INTRODUCTION
The early data after the rollout of coronavirus disease 2019 (COVID-19) vaccines revealed sufficient efficacies for protecting against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severe COVID-19 [1ŌĆō3]. However, with the emergence of variants of concern and waning of vaccine-induced immune responses, many countries are encountering a resurgence of COVID-19 despite providing two doses of COVID-19 vaccines to the majority of the populations. To overcome the reduction of vaccine efficacy, booster doses are being administered in many countries.
Multiple factors are responsible for breakthrough infections in fully vaccinated individuals, of which the short latent period (about 5 days) or incubation period (about 6 days) of SARS-CoV-2 is regarded to be important because at least several days are needed for memory B cells to proliferate and differentiate into high-affinity plasma cells [4,5]. Therefore, the rapidity of SARS-CoV-2-specific memory B or T cell responses in vaccinated individuals is important for important for our understanding of immunopathogenesis of COVID-19. We therefore compared the timing of adequate immune responses between the first and booster (the third) doses of COVID-19 vaccines in infection-naïve healthcare workers (HCWs).
METHODS
Participants and specimen collecting
This study enrolled HCWs who received two-dose of either the BNT162b2 vaccine (Pfizer-BioNTech, New York, NY, USA) or the ChAdOx1 novel coronavirus (nCoV-19) (ChAdOx1, AstraZeneca, Cambridge, UK) vaccine at a tertiary care hospital in Seoul, South Korea between March 5th and June 22nd, 2021. In accordance with the policy of the Korean government, the BNT162b2 vaccine was assigned to high-risk HCWs in direct contact with COVID-19 patients, and the ChAdOx1 vaccine was assigned to those involved in general patient care. The booster dose of the BNT162b2 vaccine was administered to the prime-BNT162b2 vaccinees between October 13rd and October 22nd, 2021, and to the prime-ChAdOx1 vaccinees between November 15th and December 10th, 2021. All participants agreed to peripheral blood sampling. A total of six blood samplings were carried out: once before the first vaccination; 7, 14, and 21 days after the first vaccination; once before the booster dose vaccination; and once between 2 and 7 days after the booster dose. The study was reviewed and approved by the Institutional Review Board of Asan Medical Center (IRB No. 2021-0170). Informed consent was received from all participants.
Measurement of S1-IgG antibody responses
SARS-CoV-2 spike 1 (S1)-specific immunoglobulin G (IgG) antibody titers were measured using an in-house-developed enzyme-linked immunosorbent assay (ELISA), and the titers are presented as IU/mL, which is standardized with the reference pooled sera from the International Vaccine Institute (Seoul, Korea). The details of the ELISA are described in previous reports [6,7]. The live-virus neutralizing antibody titer associated with 80% vaccine efficacy was 1:237 [8], which corresponds to 100 IU/mL for IgG according to the correlation curve between SARS-CoV-2 S1-specific IgG and neutralizing antibody titer calculated using microneutralization assay against live SARS-CoV-2 (╬▓CoV/Korea/KCDC/2020 NCCP43326) (equation of linear regression between S1-IgG antibody [X] and neutralizing antibody [Y]: Y = 1.010 ├Ś X + 162.8) (Supplementary Fig. 1). Therefore, 100 IU/mL of SARS-CoV-2 S1-specific IgG was arbitrarily chosen as the cut-off for meaningful IgG antibody response.
Measurement of T cell responses
Interferon gamma (IFN-╬│) enzyme-linked immunospot (ELISPOT) assays were performed to measure the SARS-CoV-2-specific T cell response of peripheral mononuclear cells (PBMCs) isolated from the participantsŌĆÖ blood samples, the details of which are described in previous reports [6,7]. Briefly, PBMCs were separated from peripheral blood by density gradient centrifugation using lymphocyte separation medium (Corning, New York, NY, USA) and stored at liquid nitrogen until analysis. The samples of 5 ├Ś 105 cells were placed in wells pre-coated with anti-human IFN-╬│ antibody (Mabtech AB, Nacka Strand, Sweden) and stimulated with overlapping peptides of SARS-CoV-2 spike protein (final concentration: 2 ╬╝g/mL, Miltenyi Biotec, Bergisch Gladbach, Germany). Spots were detected and counted with an automated ELISPOT reader (Autoimmune Diagnostika GmbH, Strassburg, Germany) and the data were expressed as spot forming cells (SFC) per 5 ├Ś 105 cells. Meaningful SARS-CoV-2-specific IFN-╬│ T cell response was defined if the SARS-CoV-2-specific IFN-╬│ T cell response in ELISPOT assays increased by two-fold or higher compared with the pre-vaccination value. In case of participants who did not measured the T cell responses at pre-vaccination, two-fold of median value were considered as a threshold.
Statistical analysis
Statistical analyses were performed using SPSS Statistics for Windows version 24.0 (IBM Corp., Armonk, NY, USA), and graphs were plotted with GraphPad Prism version 8 (Graph- Pad Software, San Diego, CA, USA). Depending on the normality of the data, we used the chi-squared test or FisherŌĆÖs exact test to analyze categorical variables, and StudentŌĆÖs t test or the Mann-Whitney U test for continuous variables. All tests of significance were two-tailed, and p values < 0.05 were considered statistically significant.
RESULTS
Baseline characteristics of the study participants
The schematic flow chart of this study is shown in Supplementary Fig. 2. A total of 51 HCWs vaccinated with two doses of the BNT162b2 vaccine were enrolled, among them, two HCWs dropped out. A total of 97 HCWs vaccinated with the ChAdOx1 vaccine were enrolled, of whom 32 dropped out and 12 postponed receiving the booster dose. The reasons for dropout included rejection of the second or booster dose, resignation from the hospital, and pregnancy. Finally, the BNT162b2 was administered as a booster vaccine in 49 HCWs vaccinated with two doses of the BNT162b2 vaccine and 53 HCWs vaccinated with two doses of the ChAdOx1 vaccine. The baseline characteristics of the participants are shown in Supplementary Table 1. The median age was higher in the ChAdOx1 group than in the BNT162b2 group (38 years vs. 29 years, p < 0.001).
S1-IgG antibody responses
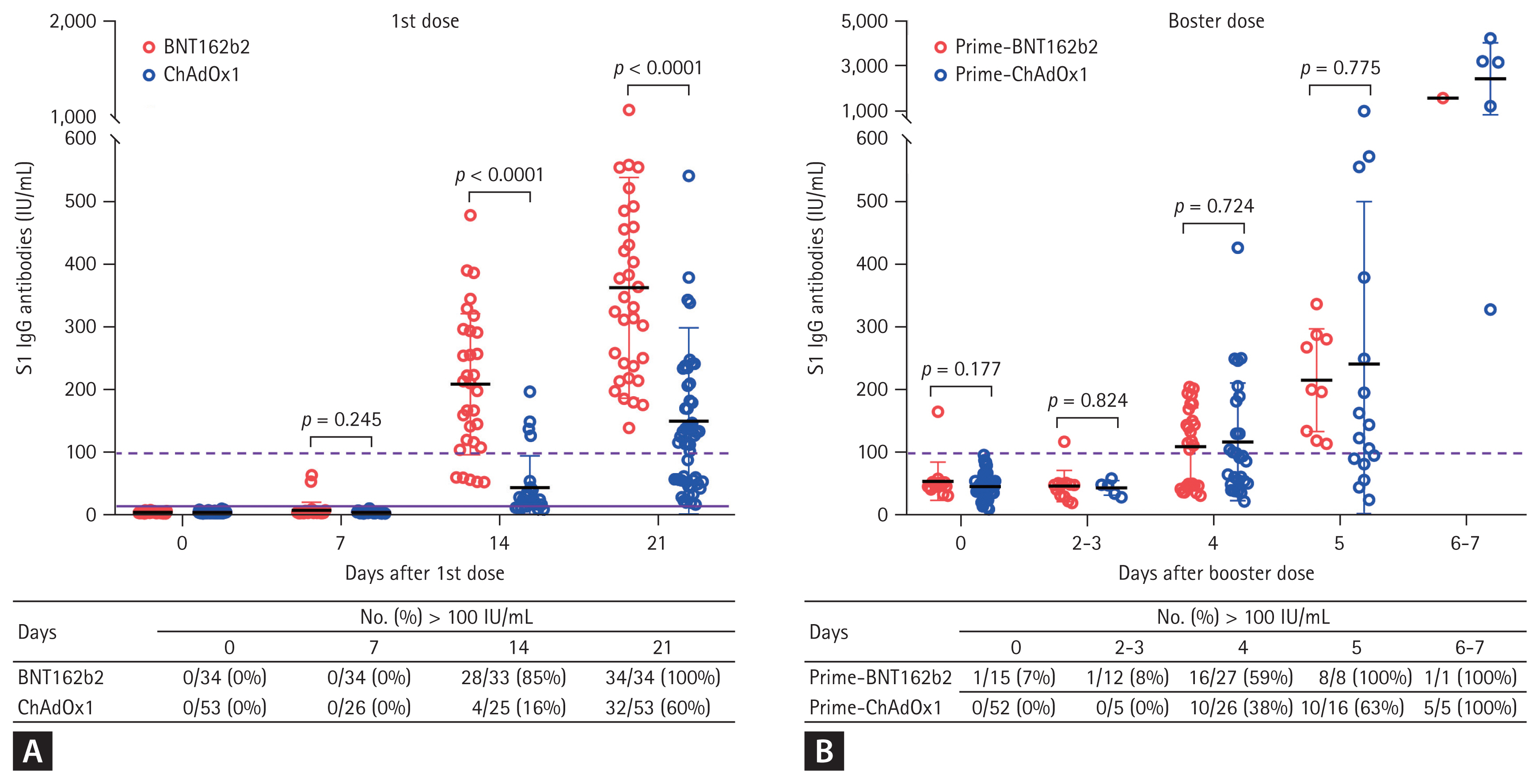
The plasma sample numbers measured SARS-CoV-2 S1-IgG responses are presented in Fig. 1. S1-IgG antibody titers were lower than the cut-off value for a meaningful value (100 IU/mL) in all participants until 7 days after their first dose vaccination. At 14 days after the first dose of vaccine, the S1-IgG responses surpassed 100 IU/mL in 85% (28 of 33) of participants in the BNT162b2 group and in 60% (32 of 53) of participants in the ChAdOx1 group (Fig. 1A). The mean S1-IgG responses were significantly higher in the BNT162b2 group than in the ChAdOx1 group at 14 days (mean ┬▒ standard deviation, 208.2 ┬▒ 112.8 IU/mL vs. 42.9 ┬▒ 50.9 IU/mL, p < 0.0001) and 21 days (362.5 ┬▒ 176.6 IU/mL vs. 148.9 ┬▒ 149.5 IU/mL, p < 0.0001) after the first dose at 21 days (Fig. 1A).
The booster dose of the BNT162b2 was administrated to the BNT162b2 group at a median of 197 days (range, 193 to 204) after the second dose of the BNT162b2 vaccine and to the ChAdOx1 group at a median of 175 days (range, 153 to 191) after the second dose of the ChAdOx1 vaccine. Prior to receiving the booster dose, the S1-IgG responses in the BNT162b2 group and the ChAdOx1 group were 52.7 ┬▒ 31.5 and 42.0 ┬▒ 19.4 IU/mL, respectively, which were below the cut-off for a meaningful response (Fig. 1B).
Until 3 days after the booster dose, the mean S1-IgG responses in both groups remained below 100 IU/mL. However, in the BNT162b2 group, the mean S1-IgG responses surpassed 100 IU/mL in 59% (16 of 27) of plasma samples and 100% (8 of 8) of plasma samples acquired at 4 and 5 days, respectively. In the ChAdOx1 group, the mean S1-IgG responses surpassed 100 IU/mL in 38% (10 of 26), 63% (10 of 16), and 100% (5 of 5) of plasma samples acquired at 4, 5, and 6 or 7 days after the booster dose. The mean S1-IgG titers of the two groups did not show significant differences at all time points after the booster dose (Fig. 1B).
IFN-╬│-producing T cell responses
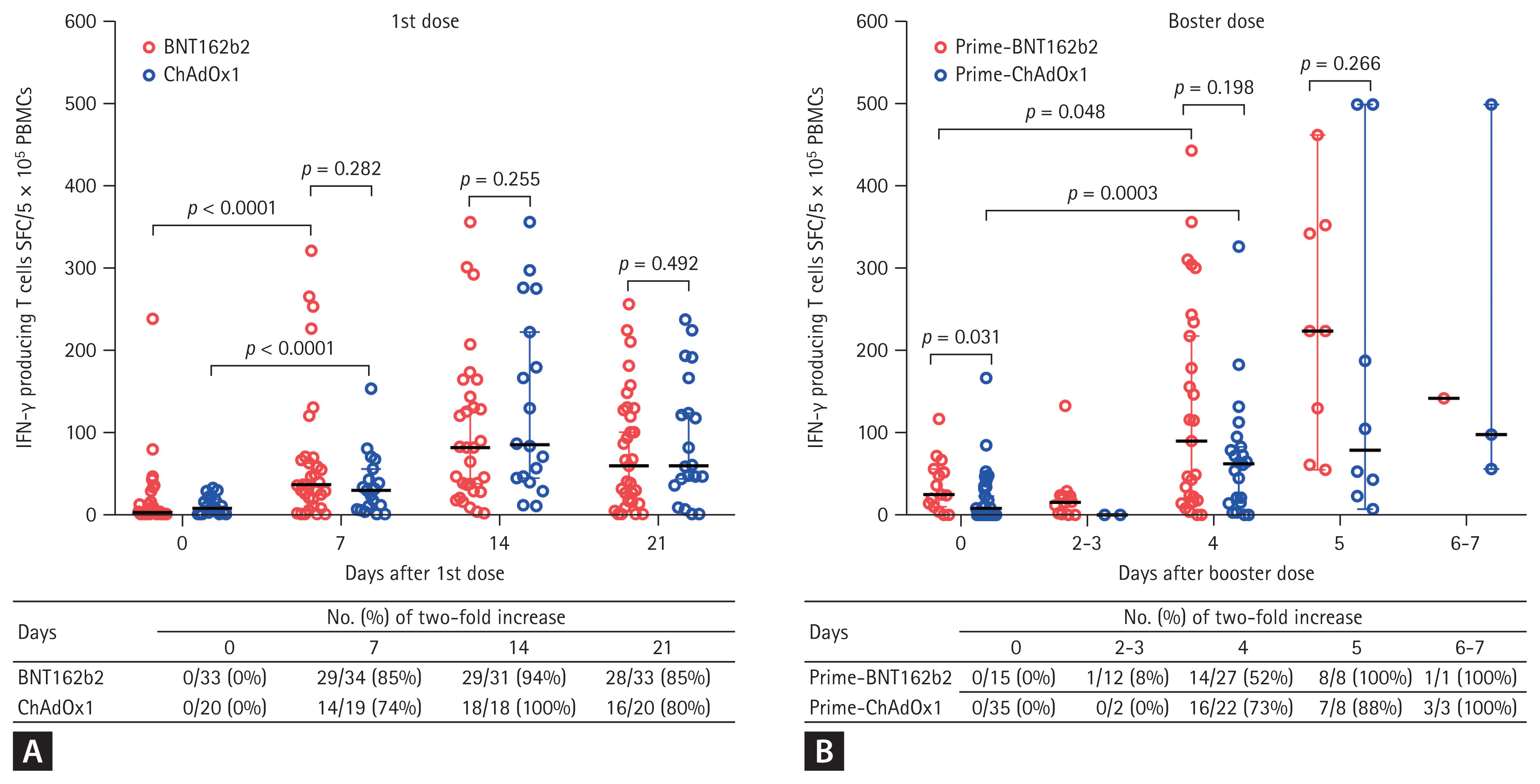
SARS-CoV-2 spike protein-specific IFN-╬│-producing T cell responses are presented in Fig. 2. Compared with the baseline value, the T cell responses increased by two-fold in 85% (29 of 34) of participants in the BNT162b2 group and in 74% (14 of 19) of participants in the ChAdOx1 group at 7 days after the first dose of vaccine (Fig. 2A).The T cell responses of the BNT162b2 and ChAdOx1 groups were not significantly different at 7, 14, and 21 days after the first dose (Fig. 2A), with the values peaking at 14 days in both groups.
Prior to receiving the booster dose, the median T cell response was significantly higher in the BNT162b2 group than in the ChAdOx1 group (median [95% confidence interval, CI], 25 [10 to 56] vs. 8 [0 to 23] SFC/5 ├Ś 105 PBMCs, p = 0.031) (Fig. 2B). Compared with the pre-booster value, the T cell responses increased by two-fold in 52% (14 of 27) of participants in the BNT162b2 group and in 73% (16 of 22) of participants in the ChAdOx1 group at 4 days after the first dose of vaccine (Fig. 2B).
The median values of the T cell responses showed meaningful increases of two-fold or more in both groups at 4 days after the booster dose (median [95% CI], BNT162b2 group, 25 [10 to 56] vs. 90 [22 to 218] SFC/5 ├Ś 105 PBMCs, p = 0.048; ChAdOx1 group, 8 [0 to 23] vs. 62 [15 to 83] SFC/5 ├Ś 105 PBMCs, p = 0.0003) (Fig. 2B). The T cell response of the BNT162b2 group was higher than that of the ChAdOx1 group after the booster dose, albeit without statistically significant difference (at 4 days, 90 [22 to 218] vs. 62 [15 to 83], p = 0.192; at 5 days, 224 [55 to 463] vs. 79 [15 to 83], p = 0.266) (Fig. 2B).
DISCUSSION
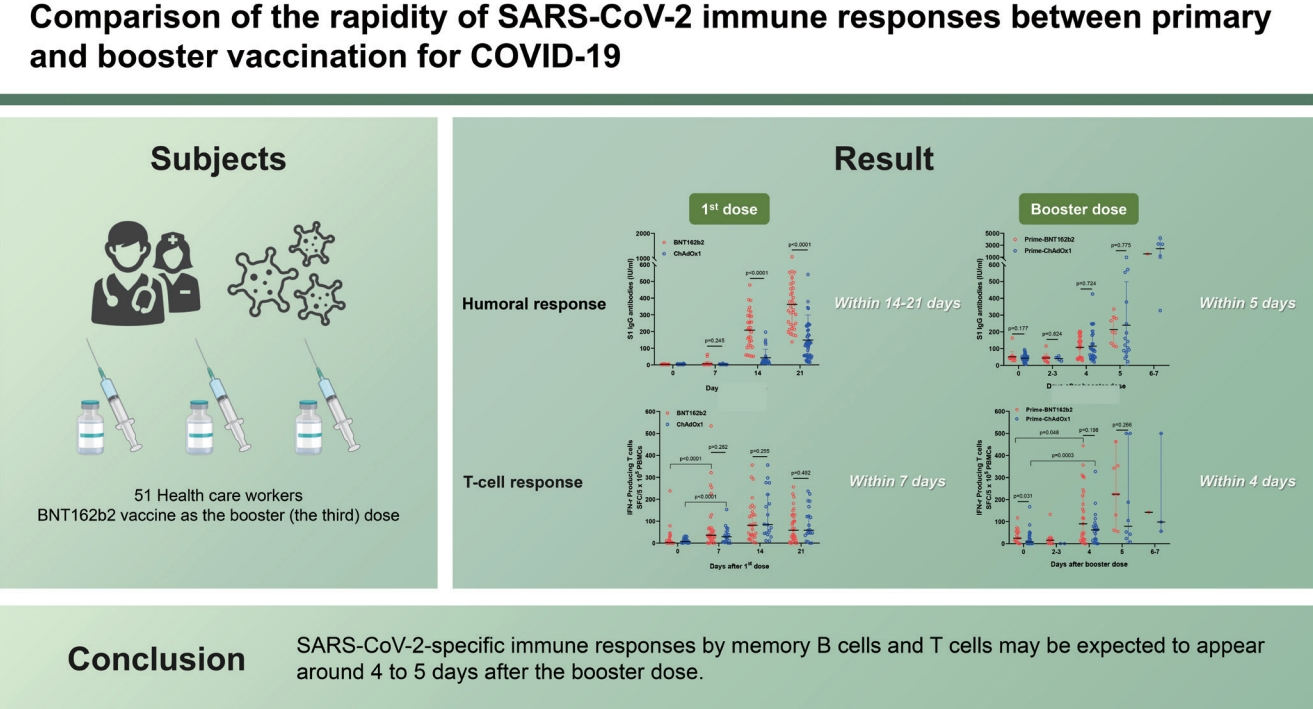
In this study involving healthy vaccinees who received the BNT162b2 vaccine as the booster dose, we found that the first doses of BNT162b2 and ChAdOx1 required 14 and 21 days to elicit clinically meaningful S1-IgG antibody responses, respectively. In contrast, after the booster dose, meaningful antibody responses were elicited as early as within 5 days in both vaccine groups. Similarly, while the first doses of the vaccines elicited significant increases in IFN-╬│ producing T cell responses within 7 days, the booster doses elicited significant increases in T cell responses within 4 days.
With the emergence of variants of concern, many countries are encountering a resurgence of COVID-19 despite providing COVID-19 vaccines to the majority of the populations. The efficacy of vaccines for humoral immune responses depends on at least two layers of defense. Pre-existing antibodies secreted by long-lived plasma cells act as the first line of defense against infections [9]. However, recent studies reported that circulating IgG antibodies induced by COVID-19 vaccines rapidly waned within 6 months [7,10,11]. In our previous study that included the same cohort used in the present study, the mean levels of S1-IgG antibody response after the second dose were 2,241 IU/mL in the BNT162b2 vaccinees and 174 IU/mL in the ChAdOx1 vaccinees [7]. At the timing of the booster dose, the S1-IgG antibody responses had significantly decreased to 53 IU/mL in the BNT162b2 vaccinees at 6.5 months after the second dose and 42 IU/mL in the ChAdOx1 vaccinees at 5 months after the second dose. Therefore, circulating SARS-CoV-2-specific antibodies produced by long-lived plasma cells might gradually become suboptimal for protecting against breakthrough infections.
The second line of defense is the reactivation of antigen-experienced memory B cells to proliferate and differentiate into plasma cells that produce high-affinity antibodies at the time of re-exposure [9]. Previous studies reported that reactive memory B cells play an essential role in eliciting responses to variants of certain viruses [12,13]. Also, it is generally agreed that memory B cell activation requires collaborative help from T cells [14,15]. Therefore, considering the currently ongoing pandemic spread of SARS-CoV-2 variants as well as the waning of circulating SARS-CoV-2-specific antibodies by long-lived plasma cells, rapidity of memory B cells reactivation is important for our understanding of immunopathogenesis of SARS-CoV-2 infection.
Considering that the booster dose of vaccination is considered a re-exposure of SARS CoV-2 antigen, our findings suggest that at least 5 days are needed to produce antibodies elicited by memory B cells. The latent period for SARS-CoV-2 infection was reported to be about 5 days, respectively [4]. Also, omicron variant of SARS-CoV-2 has a shorter incubation period. Recent studies reported that the mean incubation period of the omicron variant was 2.5 to 4.3 days in South Korea and 1.5 to 3.2 days in England [16,17]. Therefore, in cases with waning levels of circulating SARS-CoV-2-specific antibodies, the time required for production of sufficient levels of circulating antibodies might be too long to interrupt viral infection due to short incubation period, but enough to prevent severe disease.
This study has some limitations. First of all, the number of participants was relatively small. Second, the ages of the BTN162b2 and ChAdOx1 vaccinees were significantly different, which was the result of the national vaccine policy and vaccine availability. Third, the sample numbers were not consistent throughout the study period. Fourth, our findings may not be generalized to memory B cell or T cell response against new variants such as delta or omicron. Despite these limitations, our findings suggest that SARS-CoV-2-specific antibody response by memory B cells and SARS-CoV-2-specific T cell responses may be expected to appear at around 4 to 5 days after receiving the booster dose. The time window for producing an optimal immune response by memory B or T cells for overcoming breakthrough infection may be short unless a sufficient amount of circulating antibodies is maintained. Our study provides important data for policy decisions on providing booster doses of COVID-19 vaccines for protecting against breakthrough infections or severe COVID-19.
KEY MESSAGE
1. The rapidity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-speicific memory B or T cell response is a crucial factor for our understanding of immunopathogenesis of coronavirus disease 2019 (COVID-19).
2. Our findings suggest that SARS-CoV-2-specific antibody response by memory B cells and SARS-CoV-2-specific T cell responses may be expected to appear at around 4 to 5 days after receiving the booster dose.
3. The time required for production of sufficient levels of circulating antibodies might be too long to interrupt viral infection due to short incubation period, but enough to prevent severe disease.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print





