INTRODUCTION
Head and neck cancer ranks seventh highest in prevalence among Korean males, and adds 16.7 patients per 100,000 annually to the overall cancer burden in Korea [
1]. It is well-known that human papillomavirus (HPV) is a causative factor for head and neck squamous cell carcinoma (HNSCC), along with smoking and heavy alcohol consumption. HPV infection is associated with the development of oropharyngeal cancers (OPCs). Several studies, including a large meta-analysis, have reported that patients with HPV-positive HNSCC have better overall survival (OS) and disease-free survival (DFS) than patients with HPV-negative HNSCC [
2,
3]. Since the 5-year OS of HPV-positive HNSCC patients is approximately 80% to 90%, the use of de-escalation treatment for these patients is being investigated [
4]. The goal of de-escalation treatment is to maintain proper cure rates and to minimize long-term morbidity [
4]. Various approaches to less intensive treatment are being evaluated in clinical trials, including the following: (1) replacing cisplatin with an anti-epidermal growth factor receptor agent such as cetuximab; (2) decreasing the radiation dose; and (3) performing minimally invasive surgery such as transoral microendoscopic laser surgery.
However, despite these de-escalation attempts, the current practice guidelines do not recommend de-escalation treatment for HPV-positive HNSCC patients due to lack of evidence [
5]. In addition, despite the overall favorable outcomes of patients with HPV-positive OPC, some patients still relapse. To date, little is understood about the clinical and molecular features associated with poor outcomes in HPV-positive HNSCC patients, although a previous retrospective analysis found that patients with HPV-positive OPC who smoked for more than 10 pack years and had N2b-N3 disease had a 5-year survival of approximately 60% [
3]. Hence, we hypothesized that some HPV-positive HNSCC patients should not be candidates for treatment de-escalation. The aim of our study was to identify poor prognostic factors which may affect OS or DFS in HPV-positive HNSCC patients.
METHODS
Patient selection and collection of clinical data
Records of patients diagnosed with HNSCC at Seoul National University Hospital from January 2000 to February 2015 were retrospectively analyzed using an electronic database. Inclusion criteria were as follows: patients (1) with pathologically confirmed, locally advanced HNSCC (stage IŌĆōIII or stage IV M0); (2) treated with surgery, concurrent chemoradiation therapy (CCRT), or radiation therapy (RT); (3) older than 19 years at the time of diagnosis; and (4) with positive tests for HPV by liquid-bead microarray or DNA chip technology. Patients with an initial diagnosis of stage IV M1 disease or nasopharyngeal cancer were excluded. We investigated the location of primary tumor (oropharynx, hypopharynx, larynx, oral cavity, nasal cavity, or salivary gland) and the initial pathology (undifferentiated, poorly differentiated, moderately differentiated, or miscellaneous). Other baseline demographics (age, gender, smoking history, alcohol intake) and clinical data (Eastern Cooperative Oncology Group [ECOG] performance status [PS], and comorbidities including diabetes mellitus, hypertension, ischemic heart disease, liver cirrhosis, chronic obstructive pulmonary disease, and brain hemorrhage or stroke) were also obtained. We followed the guidelines outlined in the seventh edition of the American Joint Committee on Cancer to determine tumor staging [
6]. All procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards. This study was approved by the Institutional Review Board of Seoul National University Hospital (approval number: H-1508-083-695). Due to the retrospective nature of this study, patientsŌĆÖ consent to participate was waived in accordance with the Institutional Review Board.
Treatment and response evaluation
We retrospectively reviewed the electronic medical chart to determine whether induction treatment was present and the type of regimens used, the type of definitive treatment (surgery or non-surgery [CCRT or RT]), and adjuvant treatment modalities (CCRT, RT). The protocol for RT or CCRT in this cohort was described in prior reports [
7,
8]. Briefly, among the CCRT group, patients were directly given fractionated radiotherapy of more than 60 Gy for primary tumors and regional lymph nodes with concurrent use of chemotherapeutic agents (cisplatin, carboplatin, or cetuximab). The RT group followed the same protocol as that of the CCRT group, except that concurrent chemotherapy was not used.
We also evaluated the tumor response to induction treatment and definitive treatment using the Response Evaluation Criteria in Solid Tumors (version 1.1) [
9] and calculated the objective response rate (ORR) as the percentage of cases with a complete response (CR) or a partial response among all the patients whose tumor responses we were able to evaluate. During follow-up care, the disease status after the response to definitive treatment was classified into three categories: (1) relapse, defined as any evidence of disease recurrence after CR; (2) progression in non-CR, defined as disease progression when a patient did not achieve CR to definitive treatment; and (3) no relapse nor progression, defined as continued lack of evidence of disease. We also reviewed the site of relapse, including locoregional or distance relapse. If the relapsed disease was only limited to the area within the head and neck and adjacent structures including lymph nodes, the relapse was defined as a locoregional relapse, and the remaining were defined as distance relapse.
Tests for HPV infection
Tumor specimens were fixed in formalin and embedded in paraffin. After DNA was extracted from a processed specimen, either of the following HPV genotyping methods were used: (1) HPV DNA Chip [
10,
11] or (2) HPV Liquid Bead Microarray [
12].
The HPV DNA Chip is a polymerase chain reaction (PCR)-based DNA microarray system (MyGene, Seoul, Korea). Details on the methods performed after amplification were described previously [
11]. Briefly, 24 type-specific probes were utilized for HPV genotyping: 15 highrisk (HR) genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, and 68) and nine low-risk (LR) genotypes (6, 11, 34, 40, 42, 43, 44, 54, and 70). Samples appearing as a positive 150-bp band on gel electrophoresis but negative on the HPV DNA chip slide were designated as HPV-other.
The HPV Liquid Bead Microarray was performed using a PCR cycler and a Luminex analyzer (GeneFinder, Infopia Inc., Anyang, Korea). Hybridization of amplified DNA to probes coupled with beads in the Genefinder HPV Liquid Bead Microarray kit was performed according to the manufacturersŌĆÖ recommendations. Details of this process have been described previously [
12]. This method detects 32 kinds of HPV genotypes simultaneously: 18 HR types (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 69, and 73) and 14 LR types (6, 11, 32, 34, 40, 42, 43, 44, 54, 55, 62, 70, 81, and 83). An HPV copy number Ōēź 100 was designated as HPV-positive. The proportions of specimens positive for HPV genotypes 16 and 18 were determined, and other HR HPV genotypes were noted when present. Any sample positive for a HR genotype was defined as a HR type, and any sample positive for only a LR genotype was defined as a LR type.
Immunohistochemistry
Fixed and paraffin-embedded samples were evaluated by p16INK4a immunohistochemistry (IHC) using clone E6H4 of CINtec (Roche, Heidelberg, Germany) to determine the p16 status of a tumor specimen. The sample was considered positive for p16 if diffuse nuclear and cytoplasmic staining were present, and > 70% of cells were stained.
Statistical analysis
The primary outcome was OS, which was defined as the time from the date of diagnosis to the date of death, or last follow-up if censored, which was estimated using the Kaplan-Meier method. DFS was the secondary outcome, which was defined as the time from the date of diagnosis to the date of first confirmation of relapse or progression, also estimated by the Kaplan-Meier method. The independent variables used to construct a univariate logistic regression model for prognosis were demographic factors, disease-specific factors, HPV genotypes 16 and 18, and p16 status. Factors associated with OS and DFS were analyzed by univariate and multivariable Cox regression analyses, except for variables that did not satisfy the Cox proportional hazard assumption (nodal classification, definitive treatment, and the type of HPV testing method). If these variables were statistically significant in univariate analysis, they were included into multivariable model by stratification. Statistical significance was defined as p < 0.05. All statistical tests were two-sided and were carried out using STATA software version 12 (StataCorp LP, College Station, TX, USA).
DISCUSSION
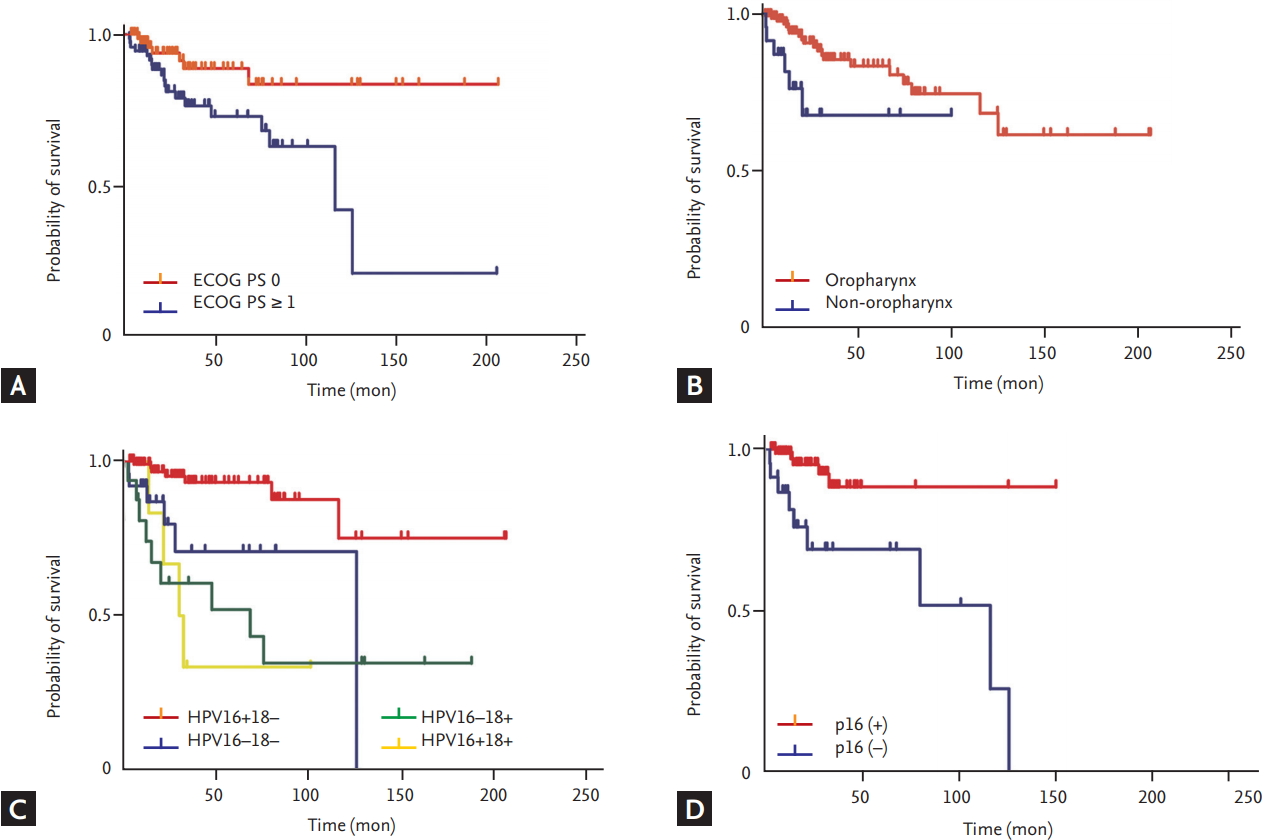
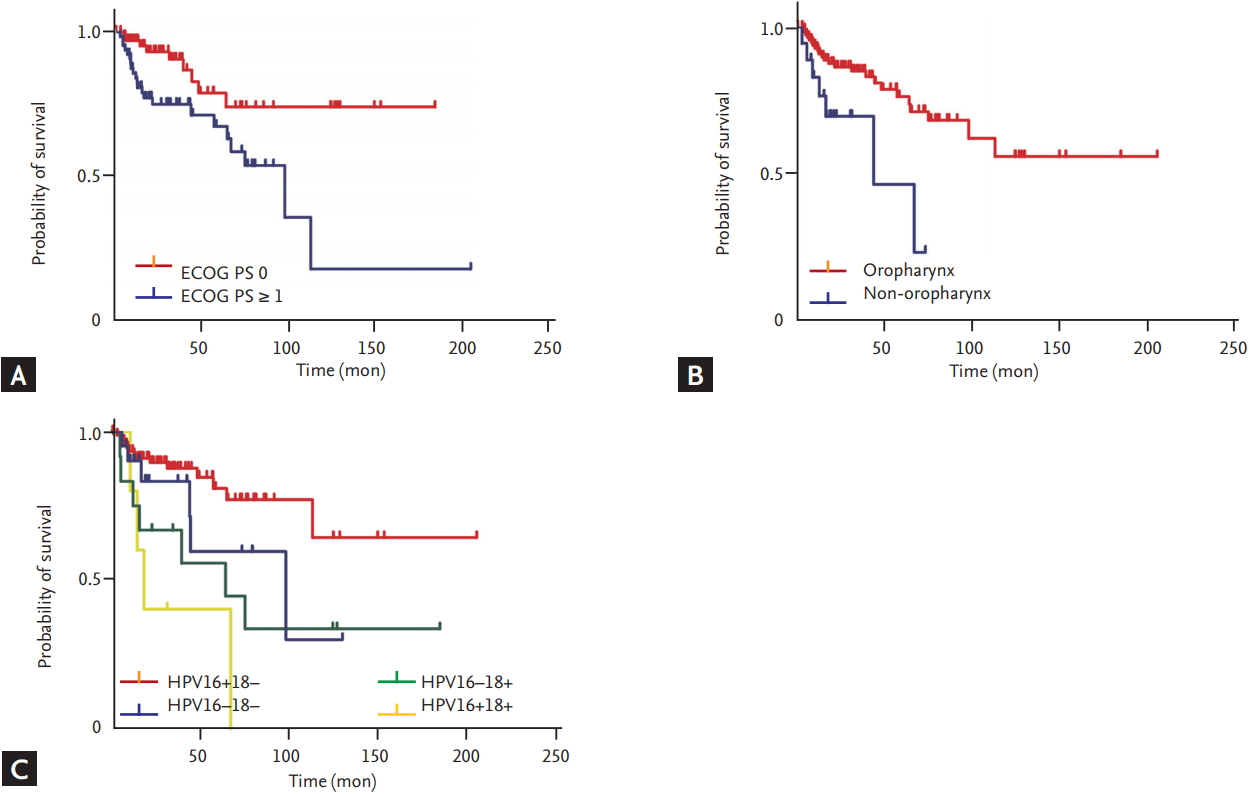
In this study, HPV-positive HNSCC had a CR rate exceeding 85%, and 70% of these patients continued to show no evidence of disease over the follow-up period. However, we found that patients with HPV genotype 18 infection, non-OPC, and poor PS relapsed and died earlier than patients without these prognostic factors.
HPV genotype 18 positivity was independently associated with poor outcome. Whereas the carcinogenicity of HPV genotype 16 is well established, the roles of the HR HPV genotype 18 in OPC and non-OPC are unclear. Single infections with HPV genotype 18 and coinfection by HPV genotypes 16 and 18 were found to be relatively less prevalent in OPCs than in non-OPCs in our study, as well as in a previous systematic review [
13]. On the other hand, HPV genotype 18 infections are known to be associated with aggressive disease and rapid progression [
14]. Moreover, HPV genotype 18 appears to have a different molecular expression pattern, which suggests a pathway to carcinogenesis different from that of HPV genotype 16 [
14]. Recent reports demonstrated that immune-related markers such as PD-L1 expression on tumor cells or immune cells may affect survival in HNSCC [
15]. However, there have been no definitive studies demonstrating that HPV genotype 18-positive HNSCC patients have different tumor immune infiltrates than HPV 16-positive HNSCC patients. According to a study comparing the survival and characteristics of HPV genotype 16 and other HPV genotypes [
16], HPV genotype 16 comprised 84% of 73 HPV-positive HNSCC samples. Among the 12 remaining samples harboring other HPV genotypes, there were eight HPV genotype 33, three HPV genotype 35, and one HPV genotype 56, and none of these samples were HPV genotype 18-postive. Several viral gene expressions differed between HPV genotype 16 and the other HPV-infected cohort, although the clinical characteristics and genomic aberration were similarly distributed. In addition, the other HPV-infected cohort had better 3-year OS than the HPV genotype 16 cohort. Taken together, these findings suggest that different levels of viral gene expression induced by HPV genotypes other than HPV genotype 16 might influence patient survival. Although limited data exist for HPV genotype 18, it is possible that similar mechanistic links may be present in correlation with this genotype. Further research is warranted to investigate the immune infiltrates in HPV genotype 18-positive HNSCC patients.
Non-OPC status predicted shorter OS and DFS than OPC in this study. The role of HR HPV in the carcinogenesis of non-OPCs remains unconfirmed [
3,
13]. A systematic review of the outcomes of patients with HPV-positive non-OPCs found inconsistent survival outcomes [
17]. This finding suggests that treatment de-escalation cannot be universally performed for patients with HPV-positive non-OPCs and indicates that HPV infection in non-OPCs may be a ŌĆ£bystanderŌĆØ infection.
Despite the good condition of our patients with HPV-positive HNSCCs overall, the subgroup with ECOG PS Ōēź 1 had shorter OS and DFS than the subgroup with ECOG PS 0. This result is similar to that of a previous study [
3].
Interestingly, the findings from our study showed that smoking and drinking were not associated with poorer survival. We suggest two explanations for this result. First, although smoking and alcohol abuse raise the risk of OPC occurrences [
18] and additional smoking increases the risk of death in HNSCC patients [
3], an association between smoking and HPV has not yet been confirmed. It has been reported previously that HPV positivity is higher in non-smokers than in smokers [
19-
21]. Second, because of our retrospective study design, limited information about smoking and drinking status were obtained. Further study is needed to determine the effect of smoking and drinking on survival in HPV-positive HNSCCs.
Our study had several limitations. First, because of its retrospective design, we did not have information about p16 expression for many of our study patients. We found that p16 positivity by IHC significantly affected OS but excluded it from the multivariable model because of the high rate of missing results and possibility of collinearity. However, a previous investigation has suggested that the link between p16 positivity by IHC and carcinogenic HPV genotype is not consistent; and some p16-positive tumors by IHC may not be positive for HR HPV [
14]. Moreover, p16 expression in non-OPC tumors (oral cavity, hypopharynx, and larynx) is present in only 14% to 24% of patients [
14]. Therefore, p16 positivity by IHC may be a poor surrogate marker. Second, there may be potential selection bias while capturing the HPV-positive population because in our institution, HPV genotyping methods, which cost approximately $120 per test, are not covered by national insurance. Therefore, for 15 years, we did not determine HPV positivity for all head and neck cancer patients. As a result, patients with higher socioeconomic status may be more heavily included in this analysis. However, to the best of our knowledge, other demographic and clinicopathologic factors did not impact whether HPV testing was performed. Third, we used either the DNA chip or liquid bead microarray assay for detecting HPV DNA, and the sensitivity and specificity of these two assays differ. It is possible that these distinct methods may raise selection bias. However, there is no gold-standard method to assess HPV genotypes to date [
22]. Moreover, to overcome the possibility of bias, we included the HPV testing method as a stratification variable in the multivariable analysis. Fourth, due to the retrospective design of this study, there might be a bias that results from differing treatment modalities. Although we have attempted to reduce the bias by including definitive treatment as a stratification variable in the analysis, its influence should be considered when interpreting the results. Finally, this study spanned 15 years, during which clinical management of HNSCC likely changed, and this may have influenced the correlates of OS and DFS.
Notwithstanding these limitations, we believe that the OS and DFS of HPV-positive patients who are identified by the present technology can be predicted based on specific clinical factors, and that patients with HPV-positive HNSCCs with unfavorable features are probably not candidates for de-escalation treatment. This study is also a valuable hypothesis-generating study that provides a basis for future in-depth studies investigating the link between HPV genotype 18 and poor prognosis.
In conclusion, HPV-positive HNSCC patients with poor PS and a primary HPV genotype 18-positive tumor located in sites other than the oropharynx may have a poor prognosis. These patients might not be candidates for de-escalation treatment.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



