 |
 |
| Korean J Intern Med > Volume 35(5); 2020 > Article |
|
Abstract
Neuropathy is the most prevalent microvascular complication of diabetes mellitus; it encompasses distal symmetric polyneuropathy, autonomic neuropathy, radiculoplexus neuropathy, mononeuropathy, and treatment-induced neuropathy. The prevalence rate of diabetic neuropathy in Korea was reported to be approximately 43%, which is similar to rates in other countries. However, the precise pathogenic mechanism underlying diabetic neuropathy is still obscure, and many clinical trials have failed to develop methods to prevent or reduce the progression of diabetic neuropathy. Nevertheless, early diagnosis and proper management of diabetic neuropathy are essential to alleviate disabling symptoms and to improve the quality of life of patients. This review discusses clinical manifestations and classification of diabetic neuropathies, bedside neurological examination, and electrophysiological tests.
Diabetic neuropathy is a common complication of diabetes and is also the most common type of acquired neuropathy. As diabetic neuropathy is not a single entity, the term “diabetic neuropathies” seems to be a more appropriate term, encompassing a wide spectrum of clinical manifestations and levels of neurological involvement. The prevalence rate of diabetic neuropathy has been reported to range from 8% to 63% in type 1 diabetes and from 13% to 51% in type 2 diabetes [1]. A recent multicenter study reported a prevalence rate of diabetic neuropathy in Korea of approximately 43% [2]. As diabetes has a prevalence of 13% in the adult population of Korea and one third of older people over 65 years of age are diabetic, the impact of diabetic neuropathy on the management of diabetic complications is significant. The precise pathogenic mechanism underlying diabetic neuropathy is still obscure, and mechanism-based treatments have failed. Nevertheless, early diagnosis and proper management of diabetic neuropathy are essential to alleviate disabling symptoms and to improve the quality of life of patients.
Diabetic neuropathies encompass a wide spectrum of neuropathies with different clinical manifestations and underlying pathophysiological mechanisms. Table 1 shows the most commonly used classification of diabetic neuropathies [3].
Distal sensorimotor polyneuropathy (DSPN) is the most common phenotype among the various types of diabetic neuropathy. Most research conducted to date has been on this “length-dependent” pattern of diabetic neuropathy. DSPN usually begins 5 to 10 years after diagnosis of diabetes, beginning insidiously at the distal part of the legs symmetrically and gradually progressing to the more proximal parts showing a “glove-and-stocking” pattern. When large-diameter sensory fibers are involved, the patient complains of tingling, pins and needles (positive symptoms), as well as numbness and heaviness (negative symptoms). Loss of small sensory fibers (Aδ and C-fibers) induces burning or lancinating pain with various symptoms or signs of autonomic dysfunction. This small fiber involvement causes painful diabetic neuropathy, which is seen in 25% of patients with DSPN [4].
Some authors differentiate between diabetic small fiber neuropathy and DSPN. However, the existence of pure small fiber neuropathy is still controversial. The term “small fiber neuropathy” usually refers to the predominant involvement of small fibers and very little large sensory and motor fiber damage. The results of nerve conduction study (NCS) are usually normal in small fiber neuropathy. Other diagnostic tests, such as autonomic function tests (AFTs), quantitative sensory tests (QST), and sudomotor tests, are necessary in such cases.
Motor involvement is usually subclinical and becomes clear late in the clinical course. Sensory symptoms beginning in the upper extremities and prominent muscle weakness raise red flags and physicians must exclude other causes of neuropathy. For example, paresthesia and burning pain beginning in the hands with gait disturbance suggest vitamin B12 deficiency caused by pernicious anemia, alcohol abuse, and occasionally metformin intake.
Autonomic nerve fibers are involved in all forms of the clinical spectrum of diabetic neuropathies. Autonomic neuropathy was reported to have prevalence rates of 16.8% in patients with type 1 diabetes and 34.3% in those with type 2 diabetes in a study using strict diagnostic criteria based on AFT [5]. However, it is considered to be more common than previously reported.
Patients often ignore or underreport their symptoms until physicians inquire about autonomic symptoms. It is reasonable to suspect autonomic neuropathy in patients with painful somatic neuropathy, because autonomic nerve fibers are thinly myelinated or unmyelinated fibers that are damaged in somatic small fiber neuropathy. Resting tachycardia may be the first symptom or sign of parasympathetic impairment, followed by dyspnea or chest pain on exercise, silent myocardial ischemia, and orthostatic dizziness. Orthostatic hypotension significantly increases the risk of falls and syncope. Therefore, it is recommended to check for the presence of cardiac autonomic neuropathy before prescribing exercise to diabetic patients [6]. Cardiac autonomic neuropathy is an independent risk factor for sudden death from cardiovascular events [7]. A recent meta-analysis showed that almost all types of supervised regular exercise training improve cardiac autonomic function in patients with type 2 diabetes [8]. The importance of annual cardiac autonomic function check-ups and proper management, including regular exercise, cannot be overemphasized.
Genitourinary dysfunction is also an important cause of poor quality of life. It is estimated that about half of all diabetic patients suffer from urinary dysfunction. Initially, the ability to sense bladder filling decreases due to damage to sensory nerve fibers innervating the bladder wall. Then, the residual amount of urine increases, resulting in overflow incontinence. Erectile dysfunction is common in men, with reported rates ranging from 35% to 90% of patients, and often appears as the first symptom of autonomic neuropathy [9]. Impotence caused by dysfunction of vascular endothelial cells requires clinical attention because it is related to the risk of cardiovascular complications.
Blurred vision in bright light, sweating abnormalities, dysphagia, constipation, and diarrhea are additional frustrating symptoms of autonomic neuropathy. Decreased sympathetic innervation to the liver and adrenal glands can be dangerous because counterregulatory responses, such as palpitation and sweating, may be absent and, thus, prevent the patient from recognizing hypoglycemia (hypoglycemic unawareness) [10].
Patients with diabetes are vulnerable to mononeuropathy, including involvement of the median, radial, or ulnar nerves. Interestingly, carpal tunnel syndrome in diabetic patients is often asymptomatic and only found in NCS [11]. Cranial nerves involving ocular motor (cranial nerve III, IV, and VI) and facial nerves are also affected, although this is rare. In diabetic oculomotor neuropathy, pupillary light reflex is preserved because superficially located pupil-constricting parasympathetic fibers can avoid ischemic insult.
Diabetic radiculoplexus neuropathy is an uncommon entity, previously called diabetic amyotrophy, which has characteristic manifestations of unilateral proximal muscle wasting and weakness following sudden onset of pain in the thigh. The brachial plexus may also be involved, but its occurrence is rarer than lumbosacral plexus. Nerve biopsy shows perivascular inflammation, focal necrosis of the perineurium, and neovascularization, suggesting ischemic nerve injury [12]. It is usually monophasic and self-limiting over several months in the majority of cases, but full recovery is unlikely. The therapeutic efficacy of immunotherapy, including plasma exchange, intravenous immunoglobulin, and intravenous methylprednisolone, is controversial [13].
Painful neuropathy following rapid correction of hyperglycemia, previously known as insulin neuritis or cachexic neuropathy, has now been designated as treatment-induced neuropathy of diabetes (TIND) [14]. Although its incidence is not yet known, in a single center study 11% of patients referred for evaluation of diabetic neuropathy were found to have TIND [15]. TIND can occur regardless of the type of treatment. Insulin, oral hypoglycemic agents, and even tight diet control have a potential risk for inducing TIND. Rapid reduction of blood glucose, i.e., a decrease in HbA1c > 3 points within 3 months, in patients with chronic hyperglycemia tends to be associated with the development of this type of neuropathy [16]. The most common symptom is “burning” or “lancinating” pain with various symptoms of autonomic dysfunction. Although the mechanism is unclear, it has been postulated that rapid reduction of blood glucose level causes relative hypoglycemia in patients with chronic hyperglycemia and results in energy crisis of the axonal transport system [16]. Small, unmyelinated nerve fibers are vulnerable to this hypoxic and ischemic condition. A history of eating disorders is extremely common in women with type 1 diabetes, and hypertension, dyslipidemia, and tobacco use are common medical comorbidities in type 2 diabetes. As there is as yet no effective treatment other than management of neuropathic pain, safe rates of glycemic change are recommended especially in chronic hyperglycemic patients at risk of developing TIND.
The diagnostic criteria used in clinical studies or research are variable, and are usually confined to DSPN. Some criteria are based on clinical symptoms or signs only, while others require diagnostic tools for objective diagnosis. The generally accepted criteria are those of the expert panels at the International Symposium on Diabetic Neuropathy in Toronto, 2009 [17]. They proposed the minimal diagnostic criteria for typical DSPN as follows: (1) possible—the presence of symptoms (decreased sensation, positive neuropathic sensory symptoms) or signs (symmetric decrease of distal sensation or unequivocally decreased or absent ankle reflexes) predominantly in the toes, feet, or legs; (2) probable—the presence of a combination of symptoms and signs of neuropathy; (3) confirmed—the presence of abnormalities on NCS with both the presence of a combination of symptoms and signs of neuropathy, and if the results of NCS are normal, other validated measures of small fiber function may be used; and (4) subclinical—no neuropathic symptoms or signs but confirmed neuropathy with NCS or other validated methods.
The onset and mode of progression of neuropathic symptoms should be determined. DSPN begins insidiously and progresses very slowly. Sudden onset or rapid worsening of symptoms or signs exclude the possibility of DSPN, and indicate a typical pattern of diabetic radiculoplexus neuropathy or other immune-mediated inflammatory neuropathies, such as Guillain-Barré syndrome. Symptom descriptions show a wide degree of interindividual variability. Careful history taking to determine whether the symptoms imply large or small fiber involvement is helpful when prescribing medication for management of symptoms. The distribution of sensory symptoms should be determined. Patients often do not exactly describe the boundaries of sensory deficits in neurological examination, so rough evaluation of subjective sensory involvement is important for differential diagnosis and should be included in history taking.
In DSPN, neurological examinations demonstrate distal symmetric loss of both vibration and pinprick sensation [18]. Vibration sensation is tested on the index finger in the upper extremity or great toe in the lower extremity. A 128-Hz tuning fork is placed on the bony prominence of the distal interphalangeal joint of the finger or toe of the patient, and they are then asked to indicate the moment when they no longer feel the vibration (Fig. 1A). The vibration sensation is considered to be normal if the examiner feels vibration for < 10 seconds on the hands. Pinprick sensation can be tested with a toothpick or broken cotton swab; a metal pin or safety pin should not be used to avoid the risk of blood-borne infection. Decreased sensory perception is evaluated to determine whether the deficit has a distal-to-proximal gradient on the tested limb or is symmetrical on both sides.
Tendon reflex is examined using a hammer. The patient’s foot should be passively dorsiflexed to obtain the maximal response. The Achilles tendon reflex may not be elicited or may be reduced at age ≥ 60 years.
Organized and validated questionnaires are commonly used for objective and consistent history taking along with physical examination. These questionnaires are useful because they represent the patient’s neurological symptoms, reduce the clinic time, and help to determine the progress of neuropathy when performed regularly. The current position statement of the American Diabetes Association recommends screening for diabetic neuropathy at the time of diagnosis and annual assessment for patients with type 2 diabetes and patients with type 1 diabetes for ≥ 5 years [19].
The Michigan Neuropathy Screening Instrument (MNSI) is the most widely used questionnaire for screening of diabetic neuropathy [20]. This questionnaire, which has been designed to make it easier for general practitioners to screen for distal symmetric neuropathy in diabetes, includes two separate chapters (Table 2). The first is a 15-item questionnaire that elicits “yes” or “no” responses regarding sensory abnormalities in a collection of questions with high sensitivity and specificity to DSPN among the items of the Neuropathy Screening Profile. Questions 4 and 10 are about vascular circulation and general asthenia, and so are excluded in scoring. A score > 7 is considered abnormal [21]. The second part consists of four items of simple physical inspection and neurological examination, which are tested on both the left and the right sides: (1) foot inspection for evidence of deformity, dry skin, callus, infection, fissure, and ulceration; (2) vibration sensation measured on the great toe using a 128-Hz tuning fork; (3) ankle reflex; and (4) tactile examination of the foot using 10 g monofilament (Fig. 1B and 1C). Eight correct answers out of 10 applications is considered normal. MNSI is a simple, semi-quantitative test that is suitable for use in busy outpatient clinics. Further objective tests should be considered to ensure accurate diagnosis in patients with a positive screening test but atypical presentation of DSPN. Michigan Diabetic Neuropathy Score (MDNS) is a tool that includes more detailed neurological examination and NCS data [20]. MDNS is used to confirm the presence of neuropathy and to evaluate its severity in patients with positive result on MNSI. Diabetic Neuropathy Examination [22] and Diabetic Neuropathy Symptom Score are additional tools designed for screening of DSPN [23], but further studies are required to validate their reliability.
Composite Autonomic Symptom Score (COMPASS-31) can be used for screening to identify autonomic dysfunction [24]. COMPASS-31 is a well-validated questionnaire for early detection of autonomic dysfunction in type 2 diabetes composed of 31 questions in six domains, i.e., orthostatic intolerance, vasomotor, secretomotor, gastrointestinal, constipation, bladder, and pupillomotor [25]. The advantages of COMPASS-31 are that each symptom is quantified differently according to clinical significance, and the score is consistent with the severity of the results of the AFT [26]. However, the symptoms of autonomic dysfunctions are diverse and not specific, and even end organ failure can mimic autonomic neuropathy. Therefore, autonomic neuropathy cannot be diagnosed based simply on the presence or absence of such symptoms. At least one objective diagnostic test is required for accurate diagnosis in patients with suspected symptoms of autonomic dysfunction.
When diabetic neuropathy is suspected, more objective tests are needed to confirm the presence of neuropathy, to measure the severity of neuropathy, and finally to exclude other possible causes of the symptoms. More detailed and accurate diagnosis is now possible with advances in diagnostic technology (Table 3).
Electrophysiological tests should be performed to confirm the presence of neuropathy, to determine its severity, and to differentiate other causes of neuropathy from diabetic neuropathy. NCS is the gold standard test for this purpose, and plays a crucial role in evaluation of the peripheral nervous system and obtaining accurate and objective data on neuropathies. Experts advocate the use of NCS as a reliable indicator of DSPN in epidemiological or clinical trials [17]. Electromyography (EMG) is often used as the general term for electrodiagnostic tests and as a mixture of NCS. However, EMG is not necessary for evaluation of neuropathy and is performed only when a referred patient seems to have another type of neuropathy or motor involvement, such as radiculopathy.
In 1982, Ewing and Clarke [27] proposed five tests for cardiovascular autonomic neurological function, i.e., heart rate response to the Valsalva maneuver, heart rate variation during deep breathing, blood pressure response to sustained hand grip, immediate heart rate response to standing, and blood pressure response to standing. The current conventional AFT includes measurement of heart rate variability during deep breathing and the Valsalva maneuver and head-up-tilt test that can assess cardiovagal and sympathetic adrenergic function. Sympathetic cholinergic function can be assessed by the quantitative sudomotor axon reflex test (QSART) in specialized laboratories [28]. Some devices provide automatic interpretation of sympathetic or parasympathetic deficit using the time and frequency domain of the heartbeat [29]. It is difficult to say whether conventional AFT or spectral analysis is better. However, power spectral analysis of the heartbeat represents data that are transformed to such an extent that this method should not be used without sufficient familiarity [30].
Heart rate variability to deep breathing (HRVDB) is the most widely used, simple, and reliable test for evaluation of cardiovagal function [31]. Deep inspiration increases intrathoracic pressure and reduced venous return accelerates heartbeat to maintain cardiac output. Exhalation decreases the heart rate and vice versa. The patient is requested to take about 5 to 6 regular slow deep breaths per minute, and continuous records of R-R interval generate sinusoidal curves (Fig. 2). The average ratio of the maximum to minimum heart rate (E:I ratio) or the average of the fastest rate minus the slowest (E-I difference) is used as HRVDB. Parasympathetic dysfunction is the first to appear in diabetic autonomic neuropathy, followed by sympathetic adrenergic impairment. Therefore, reduced HRVDB is a good indicator of early cardiac autonomic neuropathy [32].
Heart rate variability in response to the Valsalva maneuver (HRVVAL) has a somewhat complex mechanism. The heart rate and blood pressure change with the Valsalva maneuver, and can be divided into four phases (Fig. 3). Immediately after the Valsalva maneuver, the mechanical pressure on the aorta transiently increases blood pressure (phase I). During the maneuver, the decrease in venous return leads to a decrease in cardiac output (drop in blood pressure) and a compensatory increment of heart rate (early phase II). Through activation of the baroreflex, there is a return of blood pressure to baseline (late phase II). Release of the abdominal strain the maneuver causes a transient drop in blood pressure and increase in heart rate (phase III) and, similar to phase I, phase III is also a mechanically induced response and is not mediated by autonomic reflexes. Finally, there is significant overshoot of blood pressure and a sudden fall in heart rate due to the normalized venous return and increased cardiac output while peripheral vasoconstriction is still present. The Valsalva ratio is the ratio of the fastest heart rate in phase II to the slowest heart rate during phase IV. The Valsalva ratio reflects the integrity of cardiovagal and sympathetic adrenergic function, and abnormal Valsalva phase (loss of late phase II or blunting of phase IV) indicates impaired sympathetic adrenergic function [33].
Orthostatic intolerance can be assessed by the headup-tilt test. Active standing can substitute for passive tilt. The diagnosis of classic orthostatic hypotension requires a fall of > 20 mmHg in systolic blood pressure and > 10 mmHg in diastolic blood pressure within 3 minutes of standing [34]. Continuous pulse pressure monitoring can provide additional information regarding whether a decline of blood pressure is physiological (initial orthostatic hypotension caused by dehydration or medication) or pathological (neurogenic orthostatic hypotension). Compensatory tachycardia does not appear or is minimal in neurogenic orthostatic hypotension, because parasympathetic fiber loss begins earlier than sympathetic denervation.
At first glance, the AFT method seems simple, but it goes through very complex reflex pathways. In addition, the results may vary depending on the patient’s physical or psychological state at the time of the test and any medications being taken. Therefore, the results should be interpreted with knowledge of the autonomic nervous system and the factors that may affect the results.
Sudomotor function is another important autonomic function for maintenance of body temperature, and can be evaluated by QSART [28]. Using a specialized device, the nerve terminals within sweat glands are stimulated by 10% acetylcholine. This will produce axon reflex and retrograde action potential along the axon will induce sweat response to the neighboring sweat glands. The amount of sweat evaporated is measured and used as a marker of sudomotor function. QSART is the most sensitive test for detecting postganglionic sympathetic cholinergic abnormalities, and is currently used in various clinical conditions [28].
Neuropad is a simple, visual indicator test for documenting sweat production. The pad changes color from blue to pink when there is adequate sweat production, while lack of color change or incomplete response indicates hypohidrosis. Although semiquantitative, this method is easy to perform and has been reported to be useful for detection of early diabetic neuropathy or small fiber dysfunction [35]. A multicenter study including 1,010 patients with type 2 diabetes reported sensitivity of 94.9% and specificity of 70.2% for the diagnosis of small fiber neuropathy [36]. They concluded that Neuropad is an excellent screening test to exclude neuropathy in patients with diabetes.
Sudoscan is another method to assess sudomotor function. Similar to Neuropad, it is a noninvasive indicator test, based on the reaction between sodium chloride in sweat and nickel. It measures electrochemical skin conductance in the palms of the hands and soles of the feet, and low skin conductance indicates the reduced sweating seen in small fiber neuropathy. In a study to compare its accuracy for detecting small fiber dysfunction in patients with diabetes with QST and QSART, the sensitivity and specificity for diabetic neuropathy were reported to be 78% and 92%, respectively [37].
The QST is a type of psychophysiological test that assesses the sensory perception thresholds to defined sensory stimuli. Conventional QST measures the thresholds of vibration, cold, and heat pain sensation. QST is useful when the patient has predominant small fiber loss (loss of pinprick and thermal sensation) or positive symptoms (irritable nociceptors) [38]. Its clinical use is limited by its requirement for specialized equipment and considerable time consumption. As the results of QST are dependent on the concentration of the subject, the diagnostic sensitivity for diagnosis of peripheral neuropathy has been reported to range from 36% to 85% [38]. Therefore, QST is recommended for use as a complementary tool rather than as a single diagnostic test [39].
Evaluation of intraepidermal nerve fiber density (IENFD) using skin biopsy provides an objective, quantitative measure of very distal free nerve endings, mostly somatic C-fibers [40]. Skin specimens are obtained from the distal and proximal leg with a 3-mm punch biopsy [41]. The nonspecific pan-axonal marker, protein gene product 9.5, is used for immunochemical staining of intraepidermal nerve fibers [41]. IEFND is calculated by the number of intraepidermal perpendicular sprouting axons per cubic millimeter measured under microscopy (Fig. 4A). In diabetic neuropathies with small fiber loss, IENFD is markedly reduced, even in the early course of the neuropathy (Fig. 4B). Morphological changes, such as axonal swelling, demonstrate early pathological changes of neuropathy (Fig. 4C). Exploration of the dermis also provides useful information regarding innervation to the sweat glands or erector pili muscles (Fig. 4D) [42].
Although not routinely performed in clinical practice and only used for research purposes in Korea, many diagnostic criteria for small fiber neuropathy include reduced IENFD as a major feature. As IENFD reflects very early changes in degeneration and regeneration of nerve fiber axons, few studies have used skin biopsy to show the effects of interventions [43]. Recently, adrenergic and cholinergic autonomic innervation of the skin can be evaluated by immunohistochemical staining and confocal microscopy [44].
Diabetic neuropathies, the most common of which is DPSN, show a wide variety of clinical features. The most important point in diagnosis is to avoid misdiagnosing other treatable neuropathies as DSPN. It is necessary to be familiar with various clinical aspects of diabetic neuropathies and to check them through objective diagnostic tests in cases with atypical presentation. It is advisable to consult a neurologist if any other cause is suspected.
Figure 1.
Neurological examination for detecting sensory loss in diabetic neuropathy. (A) Vibration test using a 128-Hz tuning fork. (B) A 10 g Semmes-Weinstein monofilament test. (C) Ten sites recommended for monofilament test. Red points are preferentially tested sites and blue points are other recommended sites.

Figure 2.
Heart rate variability in response to deep breathing. Sinusoidal curves are generated according to the patient’s repetitive deep inspiration and expiration.
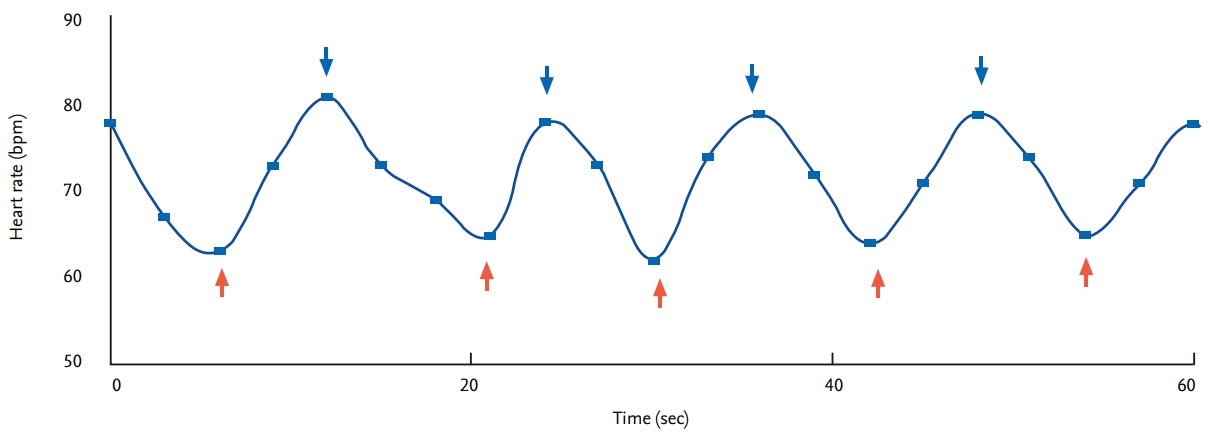
Figure 3.
Valsalva ratio (A) and Valsalva phase (B). Valsalva ratio is the ratio of the fastest heart rate in phase II to the slowest heart rate during phase IV. Valsalva phase is devided into four phases.
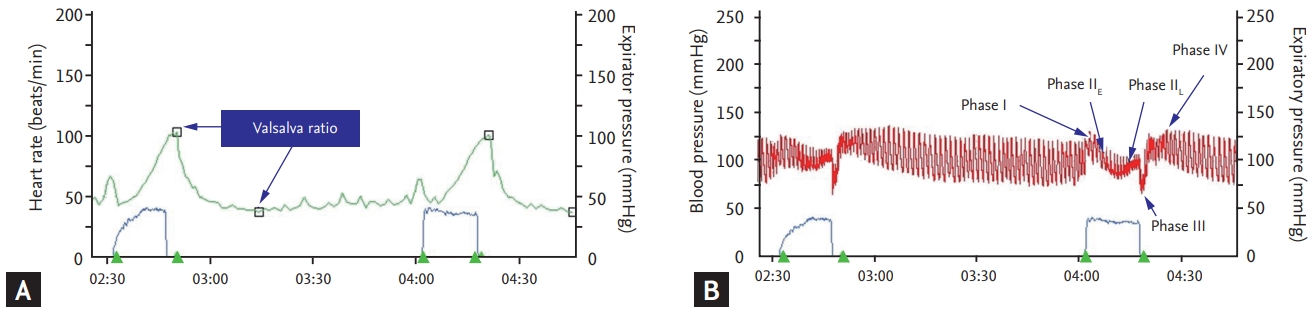
Figure 4.
Protein gene product (PGP) 9.5 immunostaining of skin. (A) Epidermal nerve fibers arising from subepidermal neural plexus (arrowhead) extend vertically with various patterns of branching in a healthy subject (arrow). (B, C) Intraepidermal nerve fibers were rarely seen with fragmented subepidermal neural plexus (white arrowhead) with prominent axonal swelling (white arrow) in a patient with diabetic neuropathy. (D) Dermal nerve plexus innervating sweat gland can be seen in the dermis. (A, B, C ×1,000; D ×400).
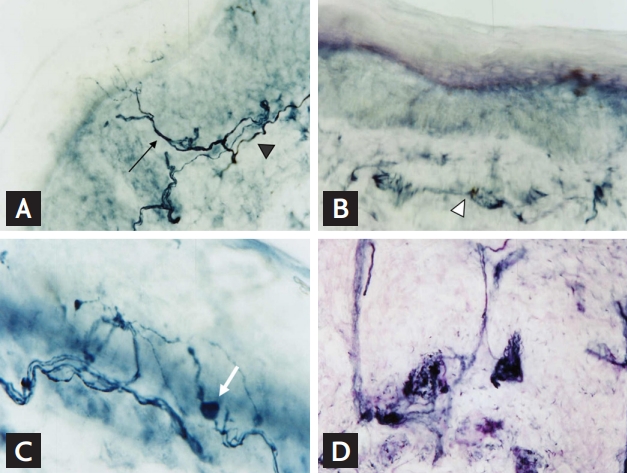
Table 1.
Classification of diabetic neuropathies
Table 2.
Michigan Neuropathy Screening Instrument
Table 3.
Advantage and disadvantage of the tests for neuropathies
REFERENCES
1. American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes. 2020. Diabetes Care 2020;43(Suppl 1):S66–S76.



2. Kim SS, Won JC, Kwon HS, et al. Prevalence and clinical implications of painful diabetic peripheral neuropathy in type 2 diabetes: results from a nationwide hospital-based study of diabetic neuropathy in Korea. Diabetes Res Clin Pract 2014;103:522–529.


3. Sasaki H, Kawamura N, Dyck PJ, Dyck PJB, Kihara M, Low PA. Spectrum of diabetic neuropathies. Diabetol Int 2020;11:87–96.



4. Alsaloum M, Estacion M, Almomani R, et al. A gain-offunction sodium channel β2-subunit mutation in painful diabetic neuropathy. Mol Pain 2019;15:1744806919849802.


5. Benarroch EE. The clinical approach to autonomic failure in neurological disorders. Nat Rev Neurol 2014;10:396–407.



6. Vinik AI, Camacho PM, Davidson JA, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on testing for autonomic and somatic nerve dysfunction. Endocr Pract 2017;23:1472–1478.


7. Azmi S, Ferdousi M, Kalteniece A, et al. Diagnosing and managing diabetic somatic and autonomic neuropathy. Ther Adv Endocrinol Metab 2019;10:2042018819826890.



8. Bhati P, Shenoy S, Hussain ME. Exercise training and cardiac autonomic function in type 2 diabetes mellitus: a systematic review. Diabetes Metab Syndr 2018;12:69–78.


9. Braffett BH, Wessells H, Sarma AV. Urogenital autonomic dysfunction in diabetes. Curr Diab Rep 2016;16:119.



10. Cryer PE. Hypoglycemia-associated autonomic failure in diabetes. Handb Clin Neurol 2013;117:295–307.


11. Rota E, Morelli N. Entrapment neuropathies in diabetes mellitus. World J Diabetes 2016;7:342–353.



12. Dyck PJ, Windebank AJ. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve 2002;25:477–491.


13. Llewelyn D, Llewelyn JG. Diabetic amyotrophy: a painful radiculoplexus neuropathy. Pract Neurol 2019;19:164–167.


14. Hwang YT, Davies G. ‘Insulin neuritis’ to ‘treatment-induced neuropathy of diabetes’: new name, same mystery. Pract Neurol 2016;16:53–55.

17. Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293.



18. Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers 2019;5:41.



19. Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017;40:136–154.


20. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–1289.


21. Herman WH, Pop-Busui R, Braffett BH, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med 2012;29:937–944.



22. Meijer JW, van Sonderen E, Blaauwwiekel EE, et al. Diabetic neuropathy examination: a hierarchical scoring system to diagnose distal polyneuropathy in diabetes. Diabetes Care 2000;23:750–753.


23. Meijer JW, Smit AJ, Sonderen EV, Groothoff JW, Eisma WH, Links TP. Symptom scoring systems to diagnose distal polyneuropathy in diabetes: the Diabetic Neuropathy Symptom score. Diabet Med 2002;19:962–965.


24. Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc 2012;87:1196–1201.



25. Singh R, Arbaz M, Rai NK, Joshi R. Diagnostic accuracy of composite autonomic symptom scale 31 (COMPASS-31) in early detection of autonomic dysfunction in type 2 diabetes mellitus. Diabetes Metab Syndr Obes 2019;12:1735–1742.



26. Ruska B, Pavicic T, Pavlovic I, et al. Performance of the COMPASS-31 questionnaire with regard to autonomic nervous system testing results and medication use: a prospective study in a real-life setting. Neurol Sci 2018;39:2079–2084.



27. Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed) 1982;285:916–918.



29. Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol 2012;8:405–416.



30. Hayano J, Yuda E. Pitfalls of assessment of autonomic function by heart rate variability. J Physiol Anthropol 2019;38:3.




31. Benichou T, Pereira B, Mermillod M, et al. Heart rate variability in type 2 diabetes mellitus: a systematic review and meta-analysis. PLoS One 2018;13:e0195166.



32. Dimitropoulos G, Tahrani AA, Stevens MJ. Cardiac autonomic neuropathy in patients with diabetes mellitus. World J Diabetes 2014;5:17–39.



33. Illigens BMW, Gibbons CH. Autonomic testing, methods and techniques. Handb Clin Neurol 2019;160:419–433.


34. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neutrally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011;21:69–72.



35. Bonhof GJ, Herder C, Strom A, Papanas N, Roden M, Ziegler D. Emerging biomarkers, tools, and treatments for diabetic polyneuropathy. Endocr Rev 2019;40:153–192.



36. Manes C, Papanas N, Exiara T, et al. The indicator test Neuropad in the assessment of small and overall nerve fibre dysfunction in patients with type 2 diabetes: a large multicentre study. Exp Clin Endocrinol Diabetes 2014;122:195–199.



37. Casellini CM, Parson HK, Richardson MS, Nevoret ML, Vinik AI. Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol Ther 2013;15:948–953.



38. Shy ME, Frohman EM, So YT, et al. Quantitative sensory testing: report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology 2003;60:898–904.


39. Muck M, Cuhls H, Radbruch L, et al. Quantitative sensory testing (QST). English version. Schmerz 2016 Jan 29 [Epub]. https://doi.org/10.1007/s00482-015-0093-2.

41. Mellgren SI, Nolano M, Sommer C. The cutaneous nerve biopsy: technical aspects, indications, and contribution. Handb Clin Neurol 2013;115:171–188.


42. Oh JY, Park KD, Kim JE, Choi YJ, Choi KG. Assessment of intraepidermal nerve fiber using skin biopsy in diabetic polyneuropathy. J Korean Neurol Assoc 2003;21:628–633.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



