 |
 |
|
|
|
Abstract
Background/Aims
Anti-phospholipase A2 receptor (PLA2R) autoantibody is the main biomarker of idiopathic membranous nephropathy (IMN). We aimed to find a new cutoff value of anti-PLA2R for patients with IMN and to explore the relevance between this antibody and baseline clinical parameters.
Methods
A total of 670 subjects including 374 IMN cases and 296 non-IMN controls were included between January 2017 and January 2020. All clinical parameters were collected at the time of renal biopsy. The levels of anti-PLA2R were detected by a commercial enzyme-linked immunosorbent assay (ELISA) kit. The optimal cutoff value was calculated by a receiver operating characteristic curve and compared in diagnostic efficiency.
Results
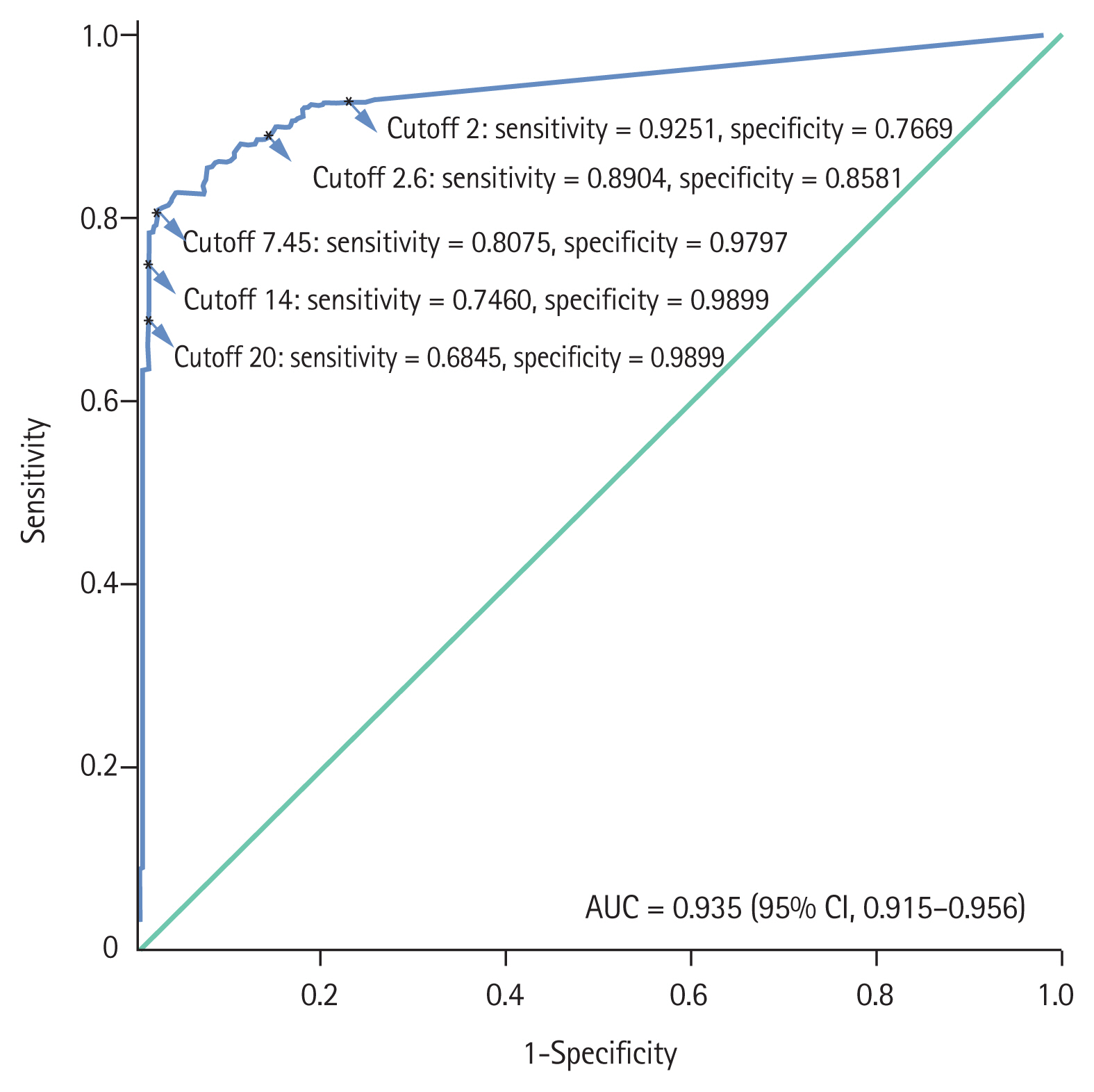
The optimal cutoff value of anti-PLA2R for IMN was 7.45 RU/mL with the highest Youden index, and the corresponding sensitivity, specificity, positive predictive value and negative predictive value were 80.75%, 97.97%, 98.05% and 80.11%, respectively. Anti-PLA2R levels in IMN patients demonstrated a significant positive correlation with serum creatinine and 24-hour urinary protein, while they showed a negative correlation with serum albumin and estimated glomerular filtration rate.
Conclusions
The recommended cutoff value of anti-PLA2R is 7.45 RU/mL using ELISA detection for distinguishing IMN from non-IMN nephropathy. The level of anti-PLA2R is related to baseline renal function in IMN. This new threshold can improve the diagnostic efficiency and facilitate early diagnosis of IMN.
Membranous nephropathy (MN) is a primary cause of nephrotic syndrome in adults [1]. The underlying causes, such as systemic autoimmune diseases, chronic hepatitis B, nonsteroidal anti-inflammatory drugs, mercury intoxication and malignant tumors, can be found in approximately 25% of MN patients diagnosed with secondary membranous nephropathy (SMN) [2]. The other cases without primary causes are diagnosed with idiopathic membranous nephropathy (IMN). This kidney-specific autoimmune disease has been confirmed, and the deposition of subepithelial immune complexes in glomeruli activates complement to destroy the filtration barrier, ultimately resulting in proteinuria [3]. Without medical intervention, 1/3 of patients can achieve spontaneous remission, 1/3 of patients will suffer persistent proteinuria, and the remaining 1/3 will progress to end-stage renal disease [4].
Histopathological examination of renal specimens is the golden standard for the diagnosis of IMN. However, renal biopsy is an invasive approach accompanied by potential complications such as bleeding, arteriovenous fistula, perirenal infection and even death [5]. Moreover, it is unsuitable for patients with uncontrolled hypertension, bleeding tendency, active renal infections or uncooperative mental state [6]. Nonetheless, distinguishing SMN from IMN is important because the treatment of SMN must target the primary disease, while certain treatments for IMN have toxic effects on the kidney or other organs. Therefore, readily available biomarkers based on pathogenesis are urgently needed to make a safer diagnosis of IMN.
In 2009, Beck et al. [7] found the main target antigen of IMN, a 185 kDa type I transmembrane glycoprotein called M-type phospholipase A2 receptor (PLA2R), in podocytes and glomerular immunoprecipitates. The positive rate of the autoantibody against PLA2R was 70% to 80% among IMN patients in their study [7]. Since then, basic and clinical studies on this target have been rapidly carried out. In earlier studies, Western blot and immunofluorescence techniques were widely used to detect serum anti-PLA2R. However, they are not suitable for clinical testing due to their semiquantitative properties and time-consuming procedures [8]. Recently, a new technique called addressable laser bead immunoassay has received attention for its high-throughput quantitative analysis of multiple indexes [9]. However, it is still a research-based assay without commercial development. In contrast, enzyme-linked immunosorbent assay (ELISA) kits have accomplished commercialization to provide quantitative results for a large population through easier and faster procedures. Thus, ELISA is now widely used in clinical laboratories to identify anti-PLA2R values higher than 20 RU/mL as positive. Considering room for improvement in diagnostic value, researchers have been exploring whether a lower cutoff value can be available. It has been proved that both the diagnostic efficiency and consistency with other immunoassay methods could be improved when the ELISA was applied at a lower cutoff value [9]. However, previous studies either investigated only values written in the instructions (2 and 14 RU/mL) or included small sample sizes.
Therefore, we aimed to set a new cutoff value of anti-PLA2R in a large-sample study by commercial ELISA and analyze the correlation between anti-PLA2R levels and clinical parameters related to renal function, such as serum creatinine, serum albumin, 24-hour urinary protein and estimated glomerular filtration rate.
This study carried out in accordance with the Helsinki Declaration and the study protocol was approved by the Ethics Review Committee of Shandong Provincial Hospital in China (LCYJ: No. 2019-105). Because of the retrospective nature of the study, patient consent for inclusion was waived.
We enrolled 670 hospitalized patients who had accepted kidney biopsy from January 2017 to January 2020 in Shandong Provincial Hospital (Jinan, Shandong, China) and collected all the clinical records retrospectively from the inpatient information system. Each patient had one of the following biopsy indications: (1) glomerular hematuria with an elevated Scr level or proteinuria; (2) proteinuria > 1 g/day with no clear comorbidity; (3) rapid elevation in proteinuria; (4) Scr did not return to baseline with 14 days of onset despite removal of culprit in acute kidney injury; (5) a rapid increase in Scr or new-onset hematuria or proteinuria in chronic kidney disease [10].
As shown in Fig. 1, these subjects were divided into the IMN group and non-IMN group according to the criteria recommended by the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guideline for glomerulonephritis [2]. Each patient had accepted medical history surveys, physical exam, thorough laboratory and imaging tests to seek evidences of primary diseases involving autoimmune diseases (such as systemic lupus erythematosus and autoimmune thyroid disease), infections (such as hepatitis virus and human immunodeficiency virus), drugs/toxins (such as penicillamine, cyclooxygenase-2 inhibitors, gold and mercury compounds) and malignancies. The diagnosis of IMN was mainly made on the kidney biopsy which featured capillary wall thickening, granular immunoglobulin G (IgG) and C3 along capillary walls on immunofluorescence, and subepithelial deposits on electron microscopy. The exclusion criteria of IMN were as follows: (1) having suspected medication history, (2) being positive for tumor markers or having undetermined imaging or gastrointestinal endoscopy evidence of the tumor, (3) antinuclear antibodies were no less than 1:320, (4) with dense deposits in the subendothelial or mesangial areas on electron microscopy. All possible primary etiologies had been excluded before the diagnosis of IMN was made. None of the patients had received immunosuppressive therapy before receiving kidney biopsy in the study.
The non-IMN group was composed of 106 patients with IgA nephropathy (IgAN), 32 patients with primary minimal change disease (MCD), 22 patients with primary focal and segmental glomerulosclerosis (FSGS), 35 patients with diabetic nephropathy (DN), 41 patients with lupus nephritis (LN) (including 25 patients with MN), 12 patients with hepatitis B virus-associated glomerulonephritis (HBV-GN) (including seven patients with MN) and other nephropathy patients. The other nephropathy patients in the non-IMN group included 19 patients with hypertensive renal damage, nine patients with interstitial nephritis, five patients with plasmacytoma-associated nephropathy, four patients with primary renal amyloidosis, four patients with purpura nephritis, two patients with membranoproliferative glomerulonephritis, one patient with endocapillary proliferative glomerulonephritis, one patient with C3 glomerulonephritis, two patients with acute tubular necrosis, and one patient with mild glomerular ischemia with chronic interstitial fibrosis.
The clinical data of all subjects came from the first sampling before immunosuppressive therapy. General information included age and sex. Laboratory parameters of blood analysis included white blood cell (WBC), hemoglobin, platelet, total protein, albumin, globulin, blood urea nitrogen (BUN), serum creatinine, estimated glomerular filtration rate (eGFR), serum cystatin C (CysC), cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, and anti-PLA2R antibodies. Values of eGFR were calculated by the Chronic Kidney Disease Epidemiology Collaboration creatinine equation [11]. The laboratory parameters of urinalysis included urinary red blood cell and 24-hour urinary protein. All patients received ultrasound-guided percutaneous renal puncture to obtain renal tissues. Experienced pathologists from Qilu Clinical Laboratory (Jinan) read tissue slices on the double-blind principle to offer a pathological diagnosis. If the final report showed two pathological stages, the higher stage was analyzed in this study.
In this study, an anti-PLA2R ELISA (IgG) kit (EUROIMMUN AG, L├╝beck, Germany) was used to detect serum anti-PLA2R IgG antibodies in subjects. The specific steps were as follows: (1) the anti-PLA2R IgG kit was placed for 30 minutes at room temperature; (2) the serum sample was diluted 1:101 with sample buffer; (3) 100 ╬╝L of diluted serum sample, prediluted controls and calibrators were added to the antigen-coated microtiter wells and incubated for 30 minutes at room temperature; (4) the microtiter wells were washed with wash buffer three times and then dried with absorbent paper; (5) 100 ╬╝L of anti-human IgG enzyme conjugate reagent was added to the microtiter wells and incubated for 30 minutes at room temperature; (6) the fourth step was repeated; (7) 100 ╬╝L of the chromogen substrate was added to the microtiter wells and then incubated in the dark for 15 minutes at room temperature; (8) 100 ╬╝L of stop solution was added to the microtiter wells; (9) the microtiter plates were placed in an automatic ELISA reader at 450 nm to obtain optical density values, and then quantitative values were calculated in relative units per milliliter (RU/mL). The EUROIMMUN manufacturer suggests reporting results lower than 14 RU/mL as negative, those between 14 RU/mL and 20 RU/mL as borderline and those no less than 20 RU/mL as positive. The assay range of this ELISA kit is 2 to 1,500 RU/mL. In this study, anti-PLA2R values lower than 20 RU/mL were defined as negative. According to the titer tertiles of positive anti-PLA2R data in the IMN group, anti-PLA2R values were considered low titer at < 78.5 RU/mL, middle titer between 78.5 RU/mL and 193.5 RU/mL and high titer at Ōēź 193.5 RU/mL.
SPSS Statistics version 24.0 (IBM Inc., Chicago, IL, USA) software was used to analyze all the data. Normally distributed data were described as the means ┬▒ SD and compared by independent t tests or one-way analysis of variance. Nonnormally distributed data were described as the median (interquartile range [IQR]) and compared by the Mann-Whitney test or Kruskal-Wallis test. Categorical variables were described as percentages, and Pearson chi-square tests were performed. Correlations between anti-PLA2R and other parameters were analyzed by the Spearman test. The sensitivity, specificity, positive predictive value (PPV), negative predictive value and Youden index of anti-PLA2R were all calculated at different cutoff values. The optimal cutoff value was determined by a receiver operating characteristic (ROC) curve. A two-sided p < 0.05 was considered statistically significant.
In this study, the clinical data of 670 patients were collected and analyzed retrospectively, including 374 (55.82%) patients in the IMN group and 296 (44.18%) patients in the non-IMN group. The average age (48 years vs. 40 years), 24-hour urinary protein (4.51 g/day vs. 2.25 g/day), eGFR (109 mL/min/1.73 m2 vs. 88 mL/min/1.73 m2), cholesterol (297.8 mg/dL vs. 223.1 mg/dL) and low density lipoprotein cholesterol (181.9 mg/dL vs. 140.6 mg/dL) in the IMN group were higher than those in the non-IMN group, while the levels of albumin (25.3 g/L vs. 36.0 g/L), BUN (13.2 mg/dL vs. 17.6 mg/dL), serum creatinine (0.71 mg/dL vs. 1.00 mg/dL) and CysC (0.87 mg/L vs 1.24 mg/L) were lower than those in the non-IMN group. The differences were statistically significant (p < 0.001). There were no statistically significant differences in the sex ratio or WBC between the two groups. Detailed information on comparison is presented in Table 1.
The median level of anti-PLA2R was 66.23 RU/mL in the IMN group, which was higher than that in the non-IMN group (< 2 RU/mL), and the difference was statistically significant (p < 0.001). In the non-IMN group, the anti-PLA2R levels of 76.7% of patients were lower than 2 RU/mL. As shown in Fig. 2, the non-IMN group was further divided into subgroups of IgAN, MCD, FSGS, DN, LN, HBV-GN and other nephropathies to explore the distribution of anti-PLA2R. And the level of anti-PLA2R in the IMN group was also significantly higher than those in all the seven subgroups (their medians were all < 2 RU/mL). The differences were statistically significant (p < 0.001). The overall positive rate of anti-PLA2R in the non-IMN group was 1.01%. The anti-PLA2R-positive proportions in FSGS, LN and HBV-GN were 4.55%, 2.44%, and 8.33%, respectively, compared with zero in IgAN, MCD, DN and other nephropathies.
There were 32 SMN patients in the non-IMN group, 25 secondary to lupus and seven secondary to hepatitis B. In SMN, the median level of anti-PLA2R was less than 2 RU/mL, and the positive rate of anti-PLA2R was only 6.25%. In Supplementary Table 1, we present detailed baseline parameters of the new non-IMN subgroups, where the SMN is presented as a separate analysis.
In this clinical study, 374 patients with IMN were divided into the anti-PLA2R negative group (n = 118, 31.6%) and anti-PLA2R-positive group (n = 256, 68.4%) according to serum anti-PLA2R levels. The median level of anti-PLA2R in the negative group was 4.00 RU/mL (IQR, 2.12 to 12.36), while that in the positive group was as high as 108.82 RU/mL (IQR, 58.40 to 259.97). Comparisons of clinical features between the two groups are listed in Table 2. The results showed that the male proportion (61.7% vs. 49.2%, p = 0.022), 24-hour urinary protein (5.31 g/day vs. 3.12 g/day, p < 0.001), serum creatinine (0.74 mg/dL vs. 0.68 mg/dL, p = 0.004), CysC (0.91 mg/dL vs. 0.82 mg/L, p < 0.001), cholesterol (311.5 mg/dL vs. 269.7 mg/dL, p < 0.001) and low density lipoprotein cholesterol (194.9 mg/dL vs. 163.2 mg/dL, p < 0.001) in the anti-PLA2R-positive group were significantly higher than those in the negative group. The levels of albumin (24.41 ┬▒ 5.45 g/L vs. 29.10 ┬▒ 6.70 g/L, p < 0.001) and eGFR (107 mL/min/1.73 m2 vs. 111 mL/min/1.73 m2, p = 0.024) in the positive group were lower than those in the negative group, and the differences were statistically significant. However, differences in other indexes, such as age, hemoglobin and BUN, had no statistical significance.
The anti-PLA2R-positive group was further divided into the low titer group (20 RU/mL Ōēż anti-PLA2R < 78.5 RU/mL), middle titer group (78.5 RU/mL Ōēż anti-PLA2R < 193.5 RU/mL) and high titer group (anti-PLA2R Ōēź 193.5 RU/mL) according to the titer tertiles. Table 2 shows that 24-hour urinary protein levels in both the low and middle titer groups were lower than those in the high titer group (3.86 and 4.79 g/day vs. 6.92 g/day, p < 0.001 and p = 0.001). However, only the level of albumin in the low titer group was higher than that in the high titer group (25.30 g/L vs. 23.60 g/L, p = 0.013), while no significant difference of it between the middle and high titer groups was found. In addition, there was no significant difference in sex, age, BUN, serum creatinine, eGFR, or CysC among the three titer groups.
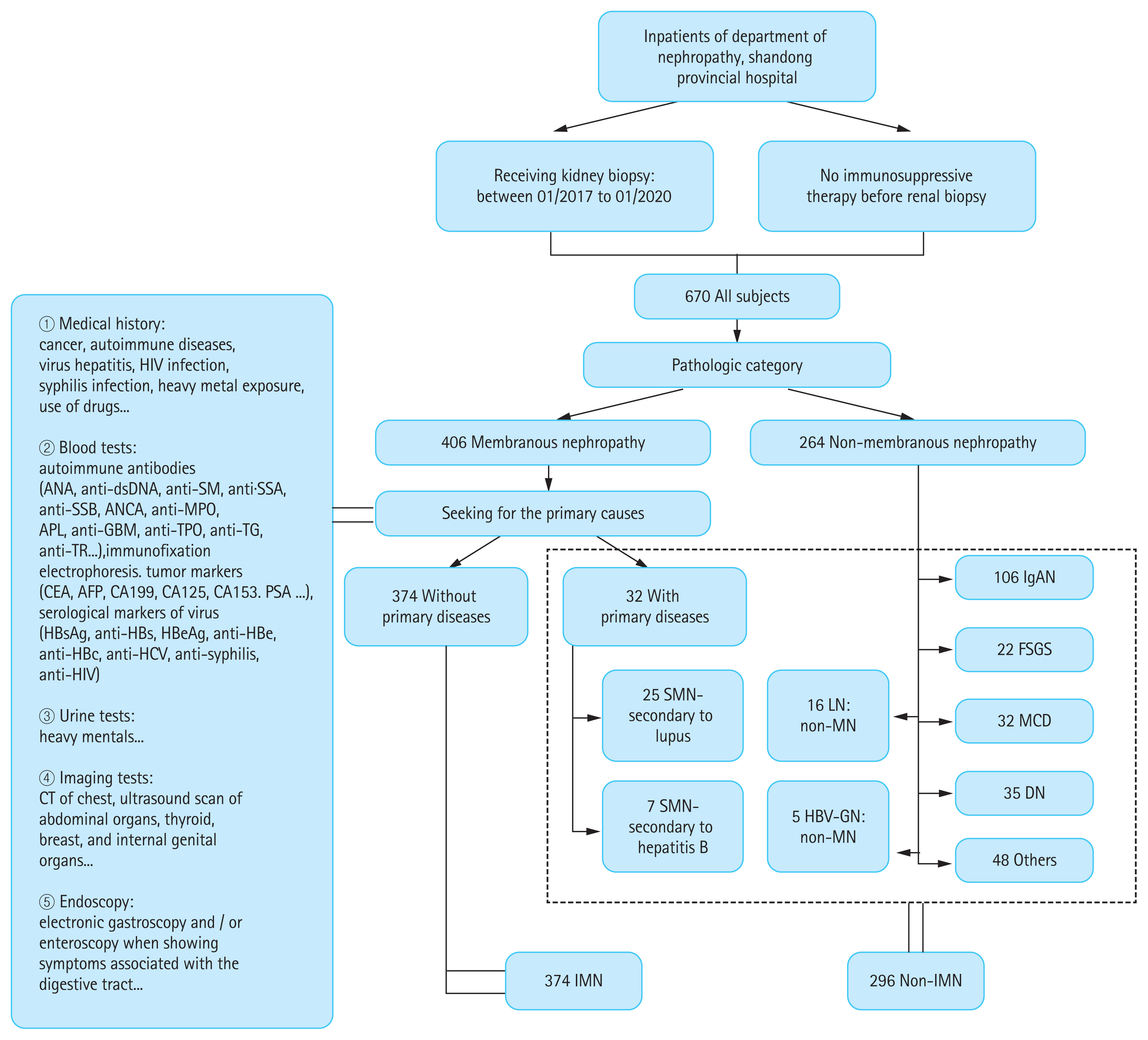
In the IMN group, we found correlations between anti-PLA2R and clinical parameters related to renal function (Fig. 3). There was a statistically significant positive correlation between anti-PLA2R and 24-hour urinary protein (r = 0.355, p < 0.001), serum creatinine (r = 0.161, p = 0.002), CysC (r = 0.228, p < 0.001) and cholesterol (r = 0.263, p < 0.001). In contrast, significant negative correlations existed between anti-PLA2R and albumin (r = ŌłÆ0.357, p < 0.001) and eGFR (r = ŌłÆ0.151, p = 0.004).
In this study, pathological stages of IMN were divided into three groups: stage I, stage II and stage III. There were 250 patients (66.84%) in stage I with an anti-PLA2R level of 61.77 RU/mL (IQR, 10.01 to 161.54) and 123 patients (32.89%) in stage II with an anti-PLA2R level of 66.95 RU/mL (IQR, 19.63 to 227.88). Only one patient whose anti-PLA2R level was 898.55 RU/mL was in stage III. Patients with higher pathological stages seemed to have higher anti-PLA2R levels, but there was no statistically significant correlation between anti-PLA2R levels and pathological stages in the IMN group or in either of its two subgroups (anti-PLA2R-positive and anti-PLA2R-negative) (Table 3).
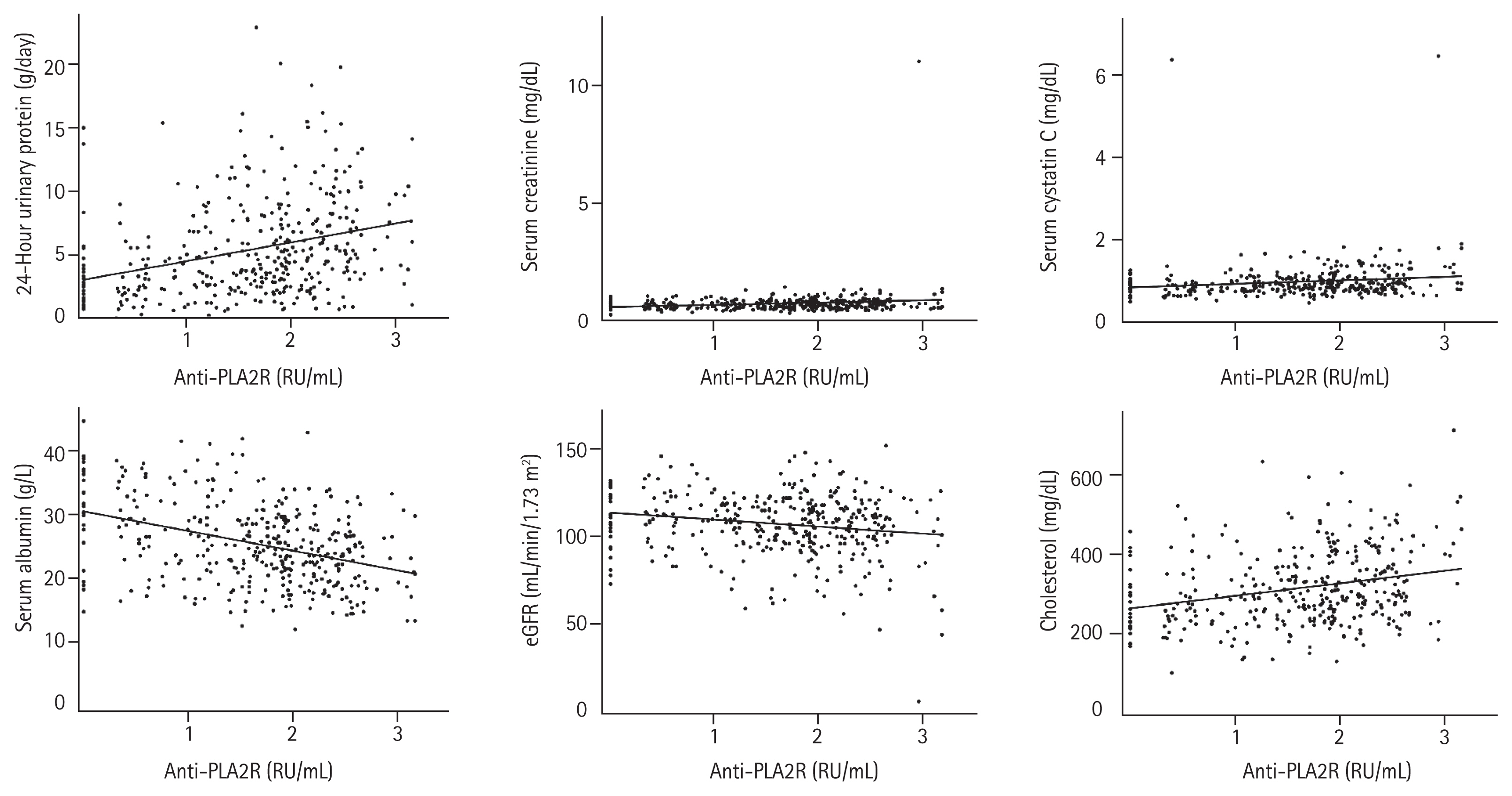
To distinguish IMN from non-IMN, the ROC curve method was chosen to validate an optimal cutoff value of anti-PLA2R. After analyzing this curve (Fig. 4), we found that 7.45 RU/mL was the cutoff with the highest Youden index.
Currently, 20 RU/mL is commonly used as the positive threshold of anti-PLA2R in clinical practice. At this value, the specificity reached 98.99%, while the sensitivity was only 68.45% in our study. Tables 4 and 5 show the diagnostic efficiency of 7.45 RU/mL and that of different cutoff values published in previous studies. When the cutoff values were 2, 2.6, 7.45, and 14 RU/mL, the sensitivities were 92.51%, 89.04%, 80.75%, and 74.60%, and the specificities were 76.69%, 85.81%, 97.97%, and 98.99%, respectively. The Youden indexes at these five values (2, 2.6, 7.45, 14, 20 RU/mL) were 0.69, 0.75, 0.79, 0.74, 0.67, respectively. The area under the ROC curve was 0.935 (95% confidence interval, 0.915 to 0.956; p < 0.001).
A disease spectrum analysis of inpatients showed that the proportion of IMN in primary glomerulonephritis (PGN) had been increasing and that IMN had become the main pathological type of PGN in northern China [12]. This nephropathy leads to proteinuria, edema, hypoalbuminemia and hyperlipidemia and brings risks of complications such as thromboembolic disease or coronary artery disease [13,14]. Thus, it is vital to realize early diagnosis and early treatment of IMN. The discovery of PLA2R was a milestone for this purpose and helped achieve noninvasive diagnosis. However, most studies included small sample sizes, and the positive threshold of anti-PLA2R by ELISA was not unified. Therefore, we analyzed the clinical characteristics of IMN through a large-sample study and aimed to set a new cutoff for the diagnosis of IMN.
IMN patients in our study demonstrated specific clinical features. For example, the male-to-female ratio in IMN was 1.37:1, which was consistent with the analysis of the Chinese national database for inpatients [12]. However, this ratio was lower than that from the Netherlands (1.72:1), which could be caused by ethnic differences [15]. In our study, serum creatinine and 24-hour urinary protein were higher, while albumin and eGFR were lower, in the anti-PLA2R-positive group than in the anti-PLA2R-negative group. However, there was no significant difference in these four indexes in the studies of Provatopoulou et al. [16] and Hofstra et al. [17]. In both Chinese studies, positive groups had higher levels of 24-hour urinary protein, while there was no difference in serum creatinine and albumin [18,19]. Kim et al. [20] found that albumin was lower in the positive group and that no significant difference existed in serum creatinine. For these differences, the effect of ethnic background cannot be ignored. Different stages and statuses of disease when subjects were enrolled also account for the discrepancies.
In the next part of this study, we found that anti-PLA2R correlated with serum creatinine, 24-hour urinary protein, albumin, eGFR, and CysC. However, correlations between anti-PLA2R and serum creatinine, eGFR and CysC were weak. Furthermore, there was no significant difference in serum creatinine, eGFR and CysC among the three titer subgroups, which also supported this result. Other related studies were not entirely consistent with our results. For example, only 24-hour urinary protein positively correlated with anti-PLA2R, while albumin, serum creatinine and CysC did not correlate in the study by Li et al. [19]. In two Japanese studies, anti-PLA2R negatively correlated with albumin, but its correlations with serum creatinine and 24-hour urinary protein differed between the two studies [21,22]. The study by Hofstra et al. [17] was consistent with our research on these correlations. However, this supportive consistency has not yet proven a definite causal relationship between anti-PLA2R and kidney damage. We can infer that a higher titer of anti-PLA2R leads to more subepithelial deposition of the immune complex, which results in more serious tissue damage, especially the destruction of the filtration barrier [3]. The differences in correlations indicate that complex links exist between anti-PLA2R and IMN, which are possibly affected by genetic or environmental backgrounds.
Since Beck et al. [7] discovered that PLA2R was the marker antigen of IMN, ELISA detection of autoantibodies to this target has been gradually promoted for commercial purposes so that more clinical laboratories can carry out objective, efficient and quantitative detection of anti-PLA2R. As reviewed in Table 5 [15,16,18ŌĆō26], associated studies usually used 2, 14, and 20 RU/mL recommended by the manufacturer as the cutoff values. In these studies, the diagnostic efficiency varied with cutoff values in the same experiment [15,16,23]. The sensitivity and specificity of the same cutoff value were also different between countries and regions. When 20 RU/mL was used as the boundary, the sensitivity in the Netherlands was relatively high at 63.30% [15]. Although Greece also belongs to Europe, the sensitivity in a Greek population was only 48.48% [16]. In two studies of the Japanese population, the sensitivities were similar, 50.00% and 52.17%, and the specificities were both 100.00% [21,22]. China held four studies, with sensitivities of 62.20%, 60.17%, 50.88%, and 67.26%, and the specificities were all more than 90% [19,23ŌĆō25]. Another study pointed out that this measurement was more sensitive for Caucasians than for Asians [27]. In China, Liu et al. [24] also conducted experiments with EuroimmunŌĆÖs ELISA kit and they recommended 2.6 RU/mL as the cutoff value of anti-PLA2R. Compared with 2.6 RU/mL and other reported cutoff values, 7.45 RU/mL was preferred in our large-sample study; it had higher diagnostic efficiency and its PPV reached 98.05%. Our study included more IMN and non-IMN nephropathy subjects and excluded data from healthy people when analyzing the ROC curve. Therefore, 7.45 RU/mL was more applicable in the differential diagnosis in clinical practice.
In our study, positive rates and levels of anti-PLA2R were significantly higher in IMN patients than in non-IMN patients, which indicates that it is a specific biomarker in the differential diagnosis of IMN. However, 31.6% of IMN patients still tested negative for anti-PLA2R. Four possible reasons are illustrated as follows. First, these IMN patients may develop lower levels of anti-PLA2R because they had entered spontaneous immune remission or possessed particular genes such as HLA alleles DQA1*05:01 and DQB1*02:01 [8]. All anti-PLA2R might have deposited outside the circulation or leaked into urine. Second, anti-PLA2R levels could be sufficient to trigger IMN but not to be reported as positive due to the higher cutoff threshold of the detection. This is partly why we aim to find a new threshold of anti-PLA2R. Third, some SMN patients might be misdiagnosed with IMN because the underlying causes were not found with current diagnostic techniques. Last, different autoantigens could be involved in the pathogenesis, such as thrombospondin type 1 domain containing 7A, aldose reductase, superoxide dismutase-2 and ╬▒-enolase. Autoantibodies against these targets have been found in IMN patients, but whether they play a direct pathogenic role remains to be confirmed [28ŌĆō31].
This study suffers from several limitations. Firstly, due to the limited observation time of the study, there could have existed unidentified misclassifications. For example, we have not found cancer patients in IMN through the thorough examination at the time of enrollment and the telephone follow-up, but some cancers are difficult to be caught in their early stages by current technology which means part of MN without primary causes could actually be secondary to the cancers. Secondly, this study lacks prognosis and survival data to assess the predictive value of anti-PLA2R. The follow-up investigation of anti-PLA2R in those who accepted medical treatments may provide more reference value for this study. Thirdly, because patients receiving immunosuppressive therapy were excluded, anti-PLAR level of them at onset could not be analysed and selection bias could not be neglected in this retrospective study. Lastly, more multicenter prospective studies with larger samples are needed to further verify these correlations and to obtain a cutoff value with higher validity.
In conclusion, the detection of anti-PLA2R is highly specific for the diagnosis of IMN. 7.45 RU/mL is recommended as the optimal cutoff value of anti-PLA2R for the diagnosis of IMN, which can improve the diagnostic efficiency and facilitate early diagnosis of IMN. Baseline parameters of renal function are associated with anti-PLA2R levels in IMN patients.
1. Anti-phospholipase A2 receptor (PLA2R) is specific for idiopathic membranous nephropathy.
2. A 7.45 RU/mL is the optimal cutoff value of anti-PLA2R by enzyme-linked immunosorbent assay for idiopathic membranous nephropathy patients.
3. Baseline renal function is associated with the serum level of anti-PLA2R.
Conflict of Interest
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
This work was supported by the Primary Research & Development Plan of Shandong Province (2018GSF118227) and the Science and Technology Plan of Shizhong District of Jinan City (673 and 741).
Figure┬Ā1
The process of patients screening and classification. IMN, idiopathic membranous nephropathy; SMN, secondary membranous nephropathy; IgAN, IgA nephropathy; FSGS, focal and segmental glomerulosclerosis; MCD, minimal change disease; DN, diabetic nephropathy; LN, lupus nephritis; HBV-GN, HBV-associated glomerulonephritis; ANA, antinuclear antibodies; SSA, Sj├ČgrenŌĆÖs syndrome-A; SSB, Sj├ČgrenŌĆÖs syndrome-B; ANCA, antineutrophil cytoplasmic antibodies; MPO, myeloperoxidase; APL, antiphospholipid antibodies; GBM, glomerular basement membrane; TPO, thyroid peroxidase; TG, thyroglobulin; TR, thyrotropin receptor; CEA, carcinoembryonic antigen; AFP, alpha-fetoprotein; CA, carbohydrate antigen; PSA, prostate specific antigen; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; anti-HBc, anti-hepatitis B core antibodies; HCV, hepatitis virus C; HIV, human immunodeficiency virus; SM, Smith; CT, computed tomography.
Figure┬Ā2
The distribution of anti-phospholipase A2 receptor antibody (anti-PLA2R) levels (log-transformed) in different nephropathies. In the idiopathic membranous nephropathy (IMN), the upper and lower short line show the 75th and 25th percentiles respectively and the middle long line shows the median. In the minimal change disease (MCD), diabetic nephropathy (DN), and lupus nephritis (LN), the upper short lines show 75 percentiles and the median (long) lines and 25 percentiles (short) lines coincide with each other. In the IgA nephropathy (IgAN), focal and segmental glomerulosclerosis (FSGS), hepatitis B virus-associated glomerulonephritis (HBV-GN), and Others, the three lines showing the 25th, 50th and 75th percentiles are all at the same level. The subgroups of Other include patients with hypertensive renal damage, interstitial nephritis, plasmacytoma-associated nephropathy, primary renal amyloidosis, purpura nephritis, membranoproliferative glomerulonephritis, endocapillary proliferative glomerulonephritis, C3 glomerulonephritis, acute tubular necrosis and mild glomerular ischemia with chronic interstitial fibrosis.

Figure┬Ā3
Correlations between the levels of anti-phospholipase A2 receptor antibody (anti-PLA2R; log-transformed) and clinical parameters in the idiopathic membranous nephropathy. eGFR, estimated glomerular filtration rate.
Figure┬Ā4
Receiver operating characteristic curve of anti-phospholipase A2 receptor antibody (anti-PLA2R) in the diagnosis of idiopathic membranous nephropathy. AUC, area under receiver operating characteristic curve; CI, confidence interval.
Table┬Ā1
General baseline parameters of the IMN group and non-IMN group
| General parameter | IMN (n = 374) | Non-IMN (n = 296) | p value |
|---|---|---|---|
| Male sex | 216 (57.8) | 167 (56.4) | 0.729 |
| Age, yr | 48 (36ŌĆō55)b | 40 (31ŌĆō51) | < 0.001 |
| WBC, 109/L | 6.00 (5.03ŌĆō7.30) | 6.20 (5.09ŌĆō7.57) | 0.546 |
| Hemoglobin, g/L | 140 (126ŌĆō151)a | 133 (116ŌĆō152) | 0.005 |
| Platelet, 109/L | 265 (226ŌĆō305)b | 247 (206ŌĆō297) | < 0.001 |
| Total protein, g/L | 49.2 (43.1ŌĆō56.0)b | 64.1 (51.7ŌĆō70.8) | < 0.001 |
| Albumin, g/L | 25.3 (21.4ŌĆō29.8)b | 36.0 (24.7ŌĆō41.1) | < 0.001 |
| Globulin, g/L | 23.87 ┬▒ 4.29b | 28.19 ┬▒ 4.66 | < 0.001 |
| BUN, mg/dL | 13.2 (10.6ŌĆō16.5)b | 17.6 (12.9ŌĆō23.7) | < 0.001 |
| Serum creatinine, mg/dL | 0.71 (0.59ŌĆō0.84)b | 1.00 (0.74ŌĆō1.44) | < 0.001 |
| eGFR, ml/min/1.73 m2 | 109 (97ŌĆō119)b | 88 (54ŌĆō109) | < 0.001 |
| CysC, mg/L | 0.87 (0.76ŌĆō1.03)b | 1.24 (0.92ŌĆō1.75) | < 0.001 |
| Cholesterol, mg/dL | 297.8 (246.6ŌĆō370.8)b | 223.1 (182.5ŌĆō300.5) | < 0.001 |
| HDL-C, mg/dL | 56.5 (47.2ŌĆō69.1)b | 48.3 (39.4ŌĆō58.4) | < 0.001 |
| LDL-C, mg/dL | 181.9 (146.7ŌĆō241.2)b | 140.6 (111.9ŌĆō193.0) | < 0.001 |
| Anti-PLA2R, RU/mL | 66.23 (13.02ŌĆō173.23)b | < 2c | < 0.001 |
| U-RBC, HPF | 6.7 (2.9ŌĆō15.5)b | 4.4 (1.5ŌĆō17.8) | < 0.001 |
| Urinary protein, g/day | 4.51 (2.54ŌĆō7.55)b | 2.25 (1.07ŌĆō4.80) | < 0.001 |
IMN, idiopathic membranous nephropathy; WBC, white blood cell; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; CysC, serum cystatin C; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; PLA2R, phospholipase A2 receptor; U-RBC, urinary red blood cell; HPF, high power field.
Table┬Ā2
Baseline characteristics of anti-PLA2R-positive patients and anti-PLA2R-negative patients
| Characteristic | Negative (n = 118) | Positive | |||
|---|---|---|---|---|---|
| Overall (n = 256) | Low titer (n = 85) | Middle titer (n = 85) | High titer (n = 86) | ||
| Male sex | 58 (49.2)a | 158 (61.7) | 54 (63.5) | 57 (67.1) | 47 (54.7) |
| Age, yr | 46 (36ŌĆō54) | 49 (36ŌĆō55) | 50 (36ŌĆō58) | 49 (40ŌĆō55) | 48 (36ŌĆō56) |
| Anti-PLA2R, RU/mL | 4.00 (2.12ŌĆō12.36)c | 108.82 (58.40ŌĆō259.97) | 44.97 (33.16ŌĆō58.52) | 107.91 (87.00ŌĆō153.23) | 343.44 (255.52ŌĆō452.86) |
| Hemoglobin, g/L | 140.53 ┬▒ 16.89 | 137.91 ┬▒ 18.95 | 137.00 (122.50ŌĆō149.00) | 139.50 (127.00ŌĆō151.75) | 140.00 (125.50ŌĆō153.00) |
| Albumin, g/L | 29.10 ┬▒ 6.70c | 24.41 ┬▒ 5.45 | 25.30 (22.15ŌĆō29.25)a | 23.40 (20.90ŌĆō28.15) | 23.60 (19.15ŌĆō26.90) |
| BUN, mg/dL | 13.2 (10.9ŌĆō15.7) | 13.2 (10.6ŌĆō17.1) | 13.2 (10.6ŌĆō16.4) | 13.7 (10.8,17.5) | 12.9 (10.5ŌĆō16.7) |
| Serum creatinine, mg/dL | 0.68 (0.57ŌĆō0.79)b | 0.74 (0.61ŌĆō0.86) | 0.72 (0.59ŌĆō0.83) | 0.75 (0.61ŌĆō0.89) | 0.75 (0.62ŌĆō0.91) |
| eGFR, mL/min/1.73 m2 | 111 (102ŌĆō122)a | 107 (96ŌĆō117) | 107 (98ŌĆō121) | 109 (96ŌĆō117) | 106 (95ŌĆō114) |
| CysC, mg/L | 0.82 (0.71ŌĆō0.96)c | 0.91 (0.77ŌĆō1.08) | 0.88 (0.76ŌĆō1.02) | 0.91 (0.77ŌĆō1.12) | 0.92 (0.78ŌĆō1.21) |
| Cholesterol, mg/dL | 269.7 (224.3ŌĆō328.4)c | 311.5 (261.9ŌĆō397.3) | 297.8 (255.2ŌĆō346.1)b | 300.9 (258.1ŌĆō397.7) | 350.3 (277.7ŌĆō425.4) |
| LDL-C, mg/dL | 163.2 (130.3ŌĆō202.2)c | 194.9 (153.9ŌĆō249.0) | 179.8 (150.3ŌĆō223.6)b | 196.4 (148.7ŌĆō242.3) | 211.3 (167.5ŌĆō295.2) |
| Urinary protein, g/day | 3.12 (1.86ŌĆō5.28)c | 5.31 (3.17ŌĆō8.28) | 3.86 (2.85ŌĆō7.97)c | 4.79 (2.72ŌĆō7.22)b | 6.92 (4.43ŌĆō9.98) |
Values are presented as number (%), median (interquartile range), or mean ┬▒ SD. Low titer, 20 Ōēż anti-PLA2R < 78.5 RU/mL; Middle titer, 78.5 Ōēż anti-PLA2R < 193.5 RU/mL; High titer, anti-PLA2R Ōēź 193.5 RU/mL.
PLA2R, phospholipase A2 receptor; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; CysC, serum cystatin C; LDL-C, low density lipoprotein cholesterol.
Table┬Ā3
Anti-PLA2R values of IMN patients in different histologic stages
| Stage | Overall (n = 374) | Anti-PLA2R positive (n = 256) | Anti-PLA2R negative (n = 118) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| No. (%) | Anti-PLA2R, RU/mL | No. (%) | Anti-PLA2R, RU/mL | No. (%) | Anti-PLA2R, RU/mL | |
| I | 250 (66.84) | 61.77 (10.01ŌĆō161.54) | 163 (63.67) | 106.16 (69.11ŌĆō228.54) | 87 (73.73) | 3.75 (< 2aŌĆō11.80) |
|
|
||||||
| II | 123 (32.89) | 66.95 (19.63ŌĆō227.88) | 92 (35.94) | 109.84 (51.98ŌĆō276.54) | 31(26.27) | 8.17 (2.29ŌĆō14.20) |
|
|
||||||
| III | 1 (0.27) | 898.55 | 1 (0.39) | 898.55 | 0 | - |
|
|
||||||
| p value | 0.067 | 0.288 | 0.229 | |||
Table┬Ā4
The efficiency of anti-PLA2R for diagnosing IMN at different cutoff values
Table┬Ā5
The diagnostic efficiency of anti-PLA2R detected by the ELISA kit (EUROIMMUN, Germany)
| Country | Time range | Cutoff, RU/mL | Sensitivity, % | Specificity, % | PPV, % | NPV, % | Youden index | AUC | References |
|---|---|---|---|---|---|---|---|---|---|
| Netherlands | 11/1979ŌĆō03/2011 | 2 | 71.56 (78/109) | 96.97 (32/33) | 98.73 (78/79) | 50.79 (32/63) | 0.69 | - | Timmermans et al. [15] |
| 20 | 63.30 (69/109) | 96.97 (32/33) | 98.57 (69/70) | 44.44 (32/72) | 0.60 | ||||
| Greece | - | 2 | 57.58 (19/33) | - | - | - | - | - | Provatopoulou et al. [16] |
| - | 14 | 48.48 (16/33) | - | - | - | - | - | ||
| - | 20 | 48.48 (16/33) | - | - | - | - | - | ||
| India | 07/2010ŌĆō02/2015 | 20 | 66.67 (76/114) | - | - | - | - | - | Ramachandran et al. [26] |
| Korea | 01/2007ŌĆō04/2014 | 14 | 44.09 (41/93) | 100.00 (67/67) | 100.00 (41/41) | 56.30 (67/119) | 0.44 | - | Kim et al. [20] |
| Japan | 06/1993ŌĆō12/2014 | 20 | 50.00 (19/38) | 100.00 (21/21) | 100.00 (19/19) | 52.50 (21/40) | 0.50 | - | Hihara et al. [22] |
| Japan | 2001ŌĆō2014 | 20 | 52.17 (12/23) | 100.00 (32/32) | 100.00 (12/12) | 74.42 (32/43) | 0.52 | - | Katsumata et al. [21] |
| China | - | 15 | 71.67 (86/120) | - | - | - | - | - | Xun et al. [18] |
| China | 01/2011ŌĆō12/2013 | 20 | 62.20 (51/82) | 91.46 (75/82) | 87.93 (51/58) | 70.75 (75/106) | 0.54 | - | Li et al. [19] |
| China | 08/2014ŌĆō12/2014 | 14 | 65.25 (77/118) | 97.30 (108/111) | 96.25 (77/80) | 72.48 (108/149) | 0.63 | 0.870a | Dou et al. [23] |
| 20 | 60.17 (71/118) | 97.30 (108/111) | 95.95 (71/74) | 69.68 (108/155) | 0.58 | ||||
| 40 | 45.76 (54/118) | 97.30 (108/111) | 94.77 (54/57) | 62.79 (108/172) | 0.43 | ||||
| China | 09/2011ŌĆō03/2016 | 2 | 82.46 (47/57) | 75.00 (63/84) | 69.12 (47/68) | 86.30 (63/73) | 0.59 | 0.879a | Liu et al. [24] |
| 2.6 | 78.95 (45/57) | 91.67 (77/84) | 86.54 (45/52) | 86.52 (77/89) | 0.71 | ||||
| 14 | 59.65 (34/57) | 95.24 (80/84) | 89.47 (34/38) | 77.67 (80/103) | 0.55 | ||||
| 20 | 50.88 (29/57) | 96.43 (81/84) | 90.63 (29/32) | 74.31 (81/109) | 0.46 | ||||
| 40 | 47.37 (27/57) | 97.62 (82/84) | 93.10 (27/29) | 73.21 (82/112) | 0.43 | ||||
| China | 04/2016ŌĆō06/2017 | 20 | 67.26 (76/113) | 98.85 (86/87) | 98.70 (76/77) | 69.92 (86/123) | 0.66 | 0.90b | Li et al. [25] |
REFERENCES
1. Ponticelli C, Glassock RJ. Glomerular diseases: membranous nephropathy: a modern view. Clin J Am Soc Nephrol 2014;9:609ŌĆō616.


3. Kerjaschki D. Pathomechanisms and molecular basis of membranous glomerulopathy. Lancet 2004;364:1194ŌĆō1196.


4. Obrisca B, Ismail G, Jurubita R, Baston C, Andronesi A, Mircescu G. Antiphospholipase A2 receptor autoantibodies: a step forward in the management of primary membranous nephropathy. Biomed Res Int 2015;2015:249740.



5. Whittier WL. Complications of the percutaneous kidney biopsy. Adv Chronic Kidney Dis 2012;19:179ŌĆō187.


7. Beck LH Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009;361:11ŌĆō21.



8. Kanigicherla D, Gummadova J, McKenzie EA, et al. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int 2013;83:940ŌĆō948.


9. Behnert A, Schiffer M, Muller-Deile J, Beck LH Jr, Mahler M, Fritzler MJ. Antiphospholipase A2 receptor autoantibodies: a comparison of three different immunoassays for the diagnosis of idiopathic membranous nephropathy. J Immunol Res 2014;2014:143274.




10. Luciano RL, Moeckel GW. Update on the native kidney biopsy: core curriculum 2019. Am J Kidney Dis 2019;73:404ŌĆō415.


11. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604ŌĆō612.



12. Li J, Cui Z, Long J, et al. Primary glomerular nephropathy among hospitalized patients in a national database in China. Nephrol Dial Transplant 2018;33:2173ŌĆō2181.


13. Glassock RJ. Diagnosis and natural course of membranous nephropathy. Semin Nephrol 2003;23:324ŌĆō332.


14. Lai WL, Yeh TH, Chen PM, et al. Membranous nephropathy: a review on the pathogenesis, diagnosis, and treatment. J Formos Med Assoc 2015;114:102ŌĆō111.


15. Timmermans SA, Damoiseaux JG, Heerings-Rewinkel PT, et al. Evaluation of anti-PLA2R1 as measured by a novel ELISA in patients with idiopathic membranous nephropathy: a cohort study. Am J Clin Pathol 2014;142:29ŌĆō34.


16. Provatopoulou S, Kalavrizioti D, Stangou M, et al. Circulating anti-phospholipase A2 receptor antibodies as a diagnostic and prognostic marker in Greek patients with idiopathic membranous nephropathy: a retrospective cohort study. Rom J Intern Med 2019;57:141ŌĆō150.


17. Hofstra JM, Debiec H, Short CD, et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol 2012;23:1735ŌĆō1743.



18. Xun C, Shuai L, Wang W, Jiang Y. Comparison of biomarkers between PLA2RAb+ and PLA2RAb- in patients with idiopathic membranous nephropathy. Int Urol Nephrol 2015;47:831ŌĆō835.


19. Li X, Wei D, Zhou Z, et al. Anti-PLA2R antibodies in Chinese patients with membranous nephropathy. Med Sci Monit 2016;22:1630ŌĆō1636.



20. Kim YG, Choi YW, Kim SY, et al. Anti-phospholipase A2 receptor antibody as prognostic indicator in idiopathic membranous nephropathy. Am J Nephrol 2015;42:250ŌĆō257.


21. Katsumata Y, Okamoto Y, Moriyama T, et al. Clinical usefulness of anti-M-type phospholipase-A-receptor antibodies in patients with membranous nephropathy and the comparison of three quantification methods. Immunol Med 2020;43:47ŌĆō56.


22. Hihara K, Iyoda M, Tachibana S, et al. Anti-phospholipase A2 receptor (PLA2R) antibody and glomerular PLA2R expression in Japanese patients with membranous nephropathy. PLoS One 2016;11:e0158154.



23. Dou Y, Zhang L, Liu D, et al. The accuracy of the anti-phospholipase A2 receptor antibody in the diagnosis of idiopathic membranous nephropathy: a comparison of different cutoff values as measured by the ELISA method. Int Urol Nephrol 2016;48:845ŌĆō849.


24. Liu Y, Li X, Ma C, et al. Serum anti-PLA2R antibody as a diagnostic biomarker of idiopathic membranous nephropathy: the optimal cut-off value for Chinese patients. Clin Chim Acta 2018;476:9ŌĆō14.


25. Li W, Guo Y, Zhang Z, et al. Comparison of 2 anti-PLA2R immunoassays for the diagnosis of primary membranous nephropathy. Lab Med 2018;49:316ŌĆō322.


26. Ramachandran R, Kumar V, Kumar A, et al. PLA2R antibodies, glomerular PLA2R deposits and variations in PLA2R1 and HLA-DQA1 genes in primary membranous nephropathy in South Asians. Nephrol Dial Transplant 2016;31:1486ŌĆō1493.


27. Du Y, Li J, He F, et al. The diagnosis accuracy of PLA2R-AB in the diagnosis of idiopathic membranous nephropathy: a meta-analysis. PLoS One 2014;9:e104936.



28. Tomas NM, Beck LH Jr, Meyer-Schwesinger C, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 2014;371:2277ŌĆō2287.



29. Tomas NM, Hoxha E, Reinicke AT, et al. Autoantibodies against thrombospondin type 1 domain-containing 7A induce membranous nephropathy. J Clin Invest 2016;126:2519ŌĆō2532.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print



