INTRODUCTION
Paraquat (PQ; 1,10-dimethyl-4,40-bipyridinium dichloride) is a highly toxic compound, and its use is rigorously restricted in many industrialized countries, such as the United States, South Korea, and the members of the European Union. Nevertheless, PQ is still widely used as an herbicide in many developing countries worldwide. Several cases of acute poisoning and even death due to this compound have been reported over the past few decades [1,2]. The lung is a main target organ because PQ is actively taken up by the alveolar epithelium, which causes pulmonary injury by acute intoxication or long-term accumulation [3,4]. The mechanism of PQ toxicity involves a series of cyclic reduction-oxidation reactions inducing many inflammatory agents, which eventually become progressive lung fibrosis; this mechanism plays an essential role in producing lethal hypoxemia after PQ poisoning [5,6].
Cyclophosphamide (CTX) is an alkylating agent with cytotoxic and immunosuppressive properties. CTX, either as a sole agent or in combination with glucocorticoids, is frequently used to treat a variety of inflammatory and fibrosis conditions [7,8]. Although prolonged low-dose CTX provides potential benefits in previous case and nonrandomized trials with a small sample size [9-12], the possibility of spontaneous recovery is undetermined. Given the lack of strong evidence of prolonged low-dose CTX therapy for PQ-induced lung injury, the beneficial effects are still unknown.
In the present study, we determined whether the prolonged low-dose CTX treatment after pulse could attenuate PQ-induced lung injury in rats. This study also aimed to observe its potential protective effects through survival rate, lung wet-to-dry weight (W/Dc), hydroxyproline (HYP) content, and fibrosis score.
METHODS
Chemicals
HYP was purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). PQ was obtained from Syngenta Chemical (Jiangsu, China). CTX was purchased from Suncadia Pharma (Jiangsu, China).
Animals
Eighty healthy Wistar rats (200 to 250 g) from the Experimental Animal Center were acclimatized to our laboratory environment for 7 days. All rats were kept under environmentally controlled conditions in an air-conditioned room at 23┬░C ┬▒ 2┬░C, with appropriate humidity and a 12-hour:12-hour light:dark cycle. These rats were fed with standard rat food and water ad libitum. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health. All experimental protocols were approved by the Ethics Committee of Cangzhou Central Hospital, China (Permit Number: 2016-110-02). All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Experimental design
PQ poisoning model was established by intraperitoneal injection with 1 mL of 25 mg/kg [13,14] body weight of PQ solution; after 60 minutes, the drug or saline solution was administered to the animals through intraperitoneal injection. Based on the results from the existing literature regarding the administration to PQ-intoxicated rats, we defined pulse treatment as 15 mg/kg/day CTX for 2 days [15,16] and prolonged low-dose therapy as 1.5 mg/kg/day CTX for 12 days [16,17]. The rats were randomly divided into four groups, and each group contained 20 rats. In the control group, rats were intraperitoneally injected with 1 mL/day saline solution for 14 days. In the PQ group, rats were intraperitoneally injected with 1 mL/day saline solution for 14 days after PQ exposure. In CTX pulse group, PQ-intoxicated rats were intraperitoneally injected with 15 mg/kg/day CTX in 1 mL of saline solution for 2 days and subsequent 1 mL/day saline solution for 12 days. In prolonged low-dose group, PQ-intoxicated rats were intraperitoneally injected with 15 mg/kg/day CTX in 1 mL of saline solution for 2 days and subsequent 1.5 mg/kg/day CTX in 1 mL of saline solution for 12 days. All animals were sacrificed 15 days after PQ exposure.
Sample collection
Prior to the sacrifice by cervical dislocation, rats were anesthetized with phenobarbital sodium (intraperitoneal injection 50 mg/kg). Subsequently, these rats underwent median sternotomy. The whole lungs (including both lobes) was dissected, separated from other tissues, washed free of blood with ice-cold saline, and placed on a sterile plastic Petri dish. Afterward, the left lung lobes of the animal lungs were preserved in 10% buffered formaldehyde solution for histopathological assessment. The right upper lung tissues were preserved for W/Dc ratio assay, and the right lower lung tissues were frozen in liquid nitrogen for HYP assay.
W/Dc ratio assay
When blood and other contaminants were removed, the right upper lung tissue was weighed, dried in a drying oven at 60┬░C for 72 hours, and weighed again according to the method of Oliveira [18]; the W/Dc ratio is expressed as the ratio of wet lung weight (mg) to dry lung weight (mg).
HYP assay
Lung collagen deposition was estimated by measuring the HYP content of lung homogenates in accordance with a previously described method [19]. Results are expressed in ╬╝g HYP per mg wet lung.
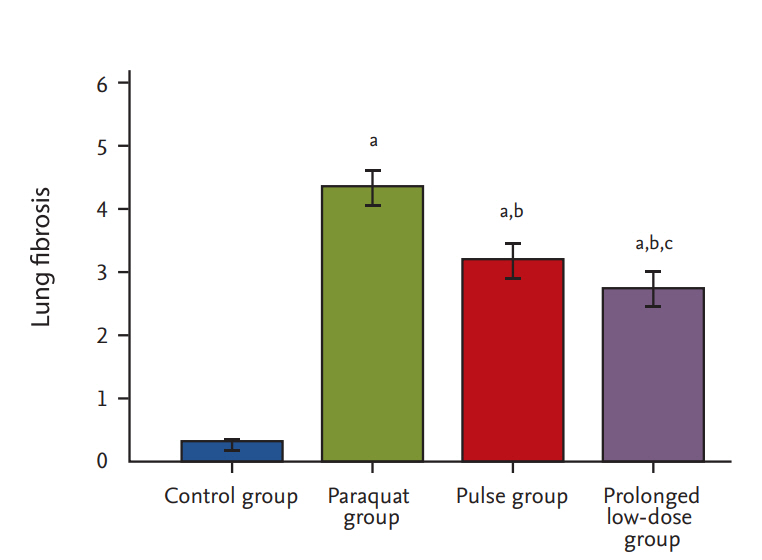
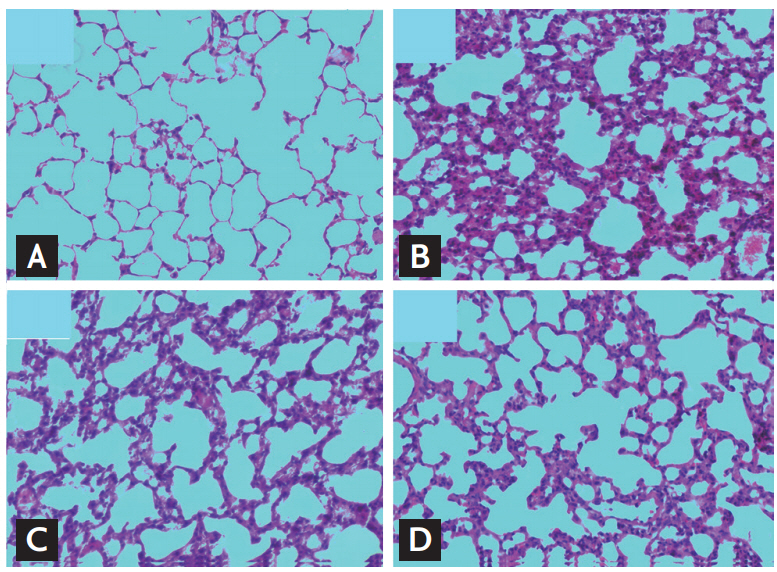
Histological assay
The left lung lobe samples were fixed in 10% formalin for 24 hours, embedded in paraffin, and sectioned (thickness, 4 ╬╝m). Slides were stained with hematoxylin and eosin. Slides were examined under light microscope (CX31, Olympus, Tokyo, Japan). The entire lung section was observed at a magnification of ├Ś100. The severity of fibrotic changes was assessed by using a semiquantitative scoring system from zero (normal lung) to eight (total fibrosis) [20]. Five randomly selected microscopic observation fields of each lung histological section were evaluated and scored independently by two investigators in blinded manner.
Data analysis
All of the data were statistically analyzed using SPSS version 19.0 (IBM Co., Armonk, NY, USA). The statistical significance of the difference between four groups of individual data (lung W/Dc ratio, HYP content, and histological score) was analyzed by one-way analysis of variance with Bonferroni post-tests for comparison between groups. Survival rates were analyzed using Kaplan-Meier method and compared using log-rank test. Lung W/Dc ratio, HYP content, and histological score are expressed as mean ┬▒ standard deviation. Statistical significance was defined as p < 0.05.
RESULTS
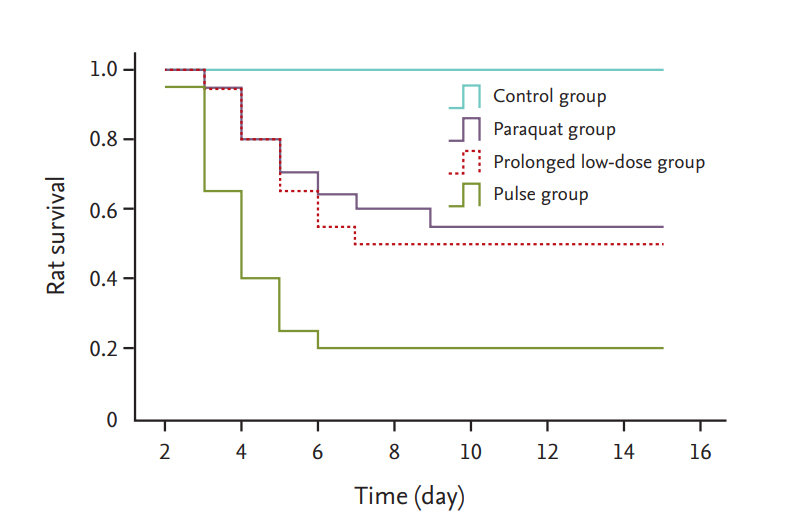
Survival rate of rats
At 15 days post-PQ exposure, the survival rates were 100%, 20%, 50%, and 55% in the control, PQ, pulse, and prolonged low-dose groups (Fig. 1). Survival rates were higher in the prolonged low-dose group (p < 0.05) and pulse group (p < 0.05) compared with that in the PQ group. The survival rates in the prolonged low-dose group were similar to those in the pulse group (p > 0.05).
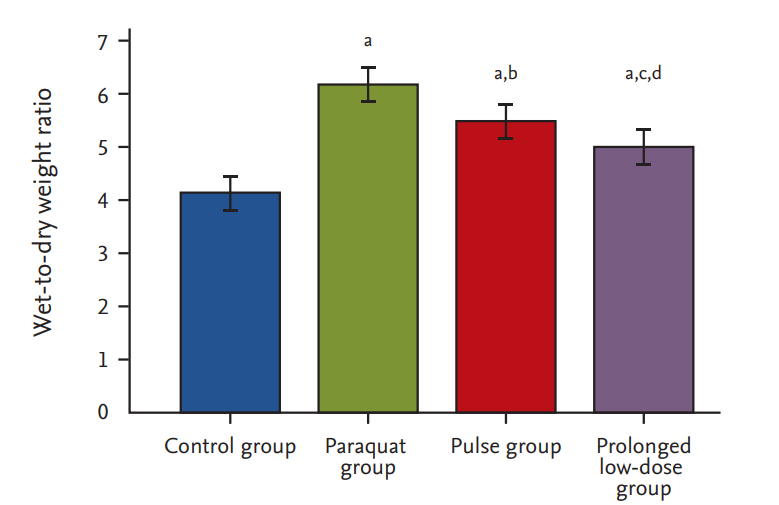
W/Dc ratio
At 15 days post-PQ exposure, the W/D ratios were 4.12 ┬▒ 0.37, 6.16 ┬▒ 0.37, 5.47 ┬▒ 0.28, and 4.94 ┬▒ 0.38 in the control, PQ, pulse, and prolonged low-dose groups, respectively (Fig. 2). The W/D ratios in the prolonged low-dose group (p < 0.001) and pulse group (p < 0.01) were lower than that in the PQ group. These ratios were lower in the prolonged low-dose group compared with that in the pulse group (p < 0.01).
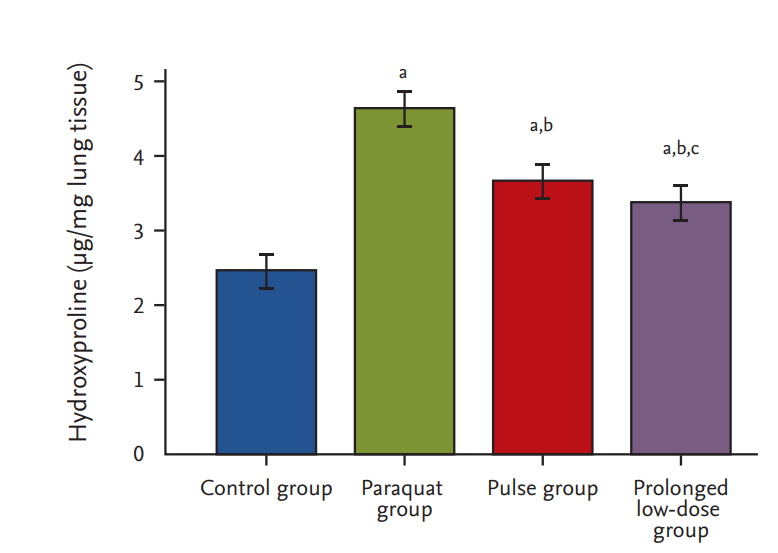
HYP content
At 15 days post-PQ exposure, the HYP content in the prolonged low-dose group was 3.34 ┬▒ 0.29 ╬╝g/mg, which was significantly lower than those in the pulse (3.65 ┬▒ 0.19 ╬╝g/mg, p < 0.001) and PQ groups (4.67 ┬▒ 0.12 ╬╝g/mg, p < 0.001) (Fig. 3).
DISCUSSION
Lung injury is one of the most common and severe clinical sequelae of PQ poisoning, which is responsible for high mortality [3]. To date, no strong evidence is available about prolonged low-dose treatment after pulse therapy of CTX attenuating and/or reversing PQ-induced lung injury. In the present study, our result suggested that prolonged low-dose CTX after pulse therapy exerts a protective effect against PQ-induced lung injury.
In the 1980s, some studies used a CTX dose of 5 mg/kg for PQ poisoning, and other investigations administered CTX at a dose of 15 mg/kg [21]. Given the small number of cases of PQ poisoning in the literature and the nonrandomized controlled trial, the use of CTX for PQ poisoning is still considered an experimental therapy. In 2013, Choi et al. [22] first approved that a CTX dose of > 15 mg/kg is effective in reducing the severity of PQ-induced lung injury, as determined by histological and micro-computed tomography tissue examination in a rat model. These results are consistent with our histological finding. However, limited evidence exists regarding the appropriate therapeutic dose and duration of treatment.
Confronted with delayed elimination of PQ [23,24], which results in progressive lung damage, some studies adopted prolonged low-dose administration of CTX. Li et al. [25] demonstrated that prolonged CTX could reduce mortality and alleviate lung fibrosis. Nevertheless, this study is a retrospective research with a small sample size and short-term follow-up, which cannot completely eliminate bias. Prolonged low-dose CTX is commonly combined with glucocorticoid to attenuate lung inflammation and fibrosis in clinical. However, clinical study results are conflicting; specifically, some studies suggested a benefit for prolonged CTX combined with glucocorticoid [9-12], and other investigations failed to determine any significant difference in survival and lung fibrosis [26]. As described in a previous report [27], we hypothesize that initial CTX pulse therapies are important for rapidly suppressing PQ-induced inflammation. Moreover, prolonged low-dose CTX treatment is necessary for the progressive and sustained reduction of inflammation to reduce the toxic effect of PQ and prevent lethal pulmonary inflammation. In addition, in contrast to high-dose immunosuppression, low-dose CTX treatment is inexpensive, has low toxicity, and easily administered. However, all patients should be carefully monitored during the therapeutic period.
The present study displays several limitations. First, this study failed to observe adverse effects, including dose-related leukopenia, hemorrhagic cystitis, infertility, secondary malignancies, and pulmonary toxicity [28]; these effects are commonly reversible, and they disappear when the CTX is discontinued. In addition, our research involves a small number of animals and a shortterm observation.
In conclusion, the current study is the first to suggest that prolonged CTX may attenuate the PQ-induced lung injury. However, further studies are needed to determine whether prolonged low-dose CTX could be used to protect against PQ-induced lung toxicity in humans.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





