A thoracic aortic aneurysm (TAA) is usually asymptomatic, but it sometimes manifests with chest pain as a chief complaint. Therefore, this disease initially mimics acute coronary syndrome. Herein, we describe a giant TAA with chest pain caused by compression of the cardiac structures that was treated by thoracic endovascular aortic repair (TEVAR).
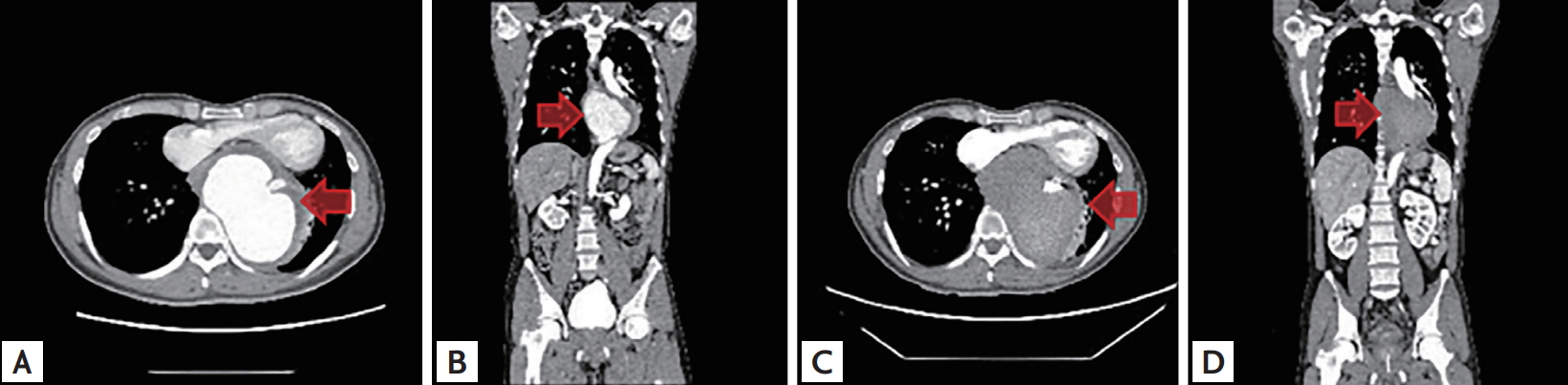
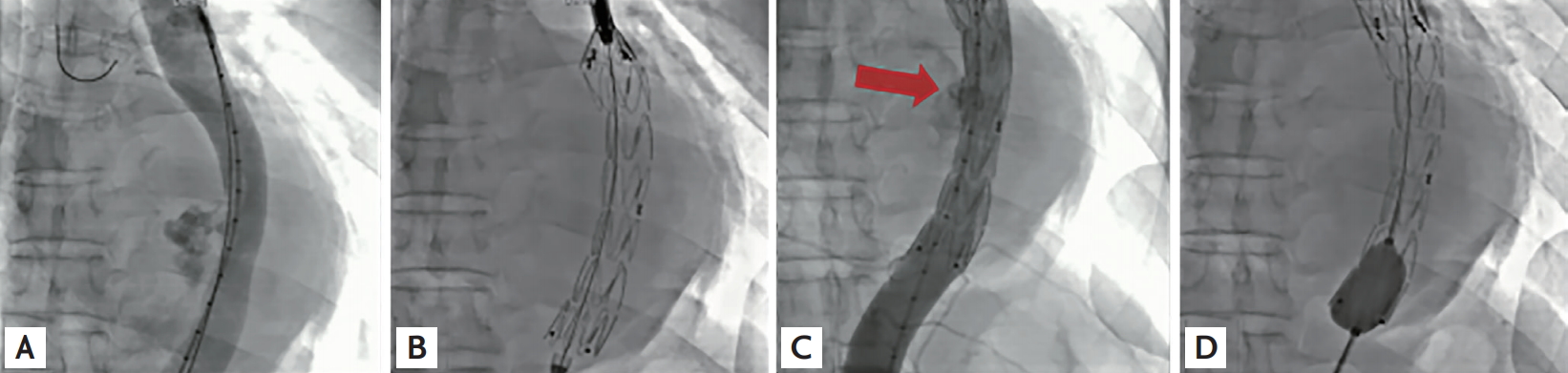
A 48-year-old man presented to our center because of squeezing chest pain. Initially, acute coronary syndrome was suspected. Computed tomography (CT) revealed a 12 cm-diameter saccular descending TAA compressing the left atrium and left ventricle (Fig. 1A and 1B). We promptly deployed a 24 × 100 mm-sized stent graft (Fig. 2A and 2B). As follow-up aortogram showed mild endoleak, additional balloon dilatation was performed (Fig. 2C and 2D). Follow-up CT revealed a well-positioned endoprosthesis with no definite endoleak (Fig. 1C and 1D). The patient was discharged from our hospital and had an uneventful recovery.
A giant aortic aneurysm is defined as an aneurysm measuring > 10 cm at its maximum diameter. There have been some case reports about unusually sized TAAs. To our best knowledge, our case is the largest TAA treated by TEVAR in South Korea.
According to the European Society of Cardiology guidelines, TEVAR is considered as first-line therapy for descending TAA. A comprehensive meta-analysis showed that TEVAR resulted in better perioperative outcomes during index hospitalization, but 1- and 5-year mortalities remained the same for TEVAR and open surgery. No comparative study about both treatments among patients with giant TAA has been performed because of the paucity of case reports available worldwide. Our case shows that TEVAR is a feasible option for managing huge TAAs.
Before the procedure, we explained the diagnosis and mentioned the positive and negative effects of the procedure to the patient. In addition, we also informed him about the preparation of a paper on such treatment, and the patient agreed with publication of his case and accompanying images. Written informed consent was obtained from patients.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





