 |
 |
|
|
|
Abstract
Background/Aims
Helicobacter pylori infection is presumably associated with iron deficiency and iron deficiency anemia (IDA). This study aimed to evaluate the relationship between H. pylori infection and the decline in iron stores in non-elderly adults during their health check-ups.
Methods
We identified a total of 1,069 subjects who were tested for iron, ferritin, and total iron-binding capacity during their health check-ups, from January 2016 to May 2017. Of these, subjects who underwent endoscopy via rapid urease test and those aged 65 years or below were finally enrolled.
Results
Overall, 281 subjects were enrolled, and 187 patients (66.5%) tested positive for H. pylori. The mean age was 36.1 years (range, 22 to 65), and 176 subjects (62.6%) were male. The mean levels of hemoglobin (14.1 ┬▒ 1.7 g/dL vs. 14.6 ┬▒ 1.4 g/dL, p = 0.019) and ferritin (121.7 ┬▒ 106.9 ng/mL vs. 151.8 ┬▒ 107.8 ng/mL, p = 0.027) in the H. pylori-positive group were significantly lower than those in the H. pylori-negative group. Iron deficiency (ferritin < 30 ng/mL) was more common in patients with H. pylori infection (p = 0.002). There was no significant difference in anemia (hemoglobin < 13 g/dL in men, < 12 g/dL in women) or IDA (anemia, ferritin < 10 ng/mL, and transferrin saturation < 16%) with H. pylori. Logistic regression analysis demonstrated that female sex (odds ratio, 197.559; 95% confidence interval, 26.461 to 1,475.015) and H. pylori infection (odds ratio, 3.033; 95% confidence interval, 1.216 to 7.567) were factors associated with iron deficiency.
Helicobacter pylori infects approximately one-half of the worldŌĆÖs population, making it one of the most common bacterial infections [1]. This bacterial pathogen is well known for its distinct etiological role in many types of gastrointestinal diseases [2]. Since the early 2000s, interest in extragastric manifestations of H. pylori has grown. Many studies have been performed to demonstrate the relationship between H. pylori and extragastric disease [3]. Extragastric ailments, including cardiovascular, neurodegenerative, autoimmune, allergic, skin, metabolic, and hematologic diseases, have been investigated [4ŌĆō6]. H. pylori infection appears to be linked to hematologic disorders, such as idiopathic thrombocytopenic purpura, vitamin B12 deficiency, and unexplained iron deficiency anemia (IDA), more than other extragastric disorders [7].
Iron is essential for biological functions, such as respiration, energy production, and cell proliferation [8]. Iron deficiency is the most common micronutrient deficiency worldwide, affecting nearly 50% of the global population [9]. Iron deficiency is the primary cause of anemia, which can lead to severe manifestations, such as IDA [9]. According to the World Health Organization (WHO) report (2002), IDA is considered one of the 10 most important contributing factors to the global burden of disease [10].
Iron deficiency and IDA are associated with multifaceted etiologies. Interestingly, several studies have indicated that H. pylori infection induces iron deficiency and IDA [11ŌĆō13]. H. pylori-associated chronic gastritis can modify gastric physiology and disrupt iron absorption [13]. Specifically, H. pylori gastritis alters gastric acid secretion [14] and gastric ascorbic acid levels [15], which are important promotors of iron absorption. Many clinical studies have evaluated the influence of H. pylori infection on iron deficiency and IDA [16]. However, the association between H. pylori infection and decline in iron stores has been disputed. To the best of our knowledge, there has not been a published study on these issues in adults who have undergone health check-ups in Korea. Therefore, this study aimed to investigate the influence of H. pylori infection on iron deficiency and IDA in non-elderly Korean adults undergoing health check-ups.
We retrospectively reviewed the medical records of patients who participated in a health check-up program at Kosin University Gospel Hospital between January 2016 and May 2017. Subjects whose hemoglobin, iron, ferritin, and total iron-binding capacity (TIBC) were evaluated and those who had undergone esophagogastroduodenoscopy with a rapid urease test on the same day were enrolled. The exclusion criteria were pregnancy, previous gastric surgery, age above 65 years, hematologic disorders related to anemia, angina, myocardial infarction, stroke, chronic kidney disease, steroid use, antiplatelet agent use, anticoagulant use, or any clinical evidence of malignancy.
Baseline demographic features, including age, sex, body mass index (BMI), hypertension, diabetes mellitus, smoking, and alcohol habits were evaluated. BMI was calculated as the weight divided by the square of height (kg/m2). Subjects were required to fast in the morning before blood sample collection for the measurement of hemoglobin, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, iron, TIBC, ferritin, and transferrin saturation.
All subjects fasted for at least 8 hours before esophagogastroduodenoscopy. Endoscopic diagnoses, such as atrophic gastritis, gastric ulcers, duodenal ulcers, gastric and duodenal ulcers, as well as others (e.g., nodular gastritis, gastric erosion, or gastric polyp), were identified by endoscopy or endoscopy with a biopsy. Peptic ulcer stages were assessed using the Sakita and Fukutomi methods [17].
The presence of H. pylori infection was confirmed by positive results in the rapid urease test (CLOtest, Delta West, Bentley, Australia) conducted during endoscopy. The antrum and corpus were the sites of gastric mucosal biopsy. We examined normal or near-normal gastric mucosa with little atrophy or intestinal metaplasia. Tissue samples were immersed in a rapid urea reagent. The rapid urease test was defined as positive when the reagent color changed from yellow to red at least 24 hours later. The CLOtest kit had a sensitivity of 91.2%, a specificity of 99.6%, a positive predictive value of 99.6%, a negative predictive value of 90.9%, and an accuracy of 95.1% [18].
Anemia was defined as serum hemoglobin levels of < 13 g/dL in men and < 12 g/dL in women, according to the WHO criteria [19]. Iron deficiency was defined as serum ferritin levels of < 30 ng/mL. IDA was defined by the presence of all three conditions: (1) anemia based on the definition above; (2) serum ferritin < 10 ng/mL; and (3) transferrin saturation < 16%, as defined by a previous review [20]. This study was approved by the Institutional Review Board (No. 2020-06-040) of the Kosin University Gospel Hospital. Written informed consent was waived by the board because of the retrospective nature of this study.
All statistical analyses were conducted using the SPSS software version 23.0 (IBM Co., Armonk, NY, USA). Categorical data are shown as numbers or percentages, and continuous data are presented as mean ┬▒ standard deviation (SD). StudentŌĆÖs t test was used to analyze continuous data. Categorical data were analyzed using the chi-square test or FisherŌĆÖs exact test. Logistic regression analysis was used to evaluate the associations between H. pylori infection and other factors, including iron status, which were expressed as odds ratios (ORs) and 95% confidence intervals (CIs). Statistical significance was set at p < 0.05.
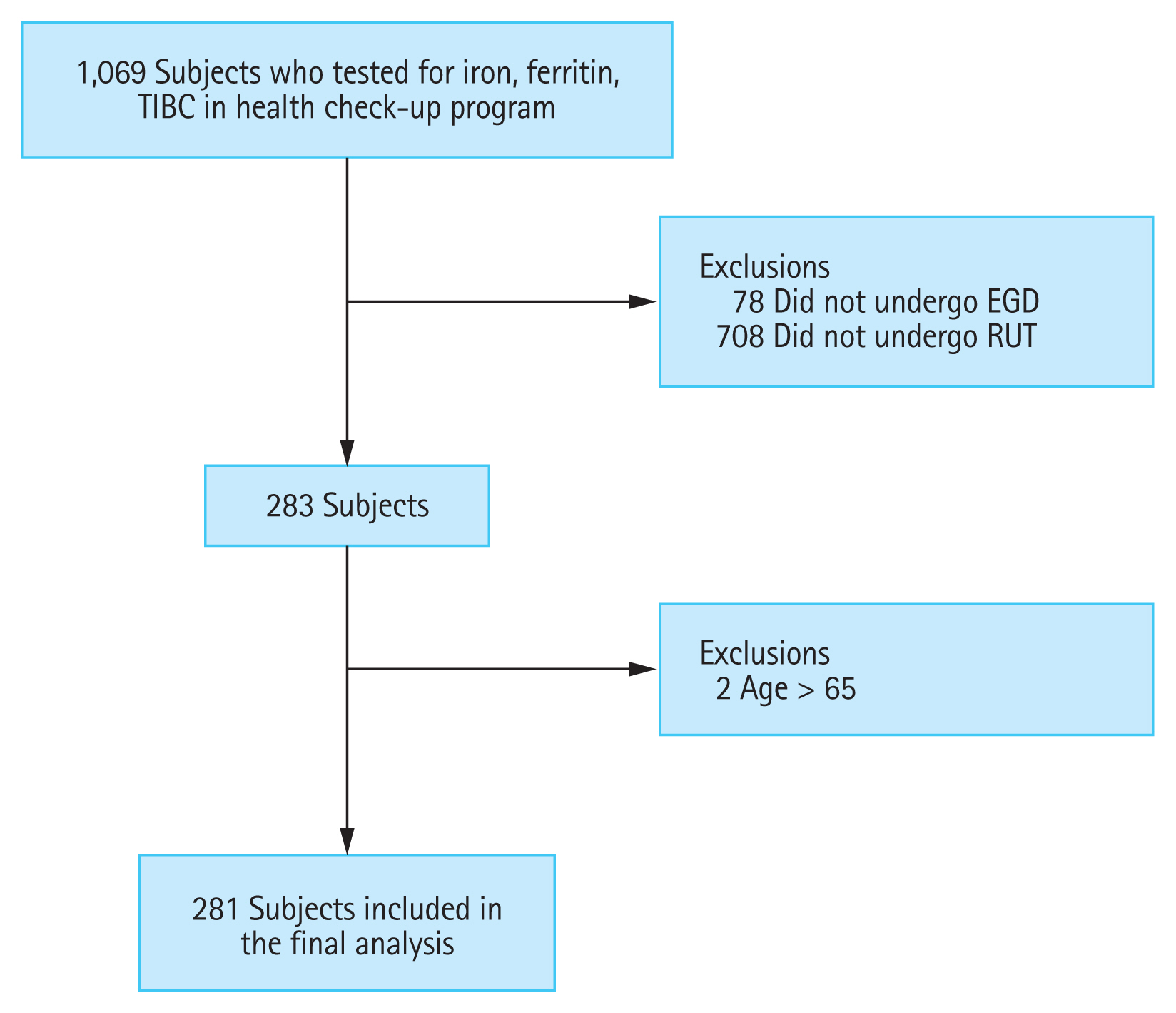
A total of 1,069 subjects were enrolled in this study, and 281 subjects were included in the final analysis (Fig. 1). The mean age of the subjects was 36.1 years (range, 22 to 65), and 176 subjects (62.6%) were male. Among the included subjects, 187 (66.5%) were positive for H. pylori. The proportion of female subjects (74.3% vs. 61.9%, p = 0.034) was significantly higher in the H. pylori-positive group. However, the percentage of subjects with hypertension was significantly lower in the H. pylori-positive group (1.6% vs. 7.4%, p = 0.018). The proportion of subjects with atrophic gastritis was significantly higher in the H. pylori-positive group (16.0% vs. 6.4%, p = 0.023). There were no significant differences in age, BMI, smoking, alcohol consumption, diabetes, or other endoscopic findings except atrophic gastritis between the H. pylori-positive and H. pylori-negative groups (Table 1).
The levels (mean ┬▒ SD) of hemoglobin, iron, TIBC, ferritin, transferrin saturation were 14.3 ┬▒ 1.6 g/dL, 129.8 ┬▒ 52.8 ╬╝g/dL, 333.4 ┬▒ 46.4 ╬╝g/dL, 131.7 ┬▒ 108.0 ng/mL, and 40.0% ┬▒ 17.1%, respectively. Hemoglobin (p = 0.019) and ferritin (p = 0.027) levels were significantly decreased in the H. pylori-infected group compared to those in the uninfected group. There were no statistically significant differences between H. pylori infection and iron-related or other laboratory findings (Table 1).
We compared the laboratory results between men and women. There were significant differences in hemoglobin (p < 0.001), iron (p < 0.001), TIBC (p < 0.001), and ferritin (p < 0.001) levels and transferrin saturation (p < 0.001) between male and female subjects (Table 2). However, these anemia profiles were not significantly related to H. pylori infection in either male or female subjects (Table 2).
We grouped the subjects based on anemia, IDA, and iron deficiency to determine the association between iron status and H. pylori infection. Based on the results of the univariate analysis, iron deficiency was statistically correlated with H. pylori infection (p = 0.002) (Table 3). The proportions of anemia and IDA were higher in the H. pylori-positive group. However, there were no significant differences between the H. pylori-positive and H. pylori-negative groups.
The association between iron deficiency and H. pylori infection is shown in Table 4. In the univariate analysis, female sex (OR, 192.500; 95% CI, 25.986 to 1,425.981) and H. pylori infection (OR, 3.171; 95% CI, 1.479 to 6.796) were related to iron deficiency. Multivariate analysis confirmed that female sex (OR, 197.559; 95% CI, 26.461 to 1,475.015) and H. pylori infection (OR, 3.033; 95% CI, 1.216 to 7.567) were correlated with iron deficiency.
In this study, H. pylori infection was associated with iron deficiency in non-elderly adult subjects. Additionally, hemoglobin and ferritin levels were significantly lower in H. pylori-positive subjects.
Serum ferritin is the major storage protein containing iron. Therefore, serum ferritin level is considered the most sensitive and specific indicator of iron deficiency [20]. Subjects with H. pylori infection showed lower serum ferritin levels in the current study than H. pylori-negative subjects. These results concurred with those of previous studies comparing serum ferritin values between H. pylori-positive and H. pylori-negative subjects. A Danish population study of 2,794 healthy adults revealed that serum ferritin levels were lower in individuals with higher H. pylori immunoglobulin antibodies (OR, 1.4; 95% CI, 1.1 to 1.8), suggesting that H. pylori infection may affect iron metabolism in humans [21]. Another study involving urban Alaskan native adults also reported that serum ferritin levels in H. pylori-positive subjects were lower than those in H. pylori-negative subjects (p = 0.04) [22]. Despite the small number of patients who were followed up, those with iron deficiency and those who received H. pylori eradication therapy showed higher ferritin levels (p = 0.02), and their iron deficiency was resolved 24 months after treatment [22]. Unlike other studies, there was no statistically significant difference in serum ferritin levels between H. pylori-positive and -negative groups (p = 0.360) in a study from Taiwan. However, the values of serum ferritin were lower in H. pylori-positive adult subjects [23].
Iron deficiency represents a reduction in iron stores that precedes IDA or persistent deficiency without progression [20]. In our study, active H. pylori infection was associated with iron deficiency (OR, 3.171; 95% CI, 1.479 to 6.796). According to a study based on the 1999 to 2000 National Health and Nutrition Examination Survey (NHANES), H. pylori infection was associated with a 40% enhanced prevalence of iron deficiency (prevalence odds ratio [POR], 1.4; 95% CI, 0.9 to 2.0) [24]. A recent meta-analysis study reported an increased likelihood of iron deficiency in individuals with H. pylori infection and a pooled OR (30 studies) of 1.33 (95% CI, 1.15 to 1.54) [16]. In contrast, a Brazilian study found no significant difference in iron deficiency between seropositive and seronegative adults [25].
Ascorbic acid and low gastric pH are crucial factors in the absorption of dietary iron [14,15]. Dietary iron is divided into heme iron (found mainly in red meat) that is immediately absorbed, and non-heme iron (white meat, vegetable, and grain), the bioavailability of which is influenced by various factors [26]. Non-heme iron exists in the oxidized ferric form under physiological pH. However, iron must be in the ferrous form or bound to a protein such as heme for membrane transport [27]. Reduction from the ferric to ferrous form is dependent on the pH of the gastric juice [27]. H. pylori infection damages the gastric mucosa and causes gastritis with altered gastric acidity and ascorbic acid levels [16]. In this study, the proportion of atrophic gastritis was higher in the H. pylori-positive group. Taguchi et al. [12] reported that the number of parietal cells in the secretory phase was significantly lower in H. pylori-positive patients than in those without H. pylori, suggesting that H. pylori-induced gastritis has a higher intragastric pH. The decrease in ascorbic acid level is associated with the pH of gastric juice, extent and severity of gastritis, presence of H. pylori, and subjectŌĆÖs cytotoxin-associated gene A (CagA) antibody status [15]. Thus, H. pylori infection may contribute to changes in human dietary iron absorption by altering gastric pH and ascorbic acid levels in the gastric juice.
Therefore, H. pylori infection is thought to affect anemia and IDA. Serum hemoglobin levels were significantly lower in subjects with H. pylori infection than in uninfected subjects in this study. However, statistical differences in anemia and IDA between H. pylori-positive and -negative groups were not detected. Compared with the current study, the previous 1999 to 2000 NHANES study reported that H. pylori infection was associated with the prevalence of IDA (POR, 2.6; 95% CI, 1.5 to 4.6) and anemia (POR, 1.3; 95% CI, 1.0 to 1.7) [24]. Although significant heterogeneity existed among the enrolled studies, an increased likelihood of IDA (pooled OR, 1.72; 95% CI, 1.23 to 2.42 in 14 observational studies) and anemia (pooled OR, 1.15; 95% CI, 1.00 to 1.32 in 23 studies) was observed in H. pylori-positive subjects compared with that in uninfected subjects in the meta-analysis [16]. There are several potential pathogenic mechanisms by which H. pylori infection is involved in anemia and IDA, such as occult blood loss secondary to H. pylori-induced chronic gastritis or increased iron uptake and usage by H. pylori infection [28]. Therefore, the relationship between H. pylori infection and IDA needs to be further investigated.
Several factors may explain the lack of effect on anemia and IDA following H. pylori infection in this study. Among them, the main factor may be related to the characteristics of the enrolled subjects. We enrolled subjects under the age of 65 years who underwent a health check-up. The proportions of anemia and IDA were relatively low, and therefore, it was difficult to demonstrate the influence of H. pylori infection on anemia and IDA in these subjects. The reported global prevalence of anemia is 32.9% according to data reported in the 2010 Study of Global Burden of Diseases, Injuries, and Risk Factors [29]. A meta-analysis revealed that the prevalence of anemia in developed countries was 1% among male adults and 14% among female adults [30]. Nonetheless, active H. pylori infection was linked to iron deficiency in the current study, suggesting that H. pylori infection may influence iron deficiency even in non-elderly adults without obvious symptoms. Similarly, H. pylori eradication therapy may be attempted in adults with iron deficiency and H. pylori infection. Several established guidelines have also recommended that subjects with unexplained IDA should be tested and treated for H. pylori infection [7,31].
The prevalence of H. pylori in this study was similar to or higher than that reported in previous Korean studies. In 2011, a nationwide prospective study reported an H. pylori prevalence rate of 54.4%, and the prevalence in the study site was 65.1% in Korea [32]. However, three recent nationwide studies in Korea reported H. pylori prevalence rates of 41.5% and 51.3% from 2015 to 2017 [33ŌĆō35]. The reasons for these discrepancies among the studies may be attributed to regional variations and differences in the diagnostic methods. Moreover, experienced endoscopists performed endoscopy in this study; therefore, the possibility that the rapid urease test was conducted in patients with a high probability of H. pylori infection cannot be ruled out.
The studyŌĆÖs limitations are worth mentioning. The main limitations include the failure to elucidate factors affecting ferritin levels, such as the use of iron supplementation or hormonal contraception, dietary history, or blood donation. We were not able to separate subjects who had previously received H. pylori eradication therapy, owing to the studyŌĆÖs retrospective design. However, H. pylori eradication was associated with significantly elevated serum hemoglobin (p = 0.01) and serum ferritin (p < 0.0001) levels in a meta-analysis [36], suggesting that this factor may have little effect on the results. Third, there may have been a selection bias in these data, because we retrospectively analyzed subjects who participated in a health check-up program, and many subjects who did not undergo a rapid urease test were excluded. The outcomes of this study may also be potentially affected by subject-related environmental factors. However, when we considered the prevalence of H. pylori infection in the current study, the selection bias was insignificant. In addition, women of reproductive age are at a high risk of anemia [37]. Understandably, there were significant differences in anemia profiles, including hemoglobin, iron, TIBC, ferritin, and transferrin saturation, between male and female subjects in this study. However, we were not able to categorize the female subjects into reproductive and postmenopausal subgroups for analysis because of the retrospective design. Lastly, we did not diagnose H. pylori according to histology. Diagnostic methods affect the diagnostic yield of H. pylori infection. However, compared with the sensitivity of H. pylori histology in the range of 80% [38], the sensitivity and specificity of the rapid urease test ranged from 90% to 95% and 95% to 100%, respectively [18,39]. The accuracy of the rapid urease test is high, and its clinical use is justified [40]. Thus, the absence of histology was unlikely to have significantly influenced the results. Most studies used serologic tests for H. pylori, whereas this study confirmed H. pylori infection via endoscopy and rapid urease tests in all enrolled subjects. Our results showed a better diagnostic accuracy for the study population.
In conclusion, this study found that H. pylori infection was associated with iron deficiency in non-elderly adults, and serum ferritin and hemoglobin levels were decreased in patients with H. pylori infection. The results suggest that active H. pylori infection affects iron status and promotes a decrease in iron stores, including iron deficiency. Further large-scale and better-designed studies are required to corroborate the relationship and mechanisms of H. pylori infection and iron status.
1. This study showed that reductions in serum ferritin and hemoglobin levels in health check-up subjects are related to Helicobacter pylori infection.
2. H. pylori infection is a risk factor for iron deficiency, postulating a decline in iron stores among H. pylori-positive non-elderly adults.
3. This is the first study on H. pylori infection and iron stores in adults who attended health check-ups in Korea.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (Grant no. 2017R1C1B5017457).
Figure┬Ā1
Schematic flow of the study. TIBC, total iron-binding capacity; EGD, esophagogastroduodenoscopy; RUT, rapid urease test.
Table┬Ā1
Baseline characteristics and laboratory findings of the subjects
| Characteristic | Overall (n = 281) | H. pylori (+) (n = 187) | H. pylori (ŌłÆ) (n = 94) | p value |
|---|---|---|---|---|
| Age, yr | 36.1 ┬▒ 7.9 | 36.3 ┬▒ 7.6 | 35.7 ┬▒ 7.7 | 0.500 |
| Sex | ||||
| ŌĆāMale | 176 (62.6) | 109 (58.3) | 67 (71.3) | 0.034a |
| ŌĆāFemale | 105 (37.4) | 78 (41.7) | 27 (28.7) | |
| BMI, kg/m2 | 24.4 ┬▒ 3.7 | 24.6 ┬▒ 3.8 | 24.1 ┬▒ 3.5 | 0.359 |
| Cigarette smoking | 86 (30.6) | 53 (28.3) | 33 (35.1) | 0.246 |
| Alcohol intake | 208 (74.0) | 137 (73.3) | 71 (75.5) | 0.682 |
| Hypertension | 10 (3.6) | 3 (1.6) | 7 (7.4) | 0.018a |
| Diabetes mellitus | 11 (3.9) | 7 (3.7) | 4 (4.3) | 1.000 |
| Endoscopic diagnosis | ||||
| ŌĆāGastric ulcer | 40 (14.2) | 24 (12.8) | 16 (17.0) | 0.563 |
| ŌĆāŌĆāActive/Healing/Scar | 1 (2.5)/5 (12.5)/34 (85.0) | 1 (4.2)/3 (12.5)/20 (83.3) | 0 (0.0)/2 (12.5)/14 (87.5) | |
| ŌĆāDuodenal ulcer | 222 (79.0) | 152 (81.3) | 70 (74.5) | 0.934 |
| ŌĆāŌĆāActive/Healing/Scar | 1 (0.5)/4 (1.8)/217 (97.7) | 1 (0.7)/2 (1.3)/149 (98.0) | 0 (0.0)/2 (2.9)/68 (97.1) | |
| ŌĆāGastroduodenal ulcer | 7 (2.5) | 5 (2.7) | 2 (2.1) | 1.000 |
| ŌĆāŌĆāActive/Healing/Scar | 0 (0.0)/1 (14.3)/6 (85.7) | 0 (0.0)/1 (20.0)/4 (80.0) | 0 (0.0)/0 (0.0)/2 (100.0) | |
| ŌĆāOthersb | 12 (4.1) | 6 (3.2) | 6 (6.4) | 0.225 |
| ŌĆāAtrophic gastritis | 36 (12.8) | 30 (16.0) | 6 (6.4) | 0.023a |
| Laboratory findings | ||||
| ŌĆāHemoglobin, g/dL | 14.3 ┬▒ 1.6 | 14.1 ┬▒ 1.7 | 14.6 ┬▒ 1.4 | 0.019a |
| ŌĆāMCV, fL | 90.2 ┬▒ 5.4 | 89.8 ┬▒ 5.9 | 90.9 ┬▒ 4.1 | 0.094 |
| ŌĆāMCH, pg | 30.0 ┬▒ 2.7 | 29.9 ┬▒ 1.8 | 30.3 ┬▒1.5 | 0.151 |
| ŌĆāMCHC, g/dL | 33.3 ┬▒ 0.8 | 33.3 ┬▒ 0.9 | 33.3 ┬▒ 0.6 | 0.584 |
| ŌĆāron, ╬╝g/dL | 129.8 ┬▒ 52.8 | 128.9 ┬▒ 56.9 | 131.5 ┬▒ 43.6 | 0.677 |
| ŌĆāTIBC, ╬╝g/dL | 333.4 ┬▒ 46.4 | 337.0 ┬▒ 48.1 | 326.5 ┬▒ 42.2 | 0.075 |
| ŌĆāFerritin, ng/mL | 131.7 ┬▒ 108.0 | 121.7 ┬▒ 106.9 | 151.8 ┬▒ 107.8 | 0.027a |
| ŌĆāTransferrin saturation, % | 40.0 ┬▒ 17.1 | 39.6 ┬▒ 18.6 | 40.8 ┬▒ 13.9 | 0.558 |
Table┬Ā2
Laboratory findings according to sex and Helicobacter pylori infection
| Male (n = 176) | Female (n = 105) | p value | Male (n = 176) | Female (n = 105) | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| H. pylori (+) (n = 109) | H. pylori (ŌłÆ) (n = 67) | p value | H. pylori (+) (n = 78) | H. pylori (ŌłÆ) (n = 27) | p value | ||||
| Hemoglobin, g/dL | 15.2 ┬▒ 1.0 | 12.8 ┬▒ 1.3 | < 0.001a | 15.2 ┬▒ 1.0 | 15.2 ┬▒ 1.0 | 0.962 | 12.7 ┬▒ 1.3 | 13.1 ┬▒ 1.1 | 0.115 |
|
|
|||||||||
| MCV, fL | 91.0 ┬▒ 3.6 | 88.8 ┬▒ 7.3 | 0.004a | 91.0 ┬▒ 3.4 | 91.0 ┬▒ 3.9 | 0.962 | 88.1 ┬▒ 7.9 | 90.7 ┬▒ 4.5 | 0.118 |
|
|
|||||||||
| MCH, pg | 30.1 ┬▒ 2.1 | 29.9 ┬▒ 2.3 | 0.581 | 30.0 ┬▒ 1.9 | 30.3 ┬▒ 2.3 | 0.409 | 30.1 ┬▒ 2.6 | 29.7 ┬▒ 2.5 | 0.443 |
|
|
|||||||||
| MCHC, g/dL | 33.5 ┬▒ 0.6 | 33.0 ┬▒ 1.0 | < 0.001a | 33.5 ┬▒ 0.6 | 33.4 ┬▒ 0.5 | 0.197 | 32.9 ┬▒ 1.1 | 33.1 ┬▒ 0.6 | 0.258 |
|
|
|||||||||
| Iron, ╬╝g/dL | 141.9 ┬▒ 47.8 | 109.5 ┬▒ 54.7 | < 0.001a | 143.5 ┬▒ 51.5 | 139.3 ┬▒ 41.2 | 0.578 | 108.6 ┬▒ 58.2 | 112.1 ┬▒ 43.9 | 0.781 |
|
|
|||||||||
| TIBC, ╬╝g/dL | 324.4 ┬▒ 35.7 | 348.5 ┬▒ 57.3 | < 0.001a | 326.2 ┬▒ 37.4 | 321.6 ┬▒ 33.0 | 0.413 | 351.9 ┬▒ 56.9 | 338.5 ┬▒ 58.2 | 0.297 |
|
|
|||||||||
| Ferritin, ng/mL | 183.0 ┬▒ 100.5 | 45.8 ┬▒ 50.0 | < 0.001a | 181.0 ┬▒ 100.1 | 186.4 ┬▒ 101.7 | 0.727 | 38.8 ┬▒ 40.4 | 65.9 ┬▒ 67.8 | 0.058 |
|
|
|||||||||
| Transferrin saturation, % | 44.3 ┬▒ 15.5 | 32.9 ┬▒ 17.4 | < 0.001a | 44.7 ┬▒ 16.8 | 43.6 ┬▒ 13.2 | 0.637 | 32.6 ┬▒ 18.7 | 33.8 ┬▒ 13.2 | 0.717 |
Table┬Ā3
Anemia, iron deficiency anemia, and iron deficiency according to Helicobacter pylori infection
| Overall (n = 281) | H. pylori (+) (n = 187) | H. pylori (ŌłÆ) (n = 94) | p value | |
|---|---|---|---|---|
| Anemiaa | 0.225 | |||
| ŌĆāNo | 261 (92.9) | 171 (91.4) | 90 (95.7) | |
| ŌĆāYes | 20 (7.1) | 16 (8.6) | 4 (4.3) | |
| IDA (anemiaa, iron < 10 ╬╝g/dL, and transferrin saturation < 16%) | 0.173 | |||
| ŌĆāNo | 271 (96.4) | 178 (95.2) | 93 (98.9) | |
| ŌĆāYes | 10 (3.6) | 9 (4.8) | 1 (1.1) | |
| Iron deficiency (ferritin < 30 ng/mL) | 0.002b | |||
| ŌĆāNo | 225 (80.1) | 140 (74.9) | 85 (90.4) | |
| ŌĆāYes | 56 (19.9) | 47 (25.1) | 9 (9.6) |
Table┬Ā4
Factors associated with iron deficiency in non-elderly adults
| Factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95% CI | p value | OR | 95% CI | p valuea | |
| Age | 0.995 | 0.957ŌĆō1.035 | 0.820 | 0.972 | 0.922ŌĆō1.024 | 0.286 |
|
|
||||||
| Female | 192.500b | 25.986ŌĆō1,425.981b | < 0.001b | 197.559b | 26.461ŌĆō1,475.015b | < 0.001b |
|
|
||||||
| H. pylori infection | 3.171b | 1.479ŌĆō6.796b | 0.003b | 3.033b | 1.216ŌĆō7.567b | 0.017b |
REFERENCES
1. Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther 1995;9:Suppl 2. 33ŌĆō39.
2. de Korwin JD, Ianiro G, Gibiino G, Gasbarrini A. Helicobacter pylori infection and extragastric diseases in 2017. Helicobacter 2017;22:Suppl 1. e12411.

3. Gasbarrini A, Carloni E, Gasbarrini G, Menard A. Helicobacter pylori and extragastric diseases: other helicobacters. Helicobacter 2003;8:Suppl 1. 68ŌĆō76.


4. Goni E, Franceschi F. Helicobacter pylori and extragastric diseases. Helicobacter 2016;21:Suppl 1. 45ŌĆō48.


5. Franceschi F, Covino M, Roubaud Baudron C. Review: Helicobacter pylori and extragastric diseases. Helicobacter 2019;24:Suppl 1. e12636.



6. Kim SE, Park MI, Park SJ, et al. The influence of iron deficiency on helicobacter pylori eradication. Korean J Helicobacter Up Gastrointest Res 2016;16:82ŌĆō87.

7. Malfertheiner P, Megraud F, OŌĆÖMorain CA, et al. Management of Helicobacter pylori infection: the Maastricht V/Florence Consensus Report. Gut 2017;66:6ŌĆō30.


8. Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell 2010;142:24ŌĆō38.


10. World Health Organization. The World Health Report 2002 reducing risks, promoting healthy life [Internet] Geneva (CH): World Health Organization, c2002. [cited 2021 Oct 25]. Available from: https://www.who.int/whr/2002/en/whr02_en.pdf
.
11. Annibale B, Marignani M, Monarca B, et al. Reversal of iron deficiency anemia after Helicobacter pylori eradication in patients with asymptomatic gastritis. Ann Intern Med 1999;131:668ŌĆō672.


12. Taguchi Y, Kaito M, Gabazza EC, et al. Helicobacter pylori inhibits the secretory activity of gastric parietal cells in patients with chronic gastritis: an ultrastructural study. Scand J Gastroenterol 1997;32:656ŌĆō663.


13. Ciacci C, Sabbatini F, Cavallaro R, et al. Helicobacter pylori impairs iron absorption in infected individuals. Dig Liver Dis 2004;36:455ŌĆō460.


14. Calam J, Gibbons A, Healey ZV, Bliss P, Arebi N. How does Helicobacter pylori cause mucosal damage?: its effect on acid and gastrin physiology. Gastroenterology 1997;113(6 Suppl):S43ŌĆōS50.


15. Zhang ZW, Patchett SE, Perrett D, Katelaris PH, Domizio P, Farthing MJ. The relation between gastric vitamin C concentrations, mucosal histology, and CagA seropositivity in the human stomach. Gut 1998;43:322ŌĆō326.



16. Hudak L, Jaraisy A, Haj S, Muhsen K. An updated systematic review and meta-analysis on the association between Helicobacter pylori infection and iron deficiency anemia. Helicobacter 2017;22:e12330.

17. Sakita T, Fukutomi H. Endoscopic diagnosis. In: Yoshitoshi Y, ed. Ulcer of Stomach and Duodenum. Tokyo (JP): Nankodo, 1971;198ŌĆō208.
18. Choi J, Kim CH, Kim D, et al. Prospective evaluation of a new stool antigen test for the detection of Helicobacter pylori, in comparison with histology, rapid urease test, (13)C-urea breath test, and serology. J Gastroenterol Hepatol 2011;26:1053ŌĆō1059.


19. Nutritional anaemias: report of a WHO scientific group. World Health Organ Tech Rep Ser 1968;405:5ŌĆō37.

21. Milman N, Rosenstock S, Andersen L, Jorgensen T, Bonnevie O. Serum ferritin, hemoglobin, and Helicobacter pylori infection: a seroepidemiologic survey comprising 2794 Danish adults. Gastroenterology 1998;115:268ŌĆō274.


22. Miernyk K, Bruden D, Zanis C, et al. The effect of Helicobacter pylori infection on iron stores and iron deficiency in urban Alaska Native adults. Helicobacter 2013;18:222ŌĆō228.



23. Shih HY, Kuo FC, Wang SS, et al. Helicobacter pylori infection and anemia in Taiwanese adults. Gastroenterol Res Pract 2013;2013:390967.




24. Cardenas VM, Mulla ZD, Ortiz M, Graham DY. Iron deficiency and Helicobacter pylori infection in the United States. Am J Epidemiol 2006;163:127ŌĆō134.


25. Alvarenga EC, Montes CG, Guerrazzi F, Zeitune JM, Grotto HZ. Helicobacter pylori infection and the severity of gastritis are not associated with iron deficiency in a group of Brazilian patients. Clin Chem Lab Med 2010;48:1809ŌĆō1812.


26. Miret S, Simpson RJ, McKie AT. Physiology and molecular biology of dietary iron absorption. Annu Rev Nutr 2003;23:283ŌĆō301.


27. Lombard M, Chua E, OŌĆÖToole P. Regulation of intestinal non-haem iron absorption. Gut 1997;40:435ŌĆō439.



28. DuBois S, Kearney DJ. Iron-deficiency anemia and Helicobacter pylori infection: a review of the evidence. Am J Gastroenterol 2005;100:453ŌĆō459.


29. Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014;123:615ŌĆō624.



30. DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat Q 1985;38:302ŌĆō316.

31. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 2017;112:212ŌĆō239.


32. Lim SH, Kwon JW, Kim N, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol 2013;13:104.



33. Lim SH, Kim N, Kwon JW, et al. Trends in the seroprevalence of Helicobacter pylori infection and its putative eradication rate over 18 years in Korea: a cross-sectional nationwide multicenter study. PLoS One 2018;13:e0204762.



34. Nam K, Shin JE, Kim SE, et al. Prevalence and risk factors for upper gastrointestinal diseases in health check-up subjects: a nationwide multicenter study in Korea. Scand J Gastroenterol 2018;53:910ŌĆō916.


35. Lee JH, Choi KD, Jung HY, et al. Seroprevalence of Helicobacter pylori in Korea: a multicenter, nationwide study conducted in 2015 and 2016. Helicobacter 2018;23:e12463.



36. Qu XH, Huang XL, Xiong P, et al. Does Helicobacter pylori infection play a role in iron deficiency anemia?: a meta-analysis. World J Gastroenterol 2010;16:886ŌĆō896.


37. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993ŌĆō2005. Public Health Nutr 2009;12:444ŌĆō454.


38. Megraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev 2007;20:280ŌĆō322.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



