 |
 |
| Korean J Intern Med > Volume 34(5); 2019 > Article |
|
Abstract
In multiple myeloma (MM), the impaired function of several types of immune cells favors the tumor’s escape from immune surveillance and, therefore, its growth and survival. Tremendous improvements have been made in the treatment of MM over the past decade but cellular immunotherapy using dendritic cells, natural killer cells, and genetically engineered T-cells represent a new therapeutic era. The application of these treatments is growing rapidly, based on their capacity to eradicate MM. In this review, we summarize recent progress in cellular immunotherapy for MM and its future prospects.
Multiple myeloma (MM) is a clonal B-cell malignancy that arises consistently from asymptomatic precursor conditions, specifically, monoclonal gammopathy of undetermined significance and smoldering MM. The proliferation of malignant plasma cells in the bone marrow and a subsequent overabundance of monoclonal paraprotein (M protein) in the serum and/or urine, renal dysfunction, anemia, hypercalcemia and lytic bone disease are the hallmarks of MM [1,2]. The incidence of the disease has increased rapidly in recent years, which, at least in Korea, accounts for it being one of the hematological malignancies currently in the medical and socioeconomic spotlight [3]. Despite significant advances in therapeutic strategies, including stem cell transplantation, proteasome inhibitors, and immunomodulatory drugs (iMiDs), which have led to improved responses to treatment and longer overall survival, most patients with MM eventually relapse and succumb to the disease [4,5]. Thus, there is a clear need to develop novel therapeutic options.
Recently, cellular immunotherapies have been recognized as an effective therapeutic modality for MM [6]. The human immune system has immense diversity and specificity that rely on a wide variety of effector mechanisms, such as those involving Fas ligands, the complement system, perforins, granzymes, and interferon-gamma (IFN-γ) [7]. However, myeloma suppresses the immune response as a whole by releasing immune suppressive molecules and cytokines, leading to the tumor’s escape from the effector immune response [8]. The goal of cancer immunotherapy is to activate, restore, and augment cytotoxic effector cells at the tumor site to effectively kill the tumor, all of which rely on the safe induction of cytotoxic cells that recognize and kill tumor cells [9]. An ideal immunotherapy should overcome the effects of an immunosuppressive microenvironment, train and recruit immune cells to eliminate all cancer cells, improve patient outcome without affecting healthy cells, and remain active in the event of recurrence. Dendritic cell (DC) vaccination and adoptive cell immunotherapy with chimeric antigen receptor (CAR) T-cells, T-cell receptor (TCR)-engineered T-cells, and natural killer (NK) cells are emerging as promising forms of cellular immunotherapy in patients with MM [10-15]. This review focuses on the efficacy and safety of recent preclinical and clinical trials in the development of DC vaccines, genetically engineered effector T-cells, and NK cell therapies for MM.
The most potent antigen-presenting cells are DCs, which play a vital role in recognizing, processing, and presenting antigens on the cell surface of naïve T-cells, and modulating tumor specific immunity [16,17]. In MM, the functional ability of DCs is abolished by several immunosuppressive cytokines and inhibitory proteins, such as vascular endothelial growth factor, interleukin 10 (IL-10), IL-6, and transforming growth factors (e.g., TGF-β), secreted by malignant plasma cells and the tumor microenvironment [18]. Thus, the retrieval of fully functional DCs against tumor cells is a promising therapeutic strategy. Among the factors that need to be considered for successful DC vaccination strategies are the selection and source of the tumor antigen, the potency of the DC vaccine formulation, the mode of delivery, adjuvants, and immunomodulation, and the treatment schedule. In general, DCs generated ex vivo for cancer immunotherapy should be mature, capable of migrating in the direction of secondary lymphoid organs, and produce type 1 helper (Th1) polarizing cytokines. In a previous study, we reported that functionally active DCs generated ex vivo from patients with MM exhibited the properties of the strong, mature DCs necessary to induce potent myeloma-specific cytotoxic T lymphocytes (CTLs) [13,19].
In early clinical trials of immunoglobulin idiotype (Id)-pulsed DCs, features indicative of myeloma- specific immune responses were observed but the clinical responses were unsatisfactory because of the weak antigenicity of the Id [20]. Tumor-associated antigens (TAAs)-loaded DCs may also induce tumor-specific CTL responses for targeting myeloma cells in vitro. Various TAAs have been identified in MM, such as mucin 1 (MUC1), New York esophageal squamous cell carcinoma 1 (NY-ESO-1), and telomerase reverse transcriptase (hTERT). However, although a single TAA may induce an antitumor immune response in MM, the myeloma cells can escape immune recognition via the down-regulation of this specific antigen over time. To overcome this problem, DCs can be loaded with whole myeloma cells to improve the antitumor immune response effectively and avoid tumor cell immune escape [13,19]. This alternative approach has been tested using DCs loaded with myeloma cell lysates or apoptotic bodies, DCs transfected with tumor-derived RNA or heat shock proteins (HSP) gp96, and DC-myeloma fusions. Regardless of the specific method, the results showed that multiple unknown epitopes were presented by the DCs for major histocompatibility complex (MHC) I recognition and the subsequent induction of polyclonal T-cell immune responses to effectively kill myeloma cells [20]. Our group was also able to generate potent DCs loaded with dying myeloma cells, which induced myeloma-specific CTLs with strong Th1 polarization [21-27]. In our previous phase I/IIa study of patients with relapsed or refractory MM, DC vaccination using cells loaded with γ-irradiated dying myeloma cells was well tolerated, did not result in any significant adverse effects, and led to disease-stabilizing activity in 66.7% of the patients and a 77.8% immunological response [28]. Fig. 1 describes DCs pulsed or loaded with different sources of myeloma antigens to induce myeloma-specific immune responses.
Recent attempts to improve the effectiveness of DC vaccines have included the use of a cocktail of several tumor antigens, genetic engineering and molecular biological modifications, and combinations with other agents. IMiDs, such as thalidomide, lenalidomide, and pomalidomide, were also shown to be effective in patients with MM. The immunological mechanism of iMiDs involves the down-regulation of regulatory T-cells (Tregs) and myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment and an enhancement of the immune response by activating NK cells and T-cells [29]. We previously reported a strong synergistic effect of DC vaccination and lenalidomide [30] or pomalidomide [31] in the induction of an anti-myeloma effect in a murine myeloma model. Another method to improve DC vaccination is to combine checkpoint blockades that modulate negative regulation in the tumor microenvironment [32]. Our data, obtained in a murine myeloma model, indicated a remarkable anti-myeloma effect of DC vaccination when combined with an antiprogrammed cell death 1 (PD-1) antibody and lenalidomide, attributable to an augmented immune response [33]. These improvements in DC vaccination may give rise to a promising cell therapy tool able to induce myeloma-specific immune responses, without significant adverse effects. A schematic representation of the future perspectives of enhanced DC vaccination strategies is shown in Fig. 2.
Approaches aimed at triggering a tumor-specific T-cell response and, thus, immunological memory against the tumor cells, include the adoptive transfer of genetically engineered T-cells. This is achieved by introducing antibody-like recognition in CARs or by modifying TCR specificity. Both methods should result in the targeting of surface antigens that are highly expressed in MM. A schematic representation of the treatment of MM with genetically engineered T-cells is shown in Fig. 3.
CAR T-cells are genetically engineered T-cells that can recognize specific antigens expressed on tumor cells and then kill the tumor cells [34,35]. A CAR consists of three domains: a single chain variable fragment (scFv) linked to a transmembrane domain, costimulatory domains, and a T-cell activation domain [36]. First-generation CAR T-cells contained only a single signaling unit, derived from the cluster of differentiation 3ζ (CD3ζ) chain or γ chains of the high-affinity IgE receptor (FcεRIγ), as an intracellular signaling domain. However, due to their restricted cytokine secretion and T-cell production, both types showed very weak antitumor activity in the killing of tumor cells [37]. Further evolutions of CARs improved their therapeutic safety and efficacy by adding one or more costimulatory molecules. Thus, second-generation CARs had a single costimulatory domain derived from either CD28 or TNF receptor superfamily member 9 (4-1BB), and third-generation CARs had two costimulatory domains, such as CD27 plus 4-1BB or CD28 plus tumor necrosis factor receptor superfamily, member 4 (OX40). (Fig. 4) [38].
The first gene-modified CAR T-cell therapy, formerly known as CTL019, yielded a remarkable response in patients with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL), resulting in approval of this therapeutic approach in the United States [39]. The excellent outcome of anti-CD19 CAR T-cell therapy against B-ALL motivated the development of myeloma cell-specific CAR T-cells. Requirements for the target antigen, a crucial factor in CAR development, were that it was expressed uniformly and specifically on all malignant cells (on-target) but, to avoid toxicity, not by normal tissues (off-target). Several antigens have been studied as feasible myeloma targets for anti-myeloma CAR T-cells, including CD44 variant 6, CD70, CD56, CD38, CD138, CD19, immunoglobulin kappa light chain, signaling lymphocytic activation molecule F7 (SLAMF7), and B-cell maturation antigen (BCMA) [40]. All have their limitations in terms of their use in MM. CD44 variant 6 is equally expressed on activated T-cells and monocytes [41], CD70 on activated lymphoid cells [42], and CD56 on NK cells, T-cells, and neuronal cells [43]. Although CD38 is normally expressed on precursor B-cells, plasma cells, T-cells, NK cells, and other tissue cells, it is highly expressed on malignant plasma cells, which has motivated the development of anti-CD38 CAR T-cells [44,45]. CD138 is also expressed on plasma cells and several tissue cells but it is nonetheless a good candidate target on myeloma cells [46,47]. The anti-CD19 CAR T-cells used in B-ALL showed impressive results against myeloma and may deplete myeloma stem cells, despite the minimal expression of CD19 on myeloma cells [48]. Immunoglobulin kappa light chain is expressed by mature B-cells but may be a target for the MM stem cell population expressing these surface immunoglobulins [49]. SLAMF7 is a promising target because of its strong expression on myeloma cells, despite its expression on plasma cells, NK cells, CD8+ T-cells, monocytes, B-cells, and DCs. Among these antigens, BCMA is currently being tested in several clinical trials and the preliminary results have been impressive. BCMA is uniformly expressed on all MM cells but not on hematopoietic stem cells or other immune cells [36].
Currently, four clinical trials of CAR T-cell therapy targeting BCMA are ongoing in patients with relapsed or refractory MM (Table 1). Raje et al. [50] presented the updated results of a multicenter study of bb2121 anti-BCMA CAR T-cell therapy in patients with heavily pretreated MM. The 43 patients received 50–800 × 106 CAR T-cells after lymphodepletion with cyclophosphamide and fludarabine. A dose-escalation study showed that a minimum of 150 × 106 CAR T-cells were needed to achieve an optimal outcome. An assessment of the overall response rate (ORR) showed that 77% of the patients had a complete response (CR), including 44% with a stringent CR (sCR), with comparable rates in patients with high- and low-BCMA-expressing tumors. Median progression-free survival following treatment with ≥ 150 × 106 cells was 11.8 months. In addition, bb2121 CAR T-cell therapy was relatively well tolerated. Cytokine release syndrome (CRS) of any grade developed in 63% of the patients, but in all cases it was manageable. Safety and efficacy data for BCMA-specific CAR T-cells (CART-BCMA) in the treatment of refractory MM were also reported [51]. CART-BCMA had activity in patients with heavily pre-treated MM, with an ORR of 46%, and its activity was not clearly associated with baseline BCMA expression or soluble BCMA concentration. The main non-hematological toxicities associated with CART-BCMA were CRS and neurotoxicity. CRS of any grade developed in 83% of the patients and ≥ grade 3 CRS in 29%. Smith et al. [52] presented the results of a phase I study of MCARH171 (human scFv-derived BCMA-targeted CAR T-cells) in patients with relapsed or refractory MM. Six patients who received 0.7–8 × 108 CAR T-cells after undergoing lymphodepletion with cyclophosphamide and fludarabine therapy experienced manageable CRS; there was no case of CRS ≥ grade 3. In addition, Chinese investigators reported the results achieved with LCAR-B38, which targets BCMA [53]. Forty pat ients who had received at least three prior lines of therapy and whose malignant plasma cells had > 10% BCMA expression were included in the study. The ORR in 22 evaluable patients was a striking 100%, with 64% of patients achieving sCR. CRS of any grade was seen in 85% and CRS ≥ grade 3 in 8.6%. Finally, the results of an interesting study of the combined infusion of CD19- and BCMA-specific CAR T-cells in patients with relapsed or refractory MM were recently reported [54]. A deep and long-term remission in patients treated with both CD19 CAR T-cells and BCMA CAR T-cells was anticipated, because of the association of disease relapse with myeloma stem cells. Nine of the 10 evaluable patients achieved a partial response or better. CRS occurred in all patients. Long-term follow-up results are needed to determine whether this combined approach improves outcomes.
TCRs are expressed on the surface of T-cells and recognize both intracellular and extracellular antigens. However, many tumor cells down-regulate the expression of MHC molecules to escape from immune cells. TCRs recognize and bind to peptides loaded onto MHCs, resulting in the activation of several signaling cascades and, in turn, protein phosphorylation. Among the proteins involved in these signaling cascades are nuclear factor of activated T-cell (NFAT) proteins and nuclear factor Fos. Their activation results in that of T-cells and, thus, the release of IFN-γ, granzyme B, and IL-2 [55]. TCR-modified T-cells are engineered to encode receptors for the tumor antigen peptide-MHC complex. Genetically engineered TCR are established by modifying the specificity of the α and β chains of the TCR to a particular tumor antigen for enhanced antigen recognition [47].
Currently, engineered TCR therapies mainly focus on cancer testis antigens (CTAs), which are highly expressed by tumors and expressed only by male germ cells in the testis and placenta but not in adult normal tissues [56]. Thus, engineered TCRs targeting CTAs are a promising therapy for cancer [56,57]. TCR-engineered T-cells targeting CTAs loaded onto human leukocyte antigen (HLA)-A have been employed in the treatment of MM. A phase I/II study of NY-ESO-1 targeting engineered TCRs was conducted in 20 patients with advanced MM and sarcoma. The results showed a significant clinical response and the remarkable safety of this approach, as none of the patients experienced CRS or other infusion-related toxicities. Recent innovative clinical trials to develop engineered TCRs against MM have focused on CTAs, neoantigens, and TAAs, such as melanoma antigen recognized by T-cells 1 (MART1), MAGE family member A3 (MAGE A3), and L antigen family member 1 (LAGE-1) [57,58].
NK cells are a subset of peripheral blood lymphocytes characterized as effector cells of the innate immune system, with potent cytotoxic activity toward cancer cells or infected cells [59]. Target-cell killing by NK cells occurs via perforin/granzymes granule-mediated lysis and death receptor interaction. NK cells also lyse target cells coated with antibodies to the antigen on the tumor cell surface, by antibody-dependent cellular cytotoxicity (ADCC). Target cell recognition is mediated by the signals delivered through several activating and inhibitory receptors of NK cells, including natural killer G2D (NKG2D), DNAX accessory molecule-1 (DNAM), and natural cytotoxicity receptors such as NKp30, NKp44, NKp46 as activating receptors, and killer-cell immunoglobulin-like receptors (KIRs), heterodimeric C-type lectin receptor (NKG2A/CD94), and check point T cell immunoreceptor with Ig and ITIM domains (TIGIT) as inhibitory receptors [60]. The advantage of NK cell therapy is that it results in the killing of various target cancer cells without prior sensitization. It also does not cause graft versus host disease (GVHD) [61]
Myeloma cells evade host immunity through various mechanisms, such as the activation of tumor suppressive pathways and suppressive cytokines (i.e., IL-6, IL-10). Among the involved immune cells are NK cells, which become weak or dysfunctional within the MM micro-environment. In patients with advanced MM, the number of circulating NK cells is reduced and their function is suppressed [62]. Advances in the ex vivo activation and expansion of NK cells to obtain adequate cell numbers have been reported, including the demonstration of an anti-MM effect of NK cells [63-65]. In attempts to develop NK cell therapies for MM, although the clinical safety of autologous ex vivo expanded NK cells infusion in patients with MM has been shown, remarkable clinical outcomes have not been achieved [66]. In contrast, allogeneic NK cells [60,61] treated with proteasome inhibitors and other anti-myeloma drugs, such as bortezomib or iMiDs, were recently shown to significantly induce NK cell effector function by inducing the expression of NK activating receptors [6]. A phase I trial evaluated the use of cord blood (CB)-derived ex vivo expanded NK cells as part of a conditioning regimen with high-dose melphalan and lenalidomide before autologous stem cell transplantation in 12 patients with MM, with high-risk characteristics. Ten of the patients achieved, at least, a very good partial response, including eight with a CR, without any significant infusion toxicities or GVHD. Interestingly, CB-NK cells were detected as an activated phenotype (NKG2D+/NKp30+) in vivo until day 21 after autologous stem cell transplantation [67]. There are several ongoing trials of NK cell therapy aimed at enhancing activity against MM, such as the use of NK cells in combination therapy with iMiDs including lenalidomide and pomalidomide, checkpoint inhibitors [68], and monoclonal antibodies to improve clinical efficiency (Fig. 5) [64,68].
Analogous to CAR T-cells, several new NK-based approaches, such as the use of NK cell lines (i.e., NK-92-CS1-CAR cells) [69], other genetic engineering approaches [60], and the generation CAR NK cells specifically targeting MM cells [70,71], have been developed. In preclinical models, CAR-engineered NK cells specific to SLAMF7 showed anti-MM activity [69], and bispecific forms redirecting NK cells to tumors have been developed for other hematological cancers [72]. With the innovative techniques in NK cell biology that are continually being developed, the prospects of novel, effective NK cell-based therapies for MM are eagerly anticipated.
Cellular immunotherapy offers great hope for the treatment of myeloma, given the encouraging results of preclinical and clinical trials. Cancer immunotherapy using DCs, NK cells, and genetically engineered T-cells has shown promise for the treatment of MM. The rapidly developing technologies support advanced strategies, including those relying on gene modification of effector cells, anti-myeloma drugs, and antibodies. These cellular immunotherapies are expected to play an important role in improving the outcome of patients with MM.
Acknowledgments
This work was supported by grants (NRF-2018R1C1B5041536, 2018R1A5A2024181) from the National Research Foundation of Korea (NRF), funded by the Korea government (MSIT).
Figure 1.
Immune response induced by different modes of tumor antigen-pulsed dendritic cells (DCs). The source of the antigen significantly influences DC function, as evidenced by the activation of tumor-specific T-cells. When DCs are pulsed with only a single tumor antigen, in the form of a single peptide, a DNA or RNA fragment encoding a single antigen, or a single idiotype (Id) protein, they induce the expansion of active monoclonal cytotoxic T lymphocytes (CTLs) that may exert cytotoxic effects on multiple myeloma (MM) cells. However, MM cells can elicit immunosuppressive and inhibitory signals or express different tumor antigens, which lead to the escape of the tumor from these monoclonal CTLs. To overcome these limitations, DCs pulsed with whole tumor lysates, multi-peptides, a cocktail of tumor-associated antigens (TAAs), fusion proteins, or whole tumor-derived DNA or RNA are used to induce the activation of multiple tumor-antigen-specific polyclonal CTLs covering almost all tumor-specific antigen targets. The goal is improved antitumor efficiency and long-term MM control. In some cases, DCs are not fully mature or are not fully activated by tumor antigens and are thus unable to induce an immune response. TA, tumor antigen.
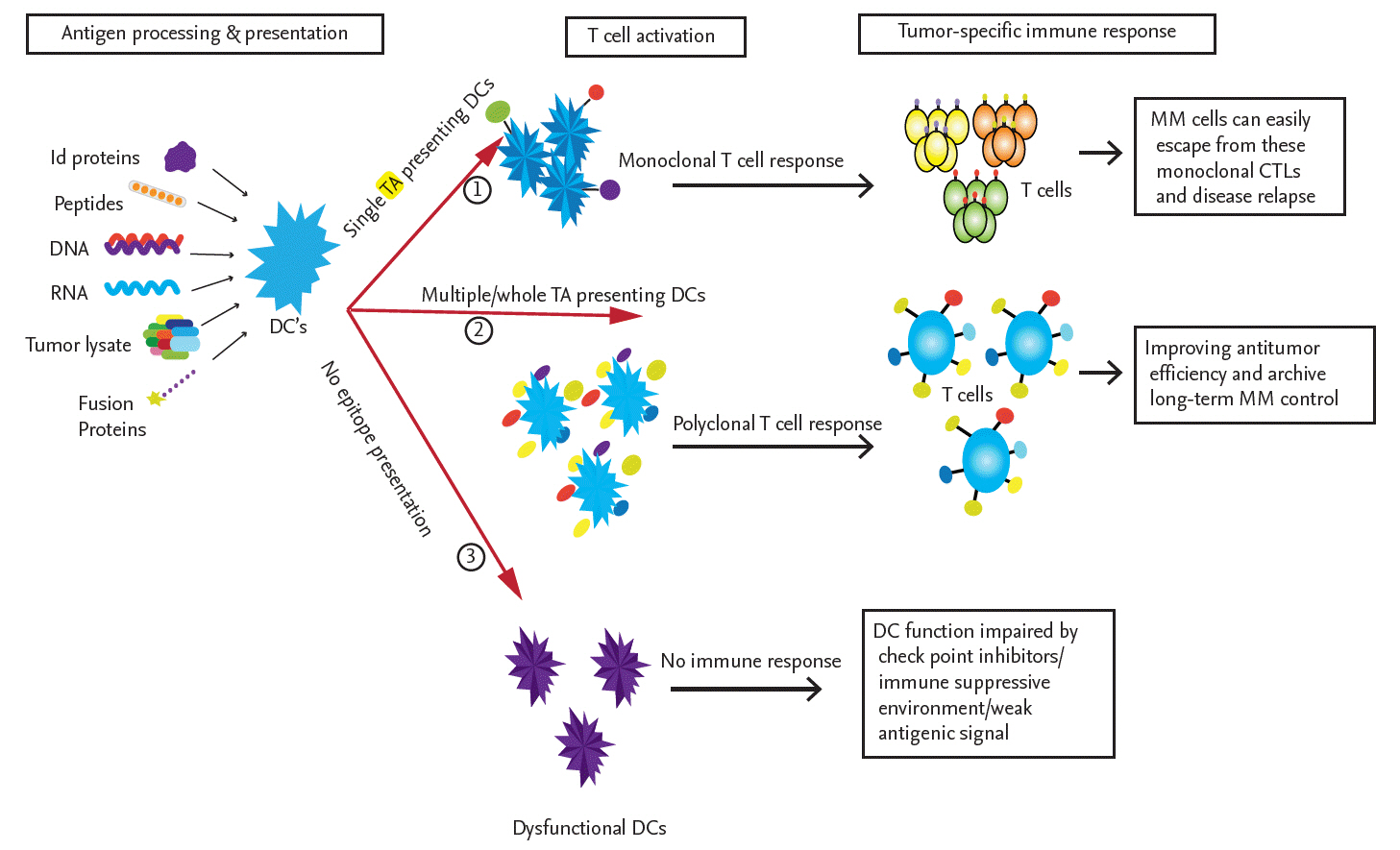
Figure 2.
Process of clinical dendritic cell (DC) vaccination in patients with multiple myeloma. The DCs of patients with multiple myeloma (MM) are functionally impaired because of the tumor microenvironment. DCs cultured ex vivo and used to vaccinate MM patients can overcome the immune dysregulation. Monocytes obtained from patients with MM are differentiated into immature DCs during their in vitro culture with interleukin 4 (IL-4) and granulocyte-macrophage colony-stimulating factor (GM-CSF). Immature DCs are then maturated with various stimuli (cytokines, cluster of differentiation 40 ligand [CD40L], survival factors or toll-like receptor [TLR] agonist) and loaded with various tumor-associated antigens using techniques such as the administration of peptides and proteins with immune adjuvants, tumor cell lysates, fusion protein, tumor cells manipulated to express cytokines, tumor cell apoptotic bodies, DNA and RNA encoding an antigen, or viral-based vectors to express antigen in the context of co-stimulatory molecules. Multiple modalities with adjuvants, immunomodulatory drugs, checkpoint blockades, and other therapeutic agents are necessary to enhance the efficacy of DC vaccination and, thus, suppress the tumor microenvironment. Numerous variables, such as dose, frequency, and route of DC vaccination also need to be optimized to induce an MM specific immune response effectively in both primary and secondary lymphoid organs. CTL, cytotoxic T lymphocyte.
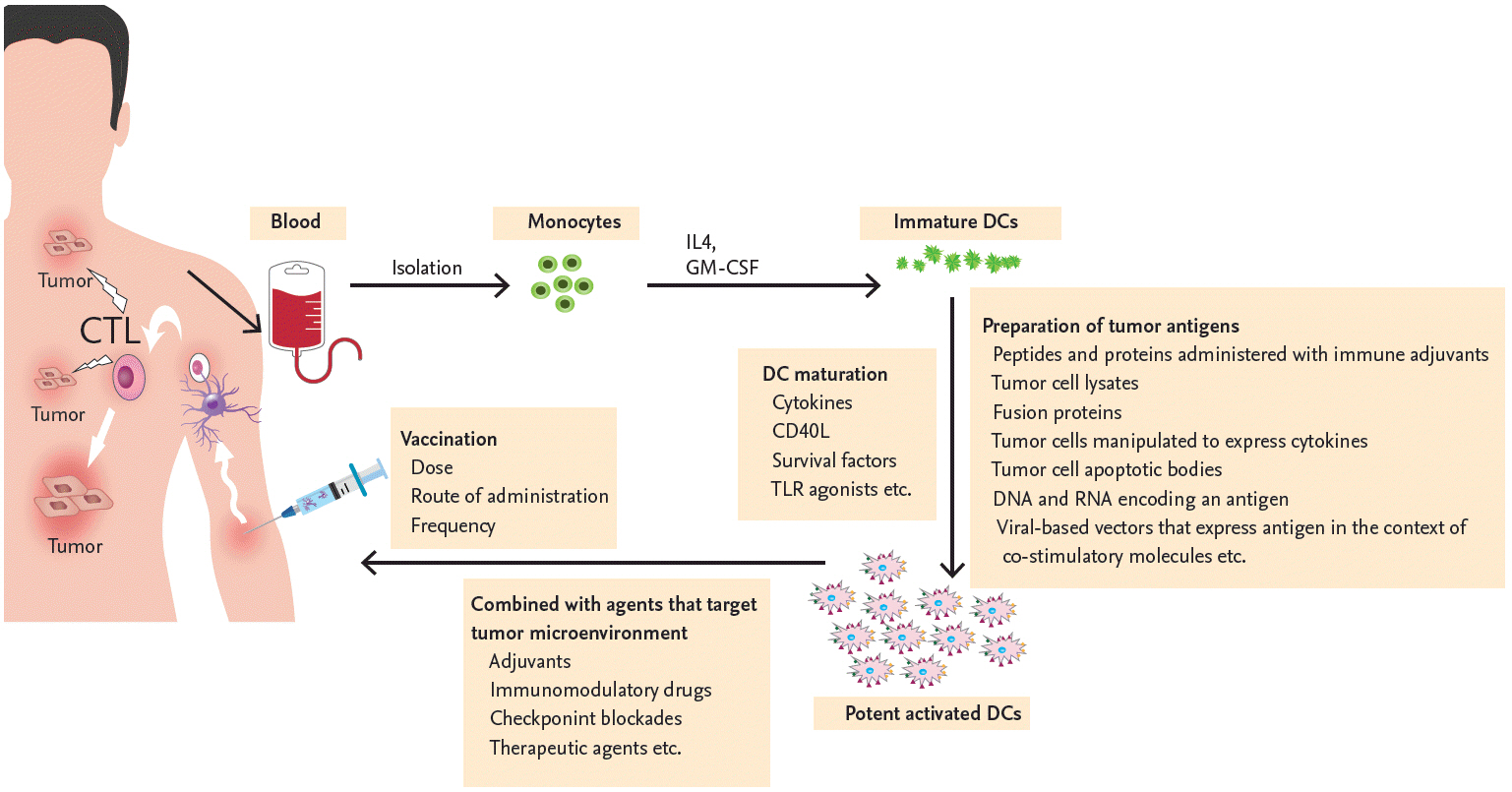
Figure 3.
Scheme of genetically engineered T-cell therapy in patients with multiple myeloma (MM). T-cells were isolated from the peripheral blood of patients with MM via apheresis and then transfected with the genes containing chimeric antigen receptor (CAR)-based tumor antigen by lentiviral, gammaretroviral or transposon/transposase approaches. Adoptive transfer of in vitro generated autologous CAR T-cells was conducted in patients with or without prior lymphodepletion. TCR, T-cell receptor.
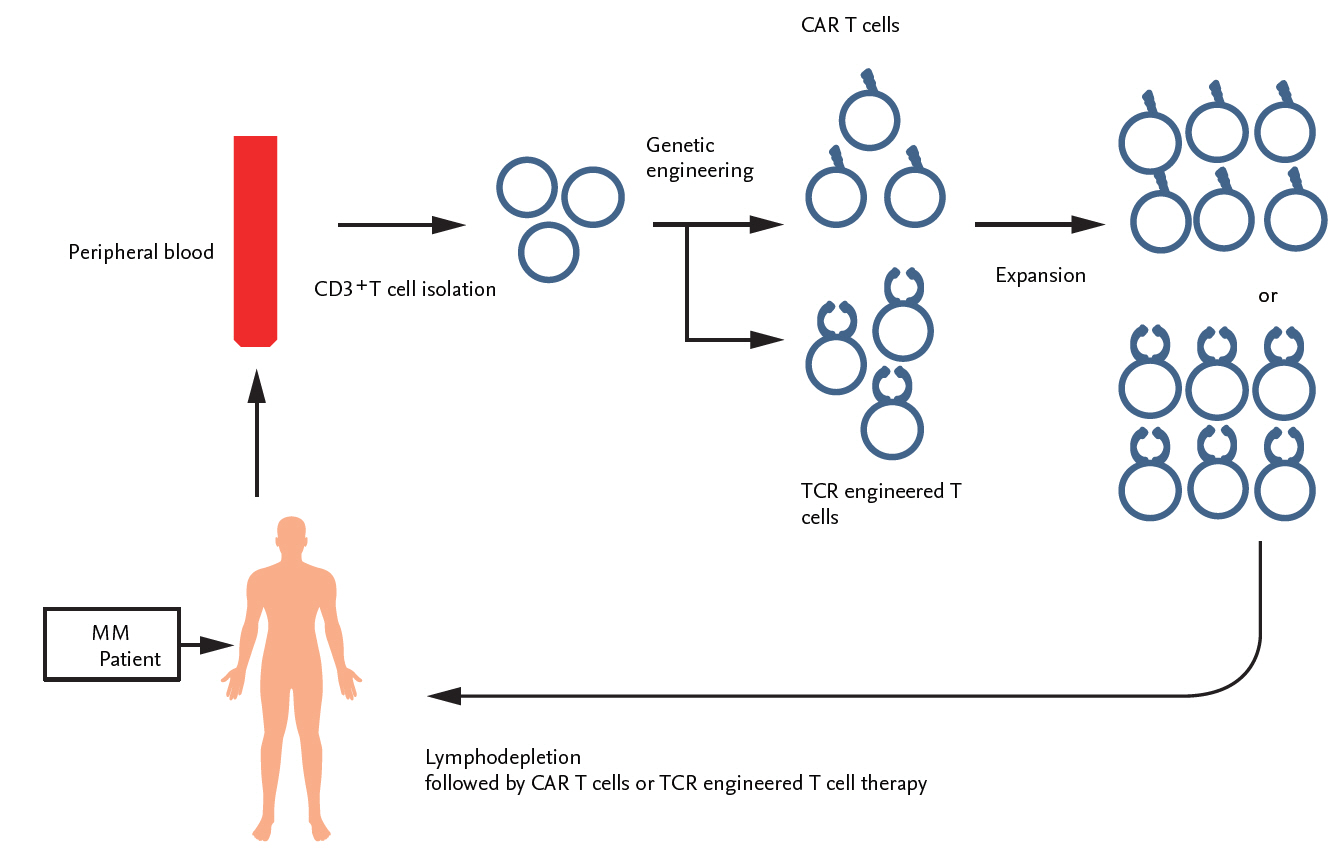
Figure 4.
The generations of chimeric antigen receptor T-cells. Chimeric antigen receptors (CARs) target tumor antigen independently of major histocompatibility complex I (MHC-I). They consist of an ectodomain, a hinge domain, a transmembrane domain, and an endodomain. First-generation CARs consisted of single chain variable fragment (scFv) (light chain variable region [VL] and heavy chain variable region [VH]) and cluster of differentiation 3ζ (CD3ζ) alone. Second-generation CARs were generated to mediate T-cell activation by the immunoreceptor tyrosine-based activation motif (ITAM) of the CD3ζ chain with a single costimulatory molecule, either CD28 or 4-1BB. Improved third-generation CARs were generated by combining the ITAM of CD3ζ chain with two costimulatory molecules, such as CD27 plus 4-1BB or CD28 plus OX40.
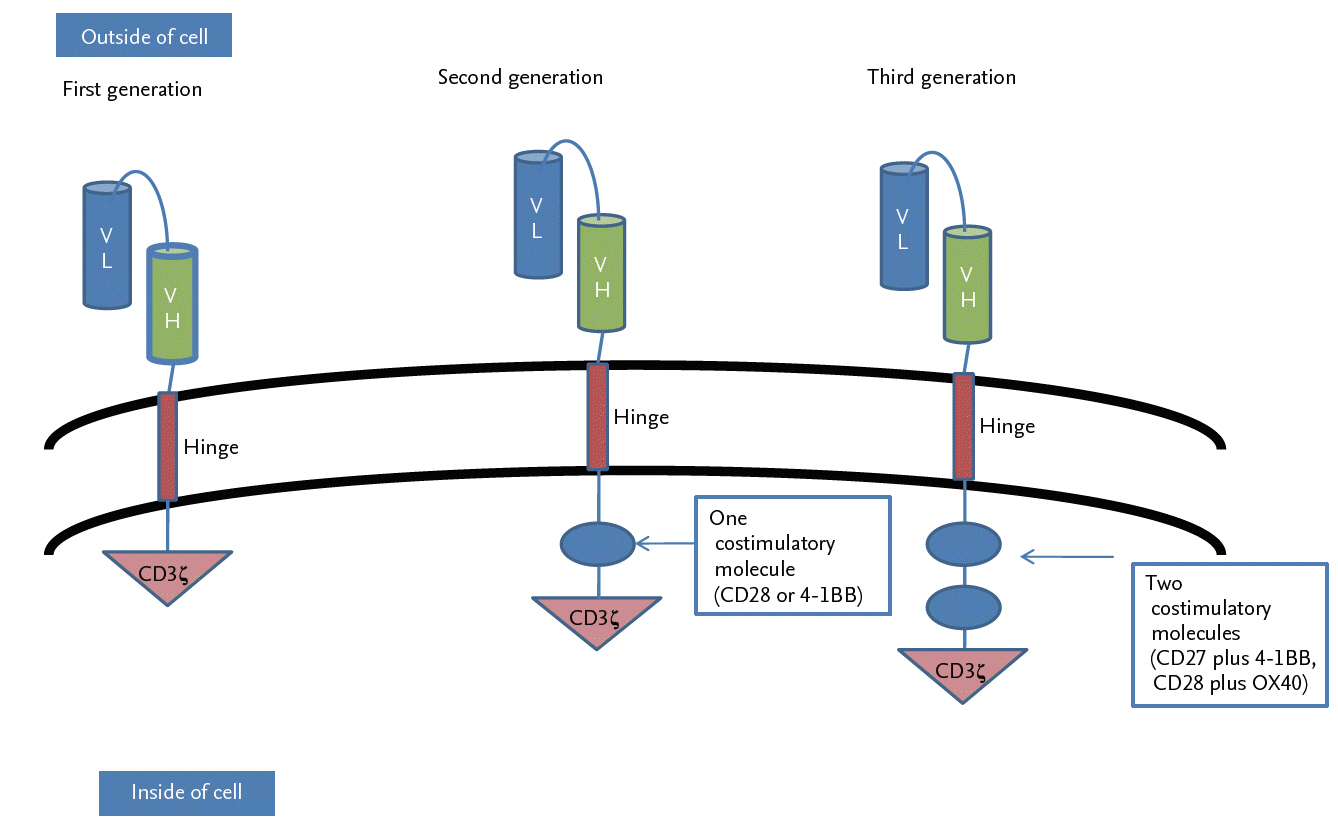
Figure 5.
Recent therapeutic approaches to enhance natural killer (NK) cytotoxicity against multiple myeloma (MM). This schematic shows how various therapeutic agents modulate NK cell-mediated cytotoxicity to target myeloma cells. Suppressive immune cells as well as bone marrow stromal cells (BMSCs) inside the tumor microenvironment are negative regulators of NK cell activation. The MM cells themselves develop several strategies to evade NK-cell-mediated killing. New immune modulators, including immunomodulatory drugs (iMiDs), immune checkpoint inhibitors, such as anti-programmed cell death 1 (PD-1)/PD-L1, anti-NKG2A, anti-TIGIT blocking antibodies, and proteasome inhibitors, target and suppress immunosuppressive factors in the MM microenvironment and enhance the cytotoxic effect of NK cells to kill MM cells. In addition, the antibodies used to treat MM, such as elotuzumab and daratumumab, induce NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC). TIGIT, T cell immunoreceptor with Ig and ITIM domains; CD16, cluster of differentiation 16; NKG2A, natural killer G2A; MDSC, myeloid-derived suppressor cell; MHC, major histocompatibility complex.

Table 1.
Results of clinical trials of CART-BCMA therapy in multiple myeloma
REFERENCES
3. Hong J, Lee JH. Recent advances in multiple myeloma: a Korean perspective. Korean J Intern Med 2016;31:820–834.




4. Lonial S, Cavenagh J. Emerging combination treatment strategies containing novel agents in newly diagnosed multiple myeloma. Br J Haematol 2009;145:681–708.


5. Attal M, Harousseau JL. The role of high-dose therapy with autologous stem cell support in the era of novel agents. Semin Hematol 2009;46:127–132.


6. Dosani T, Carlsten M, Maric I, Landgren O. The cellular immune system in myelomagenesis: NK cells and T cells in the development of myeloma [corrected] and their uses in immunotherapies. Blood Cancer J 2015;5:e306.




8. Guillerey C, Nakamura K, Vuckovic S, Hill GR, Smyth MJ. Immune responses in multiple myeloma: role of the natural immune surveillance and potential of immunotherapies. Cell Mol Life Sci 2016;73:1569–1589.



9. Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell 2017;168:724–740.



10. Bensinger W, Rotta M, Storer B, et al. Allo-SCT for multiple myeloma: a review of outcomes at a single transplant center. Bone Marrow Transplant 2012;47:1312–1317.




11. Quach H, Ritchie D, Stewart AK, Neeson P, Harrison S, Smyth MJ, et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 2010;24:22–32.




12. Vo MC, Nguyen-Pham TN, Lee HJ, et al. Combination therapy with dendritic cells and lenalidomide is an effective approach to enhance antitumor immunity in a mouse colon cancer model. Oncotarget 2017;8:27252–27262.



13. Vo MC, Nguyen-Pham TN, Lee HJ, et al. Chaetocin enhances dendritic cell function via the induction of heat shock protein and cancer testis antigens in myeloma cells. Oncotarget 2017;8:46047–46056.



14. Al-Hujaily EM, Oldham RA, Hari P, Medin JA. Development of novel immunotherapies for multiple myeloma. Int J Mol Sci 2016;17:E1506.



15. Rosenblatt J, Avigan D. Targeting the PD-1/PD-L1 axis in multiple myeloma: a dream or a reality? Blood 2017;129:275–279.



16. Jung SH, Lee HJ, Vo MC, Kim HJ, Lee JJ. Immunotherapy for the treatment of multiple myeloma. Crit Rev Oncol Hematol 2017;111:87–93.


17. Kim YS, Park HJ, Park JH, et al. A novel function of API5 (apoptosis inhibitor 5), TLR4-dependent activation of antigen presenting cells. Oncoimmunology 2018;7:e1472187.



18. Ratta M, Fagnoni F, Curti A, et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood 2002;100:230–237.



19. Vo MC, Anh-NguyenThi T, Lee HJ, et al. Lenalidomide enhances the function of dendritic cells generated from patients with multiple myeloma. Exp Hematol 2017;46:48–55.


20. Liso A, Stockerl-Goldstein KE, Auffermann-Gretzinger S, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood progenitor cell transplantation for multiple myeloma. Biol Blood Marrow Transplant 2000;6:621–627.


21. Jung SH, Lee YK, Lee HJ, et al. Dendritic cells loaded with myeloma cells pretreated with a combination of JSI-124 and bortezomib generate potent myeloma-specific cytotoxic T lymphocytes in vitro. Exp Hematol 2014;42:274–281.


22. Choi NR, Lee HJ, Jung SH, et al. Generation of potent dendritic cells with improved migration ability through p-cofilin and sarco/endoplasmic reticulum Ca(2+) transport ATPase 2 regulation. Cytotherapy 2015;17:1421–1433.


23. Hoang MD, Jung SH, Lee HJ, et al. Dendritic cell-based cancer immunotherapy against multiple myeloma: from bench to clinic. Chonnam Med J 2015;51:1–7.



24. Hoang MD, Lee HJ, Lee HJ, et al. Branched polyethylenimine-superparamagnetic iron oxide nanoparticles (bPEI-SPIONs) improve the immunogenicity of tumor antigens and enhance Th1 polarization of dendritic cells. J Immunol Res 2015;2015:706379.




25. Hong CY, Lee HJ, Choi NR, et al. Sarcoplasmic reticulum Ca(2+) ATPase 2 (SERCA2) reduces the migratory capacity of CCL21-treated monocyte-derived dendritic cells. Exp Mol Med 2016;48:e253.




26. Lee HJ, Choi NR, Vo MC, Hoang MD, Lee YK, Lee JJ. Generation of multiple peptide cocktail-pulsed dendritic cells as a cancer vaccine. Methods Mol Biol 2014;1139:17–26.


27. Vo MC, Lee HJ, Kim JS, et al. Dendritic cell vaccination with a toll-like receptor agonist derived from mycobacteria enhances anti-tumor immunity. Oncotarget 2015;6:33781–33790.



28. Jung SH, Lee HJ, Lee YK, et al. A phase I clinical study of autologous dendritic cell therapy in patients with relapsed or refractory multiple myeloma. Oncotarget 2017;8:41538–41548.



29. Neuber B, Herth I, Tolliver C, et al. Lenalidomide enhances antigen-specific activity and decreases CD45RA expression of T cells from patients with multiple myeloma. J Immunol 2011;187:1047–1056.


30. Nguyen-Pham TN, Jung SH, Vo MC, et al. Lenalidomide synergistically enhances the effect of dendritic cell vaccination in a model of murine multiple myeloma. J Immunother 2015;38:330–339.


31. Vo MC, Yang S, Jung SH, et al. Synergistic antimyeloma activity of dendritic cells and pomalidomide in a murine myeloma model. Front Immunol 2018;9:1798.



32. Rosenblatt J, Glotzbecker B, Mills H, et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother 2011;34:409–418.



33. Vo MC, Jung SH, Chu TH, et al. Lenalidomide and programmed death-1 blockade synergistically enhances the effects of dendritic cell vaccination in a model of murine myeloma. Front Immunol 2018;9:1370.



34. Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol 2013;10:267–276.




35. Ramos CA, Savoldo B, Torrano V, et al. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J Clin Invest 2016;126:2588–2596.



36. Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 2015;125:4017–4023.




37. Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov 2013;3:388–398.



38. Zhao Z, Condomines M, van der Stegen SJC, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 2015;28:415–428.



39. First-ever CAR T-cell therapy approved in U.S. Cancer Discov 2017;7:OF1.
40. Mikkilineni L, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood 2017;130:2594–2602.




41. Mackay CR, Terpe HJ, Stauder R, Marston WL, Stark H, Gunthert U. Expression and modulation of CD44 variant isoforms in humans. J Cell Biol 1994;124:71–82.




42. Kawamura T, Ogawa Y, Shimozato O, et al. CD70 is selectively expressed on Th1 but not on Th2 cells and is required for Th1-type immune responses. J Invest Dermatol 2011;131:1252–1261.


43. Van Acker HH, Capsomidis A, Smits EL, Van Tendeloo VF. CD56 in the immune system: more than a marker for cytotoxicity? Front Immunol 2017;8:892.



44. Drent E, Themeli M, Poels R, et al. A rational strategy for reducing on-target off-tumor effects of cd38-chimeric antigen receptors by affinity optimization. Mol Ther 2017;25:1946–1958.



45. Harada H, Kawano MM, Huang N, et al. Phenotypic difference of normal plasma cells from mature myeloma cells. Blood 1993;81:2658–2663.



46. Podar K, Anderson KC. The evolution and maintenance of the multiple myeloma cell clone within the liquid bone marrow compartment: therapeutic implications. In: Bradshaw RA, Dennis EA, eds. Handbook of Cell Signaling. Second Edition. Burlington (VT): Academic Press, 2010;2799–2809.
47. Guo B, Chen M, Han Q, et al. CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)-modified T cells for multiple myeloma. J Cell Immunother 2016;2:28–35.

48. Garfall AL, Stadtmauer EA, Hwang WT, et al. Anti-CD19 CAR T cells with high-dose melphalan and autologous stem cell transplantation for refractory multiple myeloma. JCI Insight 2018;3:e120505.



49. Matsui W, Wang Q, Barber JP, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res 2008;68:190–197.



50. Raje NS, Berdeja JG, Lin Y, et al. bb2121 Anti-BCMA CAR T-cell therapy in patients with relapsed/refractory multiple myeloma: updated results from a multicenter phase I study. J Clin Oncol 2018;36(15 Suppl):8007.

51. Cohen AD, Garfall AL, Stadtmauer EA, et al. Safety and efficacy of B-cell maturation antigen (BCMA)-specific chimeric antigen receptor T cells (CART-BCMA) with cyclophosphamide conditioning for refractory multiple myeloma (MM). Blood 2017;130(Suppl 1):505.
52. Smith EL, Staehr M, Masakayan R, et al. Development and evaluation of an optimal human single-chain variable fragment-derived BCMA-targeted CAR T cell vector. Mol Ther 2018;26:1447–1456.



53. Fan F, Zhao W, Liu J, et al. Durable remissions with BCMA-specific chimeric antigen receptor (CAR)-modified T cells in patients with refractory/relapsed multiple myeloma. J Clin Oncol 2017;35(18 Suppl):LBA3001.

54. Yan L, Shang J, Kang L, et al. Combined infusion of CD19 and Bcma-specific chimeric antigen receptor T cells for RRMM: initial safety and efficacy report from a clinical pilot study. Blood 2017;130(Suppl 1):506.
55. Ping Y, Liu C, Zhang Y. T-cell receptor-engineered T cells for cancer treatment: current status and future directions. Protein Cell 2018;9:254–266.




56. Kunert A, Straetemans T, Govers C, et al. TCR-engineered T cells meet new challenges to treat solid tumors: choice of antigen, T cell fitness, and sensitization of tumor milieu. Front Immunol 2013;4:363.



57. Debets R, Donnadieu E, Chouaib S, Coukos G. TCR-engineered T cells to treat tumors: seeing but not touching? Semin Immunol 2016;28:10–21.


58. Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NYESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med 2015;21:914–921.




59. Malhotra A, Shanker A. NK cells: immune cross-talk and therapeutic implications. Immunotherapy 2011;3:1143–1166.



60. Rezvani K, Rouce RH. The application of natural killer cell immunotherapy for the treatment of cancer. Front Immunol 2015;6:578.



61. Marcus A, Gowen BG, Thompson TW, et al. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol 2014;122:91–128.



62. Tamura H. Immunopathogenesis and immunotherapy of multiple myeloma. Int J Hematol 2018;107:278–285.



63. Frohn C, Hoppner M, Schlenke P, Kirchner H, Koritke P, Luhm J. Anti-myeloma activity of natural killer lymphocytes. Br J Haematol 2002;119:660–664.


64. Garg TK, Szmania SM, Khan JA, et al. Highly activated and expanded natural killer cells for multiple myeloma immunotherapy. Haematologica 2012;97:1348–1356.



65. Shah N, Martin-Antonio B, Yang H, et al. Antigen presenting cell-mediated expansion of human umbilical cord blood yields log-scale expansion of natural killer cells with anti-myeloma activity. PLoS One 2013;8:e76781.



66. Szmania S, Lapteva N, Garg T, et al. Ex vivo-expanded natural killer cells demonstrate robust proliferation in vivo in high-risk relapsed multiple myeloma patients. J Immunother 2015;38:24–36.



67. Shah N, Li L, McCarty J, et al. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br J Haematol 2017;177:457–466.



68. Leivas A, Perez-Martinez A, Blanchard MJ, et al. Novel treatment strategy with autologous activated and expanded natural killer cells plus anti-myeloma drugs for multiple myeloma. Oncoimmunology 2016;5:e1250051.



69. Chu J, Deng Y, Benson DM, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia 2014;28:917–927.




70. Liu D, Tian S, Zhang K, et al. Chimeric antigen receptor (CAR)-modified natural killer cell-based immunotherapy and immunological synapse formation in cancer and HIV. Protein Cell 2017;8:861–877.




-
METRICS

- Related articles
-
Crystalline light chain cast nephropathy in multiple myeloma2021 July;36(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


