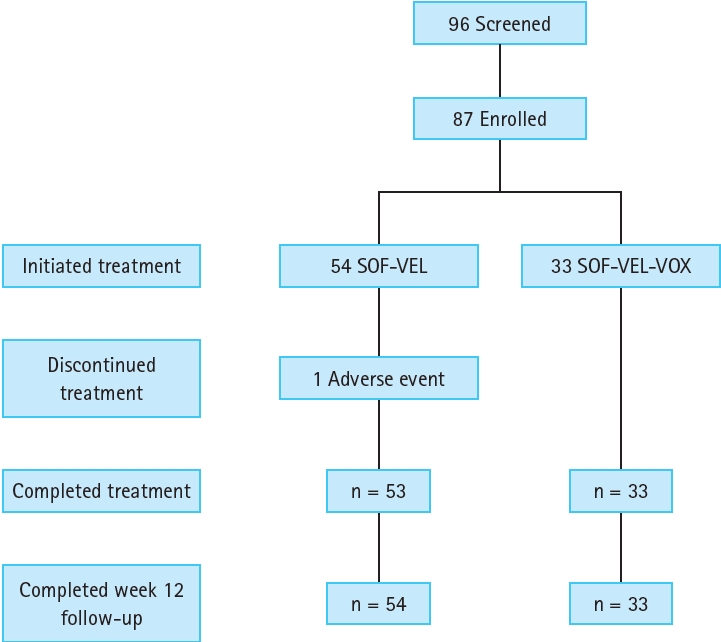
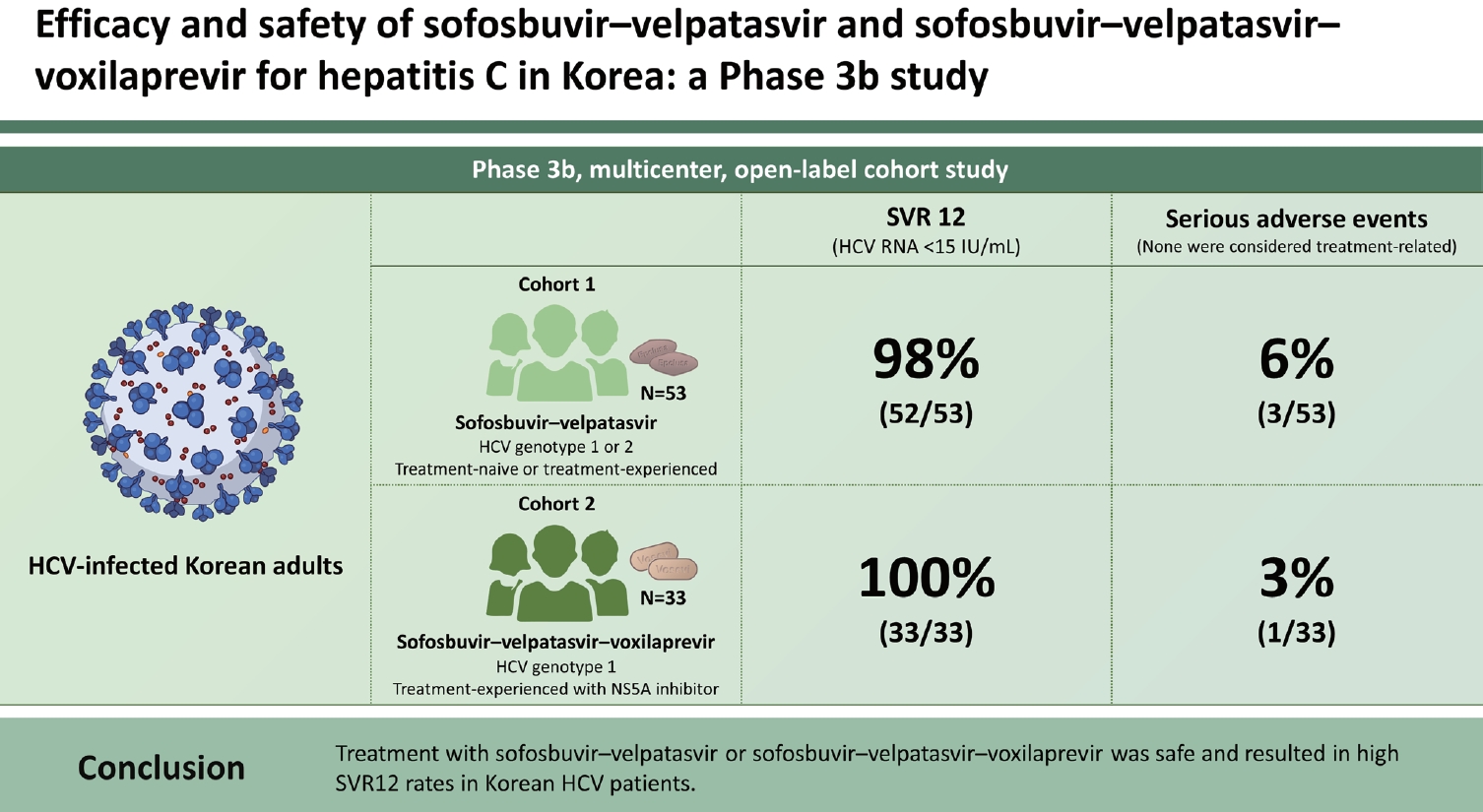
Results
Acknowledgments
Notes
Figure┬Ā1.
Table┬Ā1.
| Variable | SOFŌĆōVEL (n = 54) | SOFŌĆōVELŌĆōVOX (n = 33) |
|---|---|---|
| Age, yr | 60 (27ŌĆō85) | 62 (31ŌĆō80) |
| Sex at birth | ||
| ŌĆāFemale | 29 (53.7) | 18 (54.5) |
| ŌĆāMale | 25 (46.3) | 15 (45.5) |
| Asian race | 54 (100.0) | 33 (100.0) |
| BMI, kg/m2 | 25 (18ŌĆō36) | 25 (19ŌĆō32) |
| Genotype | ||
| ŌĆā1 | 27 (50.0) | 32 (97.0) |
| ŌĆāŌĆā1a | 1 (1.9) | - |
| ŌĆāŌĆā1b | 26 (48.1) | 32 (97.0) |
| ŌĆā2 | 27 (50.0) | 1 (3.0) |
| HCV treatment history | ||
| ŌĆāTreatment na├»ve | 46 (85.2) | - |
| ŌĆāTreatment experienced | 8 (14.8) | 33 (100.0) |
| Prior HCV treatment | ||
| ŌĆāPEG-IFN + RBV | 5 (9.3) | - |
| ŌĆāOther IFN-containing | 3 (5.6) | 1 (3.0)a) |
| ŌĆāNS5A ┬▒ DAA(s) | - | 32 (97.0) |
| ŌĆāŌĆāNS5A + NS5B | - | 2 (6.1) |
| ŌĆāŌĆāNS5A + NS3 ┬▒ NS5B | - | 29 (87.9) |
| ŌĆāŌĆāNS5A + other | - | 1 (3.0) |
| HCV RNA, log10 IU/mL | 5.9 (1.2ŌĆō7.3) | 6.5 (5.4ŌĆō7.1) |
| HCV RNA Ōēź 800,000 IU/mL | 30 (55.6) | 30 (90.9) |
| IL28B genotype | ||
| ŌĆāCC | 41 (75.9) | 23 (69.7) |
| ŌĆāCT | 13 (24.1) | 9 (27.3) |
| ŌĆāTT | - | - |
| ŌĆāMissing | - | 1 (3.0) |
| Compensated cirrhosis | 11 (20.4) | 9 (27.3) |
| ALT, U/L | 52 (9ŌĆō212) | 67 (17ŌĆō319) |
| eGFRb), mL/min/1.73 m2 | 89 (38ŌĆō179) | 86 (44ŌĆō141) |
| ŌĆā<90 | 31 (57.4) | 21 (63.6) |
| ŌĆāŌēź90 | 23 (42.6) | 12 (36.4) |
Values are presented as mean (range) or number (%).
ALT, alanine aminotransferase; BMI, body mass index; DAA, direct-acting antiviral; eGFR, estimated glomerular filtration rate; HCV, hepatitis C virus; IFN, interferon; IL28B, interleukin-28B; PEG, pegylated; RBV, ribavirin; SOF, sofosbuvir; VEL, velpatasvir; VOX, voxilaprevir.
Table┬Ā2.
| Regimen | No. of participants |
|---|---|
| Asunaprevir + daclatasvir | 26 |
| Peginterferon or interferon ┬▒ ribavirin | 9a) |
| Daclatasvir + sofosbuvir | 2 |
| Elbasvir/grazoprevir | 2 |
| Ledipasvir | 1 |
| Ombitasvir + dasabuvir + paritaprevir + ritonavir | 1 |
| Sofosbuvir + ribavirin | 1 |
a) Nine participants received peginterferon/interferon ┬▒ ribavirin as well as either asunaprevir + daclatasvir (n = 7); daclatasvir + sofosbuvir (n = 1); or sofosbuvir + ribavirin (n = 1) (Supplementary Table 1).
Table┬Ā3.
| Response | SOFŌĆōVEL (n = 53)a) | SOFŌĆōVELŌĆōVOX (n = 33) |
|---|---|---|
| SVR4 | 52 (98.1) | 33 (100.0) |
| SVR12 | 52 (98.1) | 33 (100.0) |
| ŌĆā95% CI | 90ŌĆō100 | 89ŌĆō100 |
| Virologic failure | ||
| ŌĆāOn treatment | 0 | 0 |
| ŌĆāRelapse | 1 | 0 |
| ŌĆāDiscontinued study treatment | 1 | 0 |
Table┬Ā4.
| Adverse events | SOFŌĆōVEL (n = 54) | SOFŌĆōVELŌĆōVOX (n = 33) |
|---|---|---|
| No. of participants with any | ||
| ŌĆāAdverse events | 23 (42.6) | 15 (45.5) |
| ŌĆāGrade 3 or 4 adverse eventsa) | 3 (5.6) | 1 (3.0) |
| ŌĆāTreatment-related adverse events | 5 (9.3) | 6 (18.2) |
| ŌĆāGrade 3 or 4 treatment-related adverse events | 1 (1.9)b) | 0 (0.0) |
| ŌĆāSerious adverse events | 3 (5.6) | 1 (3.0) |
| ŌĆāTreatment-related serious adverse events | 0 (0.0) | 0 (0.0) |
| ŌĆāAdverse events leading to discontinuation | 1 (1.9) | 0 (0.0) |
| Adverse events leading to discontinuation | ||
| ŌĆāLiver function test increased | 1 (1.9)b) | - |
| Adverse events in Ōēź 5% of participants in either treatment group | ||
| ŌĆāHeadache | 4 (7.4) | 3 (9.1) |
| ŌĆāNausea | 2 (3.7) | 3 (9.1) |
| ŌĆāRash | 0 (0.0) | 3 (9.1) |
| Serious adverse events | ||
| ŌĆāCerebral infarction | 1 (1.9) | - |
| ŌĆāErythema nodosum | 1 (1.9) | - |
| ŌĆāFacial bones fracture | - | 1 (3.0) |
| ŌĆāHematochezia | 1 (1.9) | - |
| ŌĆāPyrexia | 1 (1.9) | - |
| Grade 3 laboratory abnormalities | ||
| ŌĆāPlatelets, 25,000 to < 50,000/mm3 | 1 (1.9) | 1 (3.0) |
| ŌĆāALT, > 5 to 10├Ś ULN | 1 (1.9) | - |
| ŌĆāAST, > 5 to 10├Ś ULN | 1 (1.9) | - |
| ŌĆāHemoglobin, 70 to < 90 g/L or decrease Ōēź 45 g/L | 1 (1.9) | - |
| ŌĆāHyperglycemia, > 250 to 500 mg/dL | 1 (1.9) | - |
Values are presented as number (%).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal; SOF, sofosbuvir; VEL, velpatasvir; VOX, voxilaprevir.
a) Grade 3 adverse events were erythema nodosum (n = 1), facial bones fracture (n = 1), hematochezia (n = 1), liver function test increased (n = 1), and pyrexia (n = 1). There were no grade 4 adverse events.
b) Grade 3 liver function test increased. Study drug was discontinued because the increase in ALT and AST met prespecified stopping criteria. The biochemical elevations in ALT and AST were first reported on day 15, not associated with any an increase in other lab parameters (e.g., total bilirubin), and did not meet HyŌĆÖs Law. On day 140 the event was deemed resolved based on local lab results (ALT = 29 U/L, AST = 28 U/L) that were within normal ranges.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print



