 |
 |
| Korean J Intern Med > Volume 36(6); 2021 > Article |
|
Abstract
Background/Aims
Methods
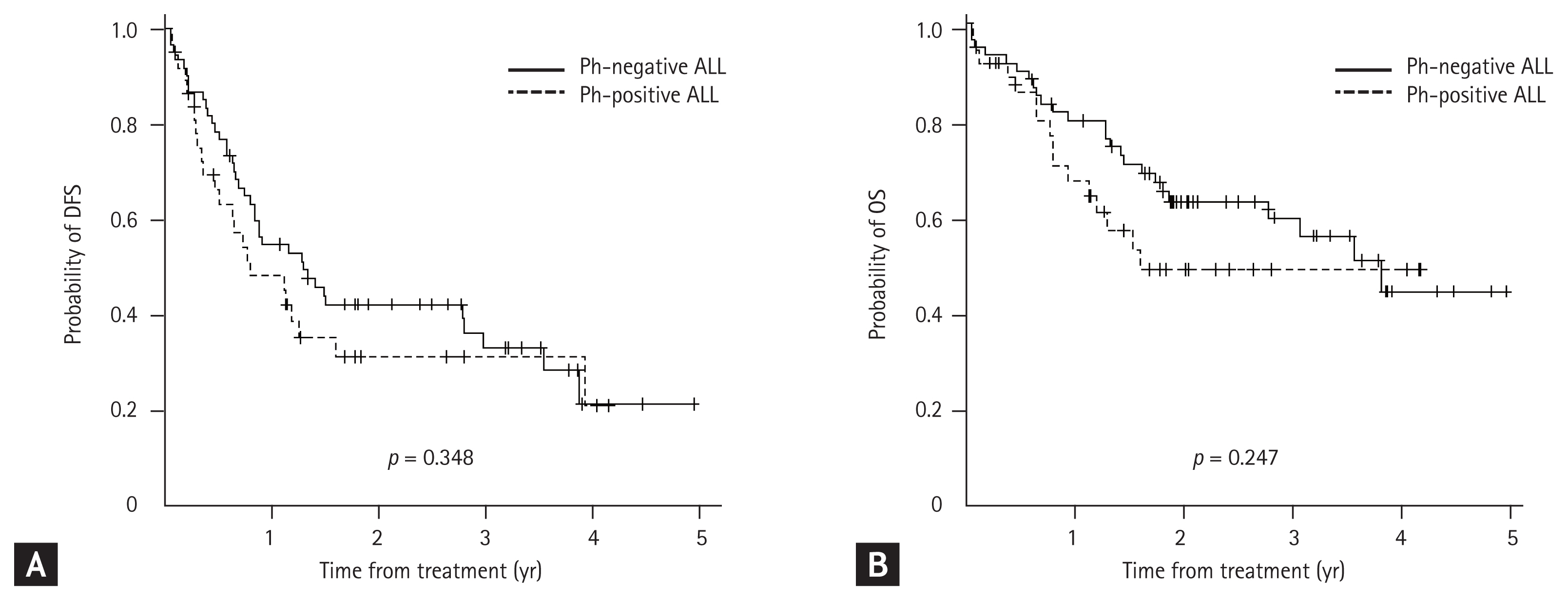
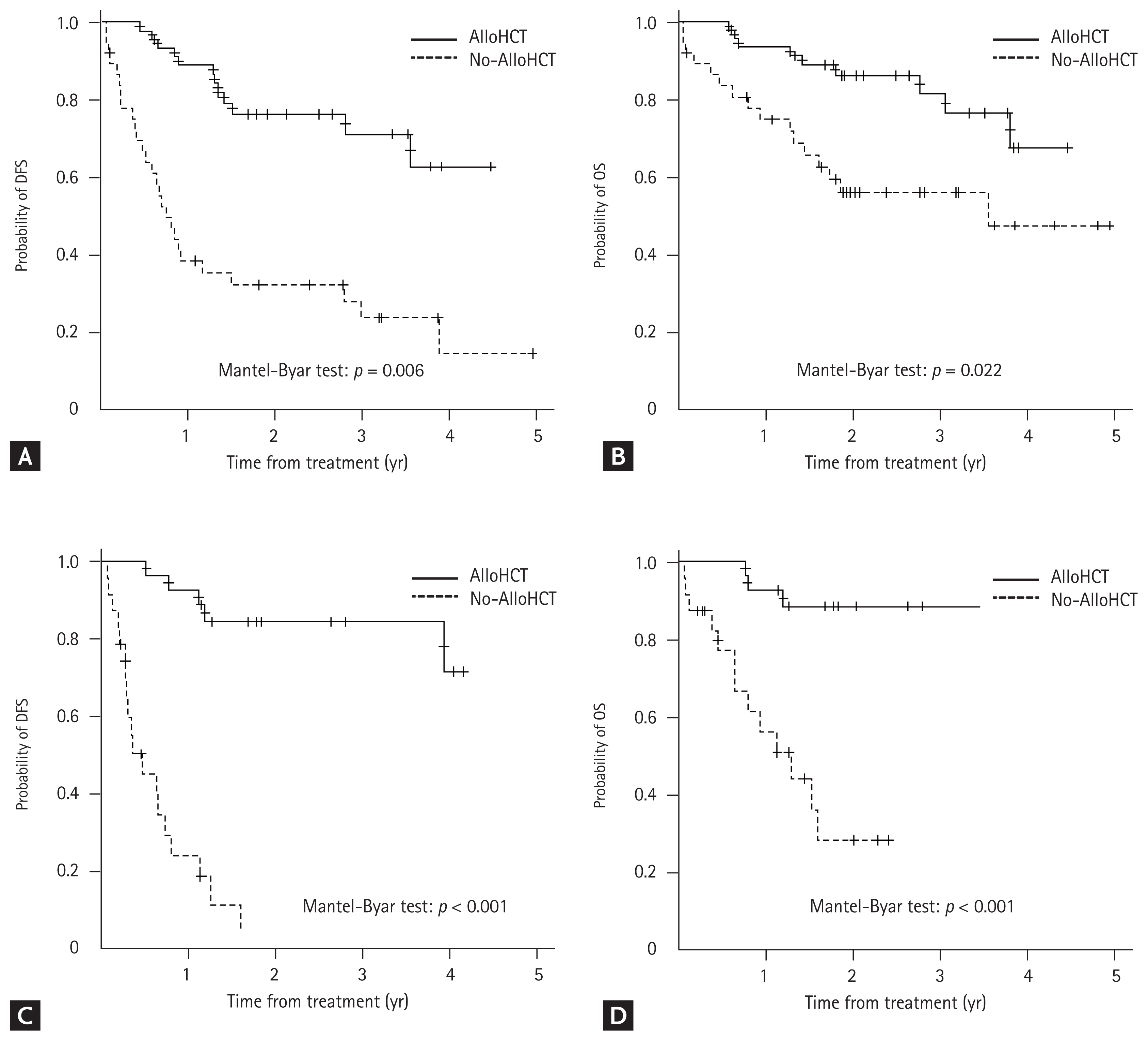
Results
Conflict of Interest
Figure┬Ā1
Figure┬Ā2
Figure┬Ā3
Figure┬Ā4
Table┬Ā1
| Schedule | Drug | Age, yr | Administration |
|---|---|---|---|
| Induction (cycle 1) | Daunorubicin | 15ŌĆō54 | 60 mg/m2/day IV, days 1ŌĆō3 |
| Ōēź 55 | 45 mg/m2/day IV, days 1ŌĆō3 | ||
| Vincristine | 15ŌĆō54 | 2 mg/day IV, days 1, 8, 15, and 22 | |
| Ōēź 55 | 2 mg/day IV, days 1, and 15 | ||
| Prednisolone | 15ŌĆō54 | 60 mg/m2/day PO, days 1ŌĆō21 | |
| Ōēź 55 | 60 mg/m2/day PO, days 1ŌĆō14 | ||
| Cyclophosphamidea | 15ŌĆō54 | 750 mg/m2/day IV, day 15 | |
| Ōēź 55 | 500 mg/m2/day IV, day 15 | ||
| L-Asparaginase (Ph-negative) | 15ŌĆō24 | 10,000 U/m2/day, IM, days 17, 19, 21, 23, 25, and 27 | |
| 25ŌĆō54 | 6,000 U/m2/day, IM, days 17, 19, 21, 23, 25, and 27 | ||
| Ōēź 55 | 4,000 U/m2/day, IM, days 17, 19, 21, 23, 25, and 27 | ||
| Imatinib (Ph-positive) | 600 mg/day PO, days 8~ | ||
| Consolidation A (cycle 2, 5) | MTX | 15ŌĆō54 | 2,380 mg/m2 IV, days 1ŌĆō2, and 15ŌĆō16 |
| Ōēź 55 | 2,380 mg/m2 IV, days 1ŌĆō2 | ||
| Dexamethasone | 15ŌĆō54 | 20 mg/ m2/day IV, days 1ŌĆō2, and 15ŌĆō16 | |
| Ōēź 55 | 20 mg/ m2/day IV, days 1ŌĆō2 | ||
| Imatinib (Ph-positive) | 600 mg/day PO, everyday | ||
| Consolidation B (cycle 3, 6) | Cytarabine | 15ŌĆō54 | 60 mg/m2/day IV, days 1ŌĆō4 |
| Ōēź 55 | 45 mg/m2/day IV, days 1ŌĆō4 | ||
| Cyclophosphamide | 15ŌĆō54 | 750 mg/m2/day IV, day 1 | |
| Ōēź 55 | 500 mg/m2/day IV, days 1 | ||
| L-Asparaginase (Ph-negative) | 15ŌĆō24 | 10,000 U/m2/day, IM, days 17, 19, 21, 23, 25, and 27 | |
| 25ŌĆō54 | 6,000 U/m2/day, IM, days 17, 19, 21, 23, 25, and 27 | ||
| Ōēź 55 | 4,000 U/m2/day, IM, days 17, 19, 21, 23, 25, and 27 | ||
| Imatinib (Ph-positive) | 600 mg/day PO, everyday | ||
| Delayed Intensification (cycle 4) | Daunorubicin | 45 mg/m2/day IV, days 1ŌĆō2 | |
| Vincristine | 2 mg/day IV, days 1, and 8 | ||
| Dexamethasone | 20 mg/m2/day IV, days 1ŌĆō2, and 15ŌĆō16 | ||
| Cyclophosphamide | 15ŌĆō54 | 750 mg/m2/day IV, day 3 | |
| Ōēź 55 | 500 mg/m2/day IV, day 3 | ||
| L-Asparaginase (Ph-negative) | 15ŌĆō24 | 10,000 U/m2/day, IM, days 1, 3, 5, and 7 | |
| 25ŌĆō54 | 6,000 U/m2/day, IM, days 1, 3, 5, and 7 | ||
| Ōēź 55 | 4,000 U/m2/day, IM, days 1, 3, 5, and 7 | ||
| Imatinib (Ph-positive) | 600 mg/day PO, everyday | ||
| Maintenance | 6-MP | 60 mg/m2 PO, days 1ŌĆō28 | |
| MTX | 20 mg/m2 PO, days 1, 8, 15, and 22 |
Table┬Ā2
| Characteristic | Total (n = 99) | Ph-negative ALL (n = 62) | Ph-positive ALL (n = 37) | p value |
|---|---|---|---|---|
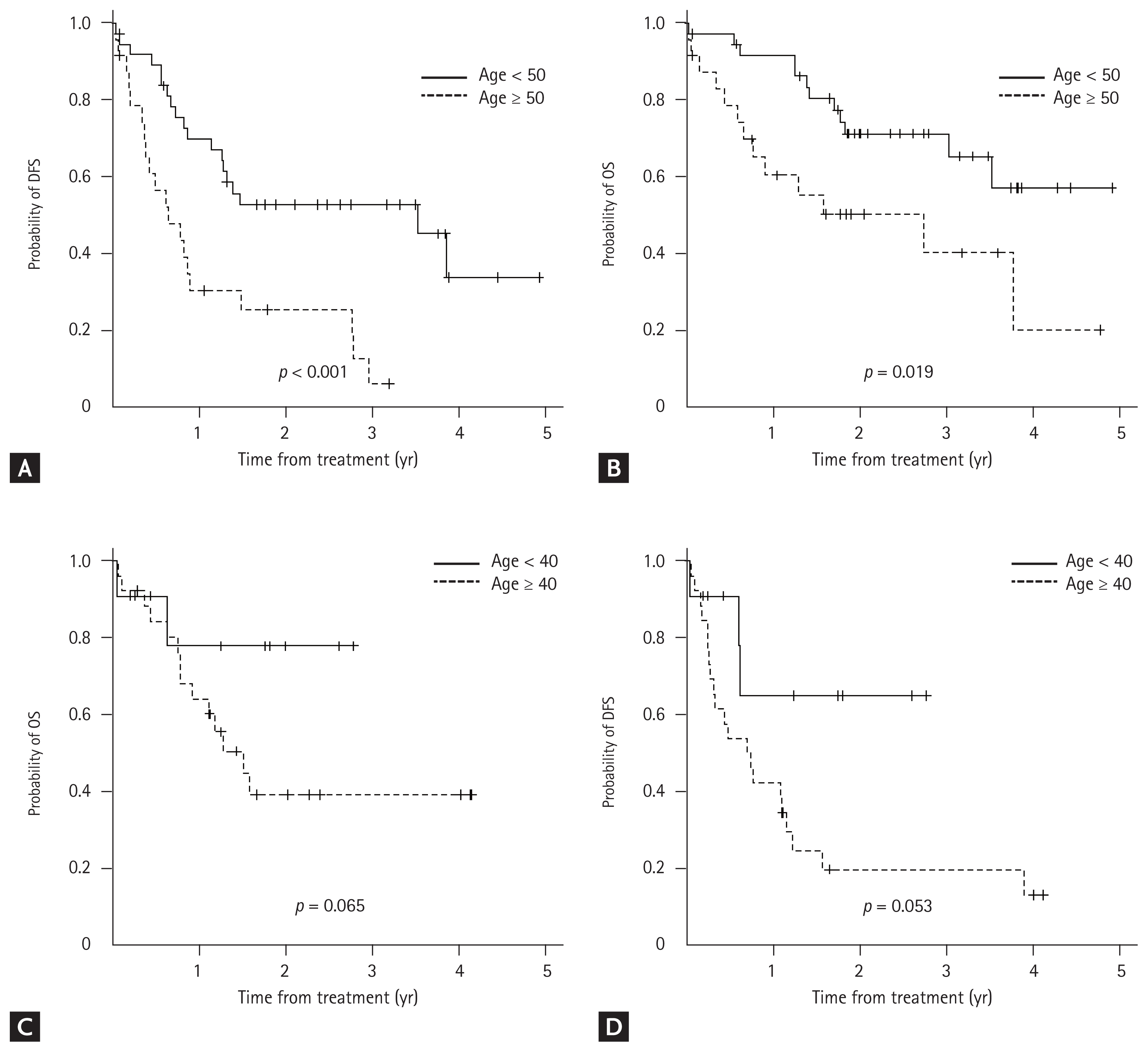
| Age, yr | 47 (17ŌĆō82) | 46 (17ŌĆō82) | 49 (20ŌĆō73) | 0.051 |
| ŌĆā< 25 | 17 (17.2) | 16 (25.8) | 1 (2.7) | |
| ŌĆāŌēź 25 and < 55 | 46 (46.4) | 27 (43.5) | 19 (51.4) | |
| ŌĆāŌēź 55 | 36 (36.4) | 19 (30.6) | 17 (45.9) | |
| Sex | 0.364 | |||
| ŌĆāMale | 49 (50) | 28 (45) | 21 (57) | |
| ŌĆāFemale | 50 (50) | 34 (55) | 16 (43) | |
| BM blasts, % | 90 (21ŌĆō99.8) | 90.3 (23ŌĆō99.8) | 90 (21ŌĆō99.4) | 0.104 |
| Cytogenetic risk groupsa | < 0.001 | |||
| ŌĆāGood risk | 7 (7) | 7 (11) | 0 | |
| ŌĆāIntermediate risk | 42 (42) | 42 (68) | 0 | |
| ŌĆāPoor risk | 50 (51) | 13 (21) | 37 (100) | |
| WBC, ├Ś 103/╬╝L | 11.9 (0.27ŌĆō442.8) | 10.1 (0.27ŌĆō339.2) | 21.3 (2.8ŌĆō442.8) | 0.007 |
| ŌĆā< 30.0 | 68 (69) | 49 (79) | 19 (51.4) | |
| ŌĆāŌēź 30.0 | 31 (31) | 13 (21) | 18 (48.6) | |
| Increased LDH | 73 (74) | 43 (69) | 30 (81) | 0.295 |
| Extramedullary involvement | 0.302 | |||
| ŌĆāLymph nodes | 18 (18) | 14 (23) | 4 (11) | |
| ŌĆāCNS | 6 (6) | 3 (5) | 3 (8) | |
| No. of cycles | 3 (1ŌĆō6) | 3 (1ŌĆō6) | 3 (1ŌĆō6) | 0.006 |
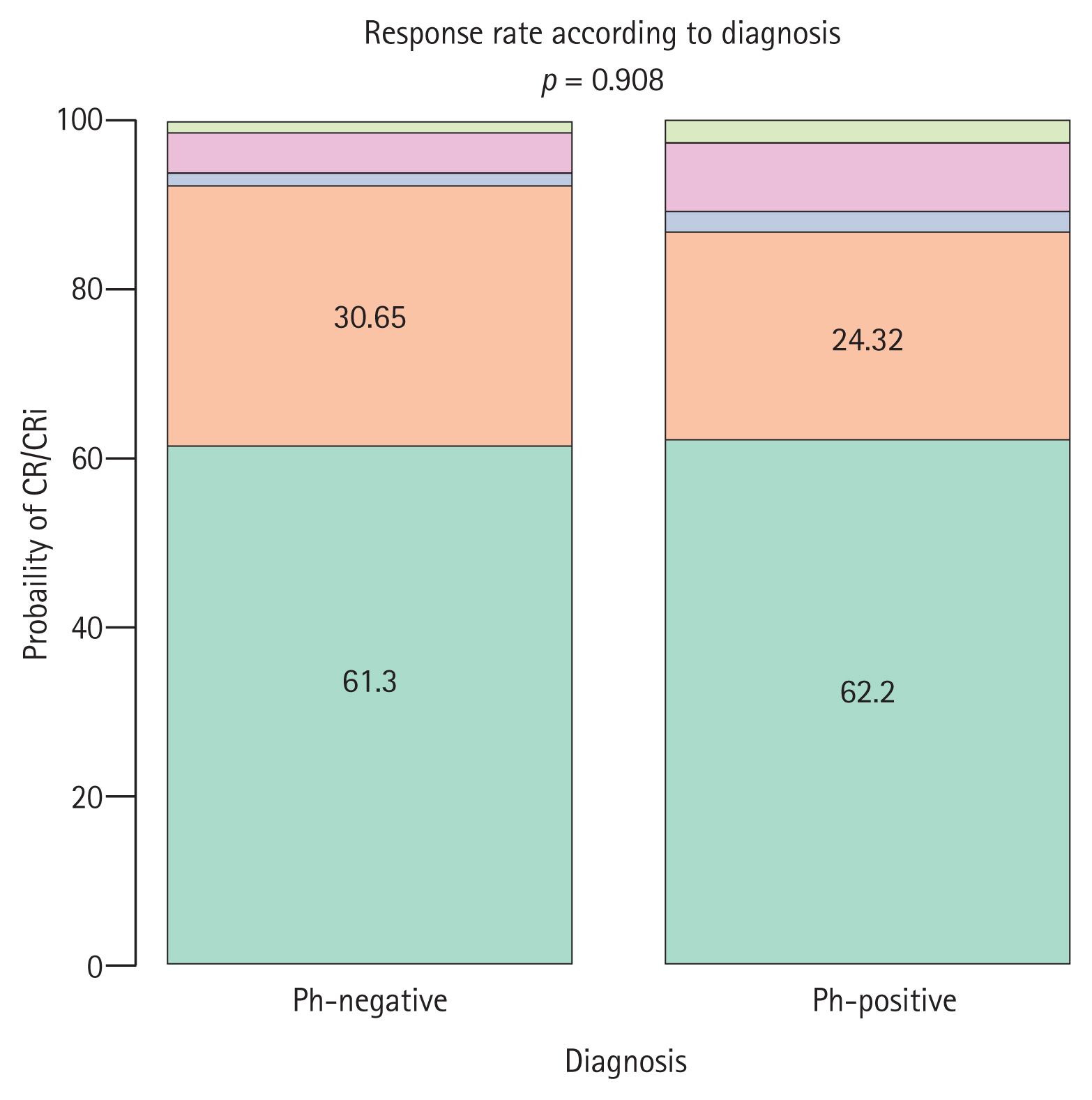
| Response to induction | 0.908 | |||
| ŌĆāCR | 61 (62) | 38 (61) | 23 (62) | |
| ŌĆāCRi | 28 (28) | 19 (31) | 9 (24) | |
| ŌĆāPrimary refractory | 2 (2) | 1 (2) | 1 (3) | |
| ŌĆāDeath | 6 (6) | 3 (5) | 3 (8) | |
| ŌĆāUnknown or missing data | 2 (2) | 1 (2) | 1 (3) | |
| Allogeneic HCT | 39 (39) | 25 (40) | 14 (38) | 0.974 |
| Relapse | 63 (64) | 39 (63) | 24 (65) | 1.000 |
| Death | 41 (41) | 25 (40) | 16 (43) | 0.941 |
Values are presented as median (range) or number (%). Good risk: hyperdiploidy (> 50 chromosomes); triosomy of chromosome 4, 10, or 17; t(12;21)(p13;q22): ETV6-RUNX1; t(1;19)(q23;p13): TCF3-PBX1. Poor risk: Hypodiploidy (< 44 chromosomes); t(v;11q23) (e.g., t(4;11) and others), t(11;19): KMT2A rearranged; t(9;22)(q34;q11.2): BCR-ABL1; complex karyotype (5 or more chromosomal abnormalities); Ph-like ALL; intrachromosomal amplification of chromosome 21 (iAMP21). Intermediate risk: the others with normal karyotypes.
Table┬Ā3
Table┬Ā4
Table┬Ā5
Table┬Ā6
| Study | Patients (n) | Age, median (range), yr | Regimen | Response to induction therapy | Long-term outcome |
|---|---|---|---|---|---|
| Kantarjian et al. (2004) [27] |
Ph-negative ALL (240) Ph-positive ALL (48) |
40 (15ŌĆō92) | Hyper-CVAD | CR: 92% | 5-year OS: 51% (age < 40), 30% (age Ōēź 40, < 59), 17% (age Ōēź 60) |
| Thomas et al. (2010) [28] | Ph-negative ALL (282) | 41 (13ŌĆō83) | Modified hyper-CVAD ┬▒ rituximab | CR: 95% | 3-year CRD: 60%, OS: 50% |
| Huguet et al. (2018) [29] |
BCP-ALL (525) T-ALL (262) |
36.1 (18ŌĆō59) | GRAALL-2005 trial | CR: 91% | 5-year EFS: 55.7% (age < 55), 25.8% (Ōēź 55) |
| Tanguy-Schmidt et al. (2013) [30] | Ph-positive ALL (45) | 45 (16ŌĆō59) | GRAAPH-2003 (imatinib combined chemotherapy) | CR: 96% | 4-year DFS: 43%, OS: 52% |
| Kim et al. (2015) [31] | Ph-positive ALL (90) | 47 (17ŌĆō71) | Nilotinib based multiagent chemotherapy | CR: 91% | 2-year OS: 72% |
| Wieduwilt et al. (2018) [32] | Ph-positive ALL (64) | 60 (22ŌĆō87) | Alliance/CALGB Study 10701 (dasatinib combined chemotherapy) | CR: 97% | 3-year DFS: 43%, OS: 55% |
| Ravandi et al. (2010) [33] | Ph-positive ALL (35) | 53 (21ŌĆō79) | Hyper-CVAD + dasatinib | CR: 94% | 2-year OS: 64% |
| Present study |
Ph-negative ALL (62) Ph-positive ALL (37) |
47 (17ŌĆō82) |
KALLA 1406 KALLA 1407 |
CR/CRi: 92% CR/CRi: 86% |
2-year DFS: 42%, OS: 63% 2-year DFS: 31.2%, OS: 49.1% |
Ph, Philadelphia; ALL, acute lymphoblastic leukemia; CVAD, cyclophosphamide, vincristine, doxorubicin, and dexamethasone; CR, complete remission; OS, overall survival; CRD, CR duration; BCP, B-cell precursor; EFS, event-free survival; DFS, disease free survival; CRi, CR with incomplete hematologic recovery.
REFERENCES
- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



