INTRODUCTION
Pleuroparenchymal fibroelastosis (PPFE) is a rare type of idiopathic interstitial pneumonia (IIP) [
1], and was first reported in 2004 by Frankel et al. [
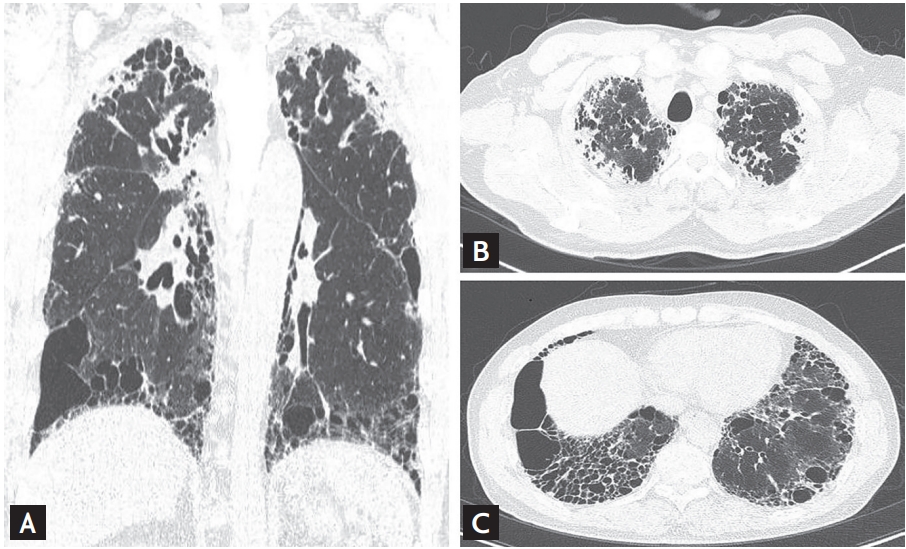
2]. Pathological findings for PPFE include pleural fibrosis with subjacent intra-alveolar fibrosis and alveolar septal elastosis, while radiological findings include pleural thickening of the upper lobe, with subpleural fibrosis, on high-resolution computed tomography (HRCT) [
3,
4]. PPFE is known to predominantly involve the upper lobes; however, there is increasing evidence for its coexistence with interstitial lung disease (ILD) in the lower lobe (43% to 89%), with the usual interstitial pneumonia (UIP) pattern being the most common [
3-
5]. PPFE shows diverse clinical courses, ranging from slow to rapid progression [
6,
7]. Previous studies have reported that the median survival period for patients with PPFE ranges from 35.3 months to 11 years, which is similar to that of patients with other IIPs [
6,
8-
11].
Recently, Cha et al. [
12] reported clinico-pathologic features of seven Korean patients with histologically proven PPFE. In this study, all patients showed a restrictive pattern in lung function and had underlying clinical conditions (four connective tissue disease, three organ transplantation, and one fungal infection). In five patients (71.4%), lower lobe involvement was observed on HRCT. The UIP pattern (40%) was the most common pathological finding in the lower lobe [
12]. However, these results are limited by a small number of cases and the absence of follow-up data. In addition, it is still uncertain whether lower lobe involvement impacts the prognosis of PPFE patients. Therefore, the aim of this study was to evaluate the clinico-radiologic-pathologic features and clinical course in a larger number of Korean patients with PPFE, and to evaluate the clinical differences between patients with and without lower lobe involvement.
DISCUSSION
In our study, the clinical characteristics of Korean patients with PPFE were similar to those reported previously. Most patients showed a low body mass index (BMI), restrictive lung function, and frequent pneumothorax during follow-up. However, lower lobe involvement was frequently observed; patients with lower lung involvement were older and had a poorer exercise capacity compared with those without lower lung involvement. FVC was a significant prognostic factor in Korean patients with PPFE.
In previous studies, relatively lower BMI and higher residual volume (RV)/TLC in PPFE patients than in other IIP patients were reported as typical features of PPFE [
9,
16,
17,
19,
20]; our results support these findings. Tanizawa et al. [
21] reported that, in 118 patients with fibrotic ILD who registered for lung transplantation, BMI was significantly different between the radiologically defined PPFE and no PPFE groups (15.9 kg/m
┬▓ vs. 21.5 kg/m
┬▓,
p < 0.001). Oda et al. [
16], in 108 IPF patients, reported that the combined PPFE group showed lower BMI (18.6 ┬▒ 1.8 kg/m
┬▓ vs. 25.1 ┬▒ 3.6 kg/m
┬▓,
p < 0.01) and higher RV/TLC (42.6% ┬▒ 8.7% vs. 33.1% ┬▒ 7.6%,
p < 0.01) than the IPF only group. The reason for lower BMI in patients with PPFE than in other IIP patients is not well known; however, Suzuki et al. [
22], on comparing body composition changes between 43 PPFE and 131 IPF patients, suggested that the characteristics of upper lobe predominant fibrosis and more severe restrictive defects in patients with PPFE might exacerbate the impaired energy and protein balance, resulting in weight loss and muscle wasting. In terms of RV/TLC, Oda et al. [
16] suggested that, despite upper lobe volume loss, RV/TLC was higher in PPFE patients than in other IIP patients because RV was relatively more increased than TLC owing to compensatory hyperinflation of the middle and lower lobes.
PPFE could be related to relevant underlying conditions including infection, drugs, autoimmune disease, and organ transplantation, and the reports of PPFE after organ transplantation have increased [
23-
26]. In our study, among 26 patients, two patients (7.7%) had a history of organ transplantation (one heart and one kidney), consistent with previous results; in the study including 12 patients with PPFE, Reddy et al. [
3] reported that one patient (8.3%) had a history of kidney transplantation. Cha et al. [
12] also reported that among seven Korean patients with PPFE, three patients (42.9%) had a history of organ transplantation (two bone marrow and one liver).
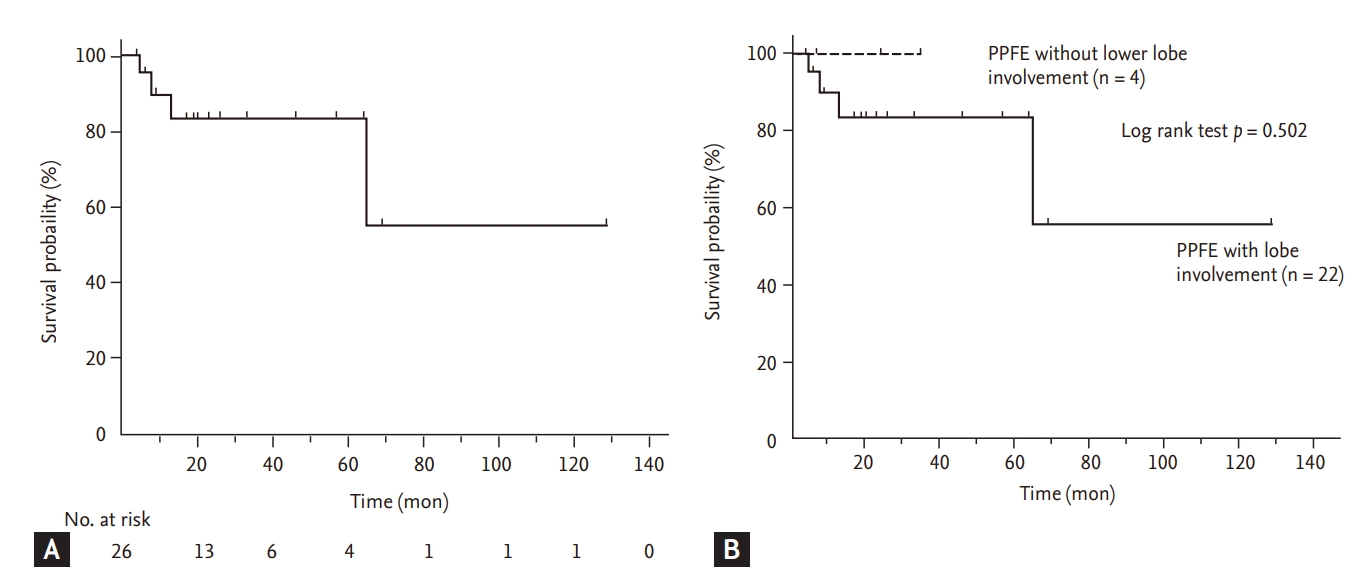
In patients with PPFE, the coexistence of other ILDs in the lower lobes has been increasingly reported [
3,
16,
27], and the prevalence of coexistent ILD is relatively high (43% to 89%), especially in Japanese reports [
9,
17]. In a study including patients with PPFE from western countries, Reddy et al. [
3] reported that among 12 patients with PPFE, lower lobe involvement was identified in 50% of the subjects. Our study also confirmed the high prevalence (84.6%) of lower lobe involvement reported by in the Japanese studies. In our study, PPFE patients with lower lobe involvement were older and had a poorer exercise capacity compared with those without lower lobe involvement; however, survival did not differ between the two groups. In a recent study of 40 Japanese patients with clinically diagnosed PPFE, Kono et al. [
28] showed results similar to those of our study. PPFE patients with lower lobe involvement (n = 21) were significantly older (74.3 ┬▒ 9.3 years vs. 60.2 ┬▒ 9.9 years,
p = 0.002) and had lower lung function including RV (99.2% ┬▒ 27.4% vs. 130.7% ┬▒ 27.8%,
p = 0.03) and TLC (72.3% ┬▒ 13.7% vs. 93.5% ┬▒ 16.7%,
p = 0.007) than those without lower lobe involvement (n = 19). However, survival was significantly worse in PPFE patients with lower lobe involvement than in those without lower lobe involvement (log-rank test,
p = 0.014) [
28].
In our study, among patients with lower lobe involvement, EFs were detected in the lower lobes of one-third of patients (38.5%). Nakatani et al. [
4] also reported that, among 12 patients with PPFE, fibroelastosis was observed in the lower lobe in two patients (16.7%). In addition, one study comparing the amount of lung EFs (EF) in PPFE patients (n = 6) and in IPF patients (n = 28) found that PPFE patients presented significantly more EFs in the lower lobe (EF scores, 23.6% ┬▒ 2.4% vs. 12.2% ┬▒ 4.4%,
p < 0.0001) [
29]. These results suggest that, in some patients, lower lobe involvement could be due to PPFE progression rather than due to the coexistence of other types of ILD.
Our study showed that only FVC was a significant predictive factor for mortality in patients with PPFE. The results of a previous report support our findings: Shioya et al. [
30], in a study of 29 PPFE patients, showed that FVC (HR, 0.093; 95% CI, 0.083 to 0.982;
p = 0.017) was an independent factor predicting mortality, along with sex, age, and physiology (GAP) index (HR, 2.510; 95% CI, 1.245 to 5.059;
p = 0.010), in a multivariate Cox proportional hazard model. Previous studies have also reported other prognostic factors in PPFE patients [
17,
19,
31]. Kato el al. [
19] reported that, in 36 Japanese patients with PPFE, the UIP pattern in the lower lobe on HRCT was a significant risk factor for poor prognosis (odds ratio, 23.670; 95% CI, 1.505 to 372.268;
p = 0.024) after adjusting for Krebs von den Lunen-6, modified Medical Research Council, and fibrosis scores. Additionally, Khiroya et al. [
31] reported that, in 43 Japanese patients with PPFE, male sex was an independent predictor of mortality (HR, 5.31; 95% CI, 1.08 to 26.12;
p = 0.04) after adjusting for age.
In our study, most patients were treated with anti-inflammatory or antifibrotic agents, but no differences in survival were observed between patient groups. At present, no effective therapy exists for patients with PPFE. There are only some case reports showing that pirfenidone, an antifibrotic agent used for IPF, reduced the decline in lung function in PPFE patients [
32,
33]. In patients with PPFE, a UIP pattern in lower lobe involvement is common; therefore, pirfenidone might be useful for PPFE patients with a coexistent UIP pattern in their lower lobes. In the near future, large-scale studies are needed to develop further effective treatments.
This study has some limitations. First, this was a retrospective study conducted in a single center with a relatively small number of patients and short follow-up period. However, the baseline characteristics of our subjects were similar to those of patients in previous reports. Second, pathologic diagnosis was performed using transbronchial lung biopsy in around half (42.3%) of the subjects. However, a previous study suggested that patients with a clinical diagnosis of PPFE had similar characteristics to those with histopathologically confirmed PPFE patients [
9], and in our study, the diagnosis of PPFE was confirmed by multidisciplinary discussion using clinical radiologic and pathologic data. Third, the number of patients without lower lobe involvement was small (n = 4, 15.4%), and follow-up duration was short (mean 7.3 months), which could reduce statistical power when comparing patients with and without lower lobe involvement. However, despite the small sample size, patients with lower lobe involvement were characterized by older age and poorer exercise capacity, compared with those without lower lobe involvement.
In conclusion, Korean patients with PPFE showed clinical characteristics and prognoses similar to those reported previously; however, lower lobe involvement was frequently observed. Patients with lower lung involvement were older and had a poorer exercise capacity compared with those without lower lung involvement.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



