 |
 |
| Korean J Intern Med > Volume 33(6); 2018 > Article |
|
Abstract
Background/Aims
Methods
Results
Figure 1.
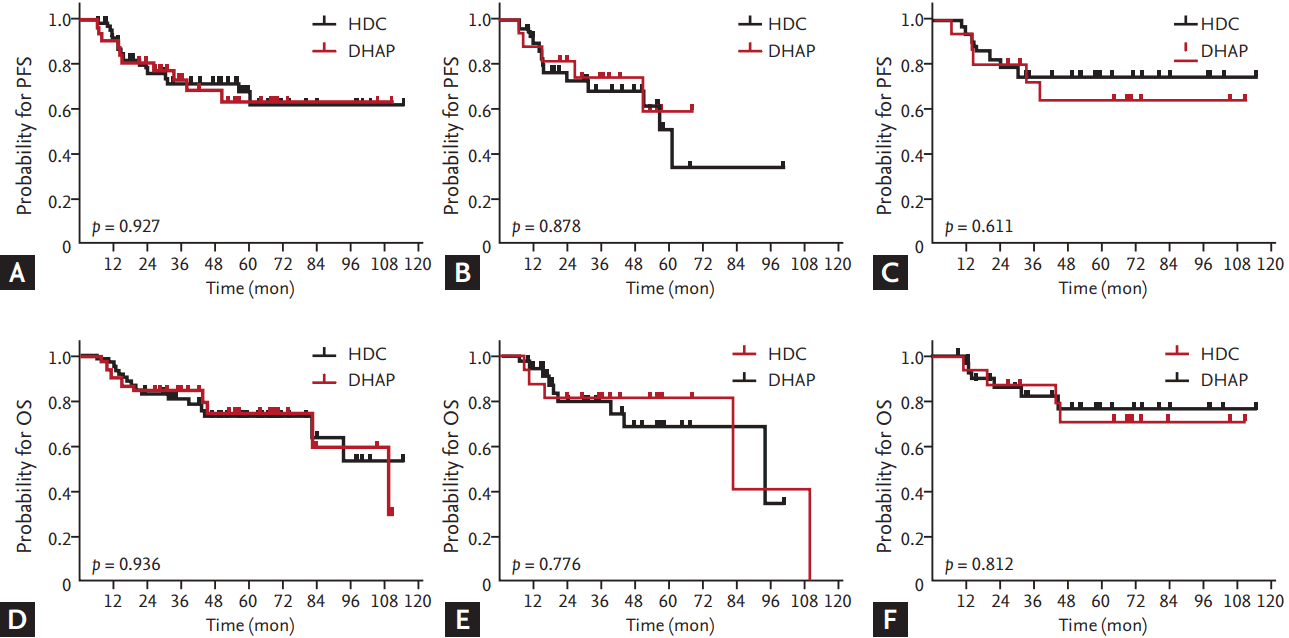
Figure 2.
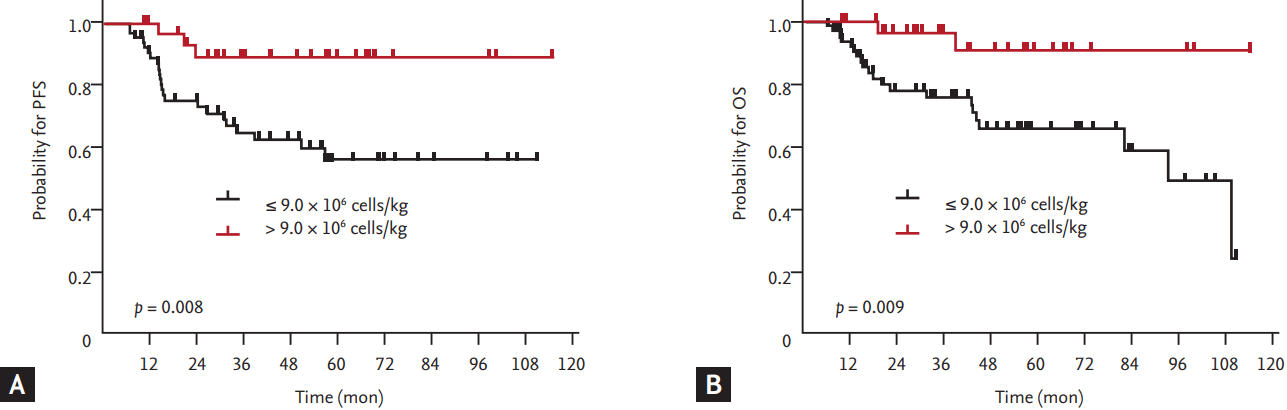
Table 1.
Values are presented as median (range) or number (%).
DHAP, dexamethasone, cytarabine, and cisplatin; HDC, high-dose cyclophosphamide; DLBCL, diffuse large B-cell lymphoma; PTCL, peripheral T-cell lymphoma; NOS, not otherwise specified; ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma kinase; AITL, angioimmunoblastic T-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; aaIPI, age-adjusted International Prognostic Index; BM, bone marrow; R, rituximab; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete response; PR, partial response.
Table 2.
Table 3.
| Variable | No. | The rate of successful mobilization, % (95% CI) |
Univariate analysis |
Multivariate analysisa |
||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |||
| Age, yr | ||||||
| ≥ 48 | 48 | 70.8 (49.1–99.0) | 1 | |||
| < 48 | 48 | 68.8 (47.3–96.6) | 0.91 (0.38–2.17) | 0.824 | ||
| Sex | ||||||
| Male | 57 | 73.7 (53.1–99.6) | 1 | |||
| Female | 39 | 64.1 (41.5–94.6) | 0.64 (0.26–1.54) | 0.317 | ||
| Histologic types | ||||||
| DLBCL | 52 | 67.3 (46.9–93.6) | 1 | |||
| PTCL | 44 | 72.7 (49.7–100.0) | 1.30 (0.54–3.13) | 0.565 | ||
| Ann Arbor stage | ||||||
| I to III | 49 | 73.5 (51.5–100.0) | 1 | |||
| IV | 47 | 66.0 (44.8–93.6) | 0.70 (0.29–1.68) | 0.424 | ||
| LDH level | ||||||
| Normal | 28 | 71.4 (43.6–100.0) | 1 | |||
| Elevated | 68 | 78.3 (57.6–100.0) | 1.12 (0.42–2.94) | 0.823 | ||
| aaIPI | ||||||
| Low to LI | 48 | 69.7 (44.2–100.0) | 1 | |||
| HI to high | 47 | 69.8 (50.7–93.8) | 1.01 (0.40–2.52) | 0.988 | ||
| Baseline BM involvement | ||||||
| Yes | 17 | 52.9 (24.2–100.0) | 1 | 1 | ||
| No | 79 | 73.4 (55.7–94.9) | 2.46 (0.84–7.20) | 0.098 | 4.60 (1.20–17.61) | 0.026 |
| Disease status before chemo-mobilization | ||||||
| PR | 10 | 50.0 (16.2–100.0) | 1 | |||
| CR | 86 | 72.1 (55.3–92.4) | 2.58 (0.69–9.73) | 0.161 | ||
| Chemotherapy cycles | ||||||
| > 6 | 25 | 52.0 (27.7–88.9) | 1 | |||
| ≤ 6 | 71 | 76.1 (57.1–99.2) | 2.93 (1.13–7.62) | 0.027 | NS | NS |
| Previous radiotherapy | ||||||
| Yes | 8 | 37.5 (7.7–10.0) | 1 | |||
| No | 88 | 72.7 (56.0–92.9) | 4.44 (0.99–20.04) | 0.052 | 11.78 (1.68–82.39) | 0.013 |
| WBC counts at first apheresis day | ||||||
| ≤ 1.785 × 103/μL | 40 | 47.5 (28.6–74.2) | 1 | |||
| > 1.785 × 103/μL | 56 | 85.7 (63.2–100.0) | 6.63 (2.51–17.53) | < 0.001 | 9.25 (2.89–29.58) | < 0.001 |
| Mobilization regimen | ||||||
| HDC | 65 | 61.5 (44.0–83.8) | 1 | 1 | ||
| DHAP | 31 | 87.1 (57.4–100.0) | 4.22 (1.32–13.50) | 0.015 | 4.12 (1.12–15.17) | 0.033 |
CI, confidence interval; OR, odds ratio; DLBCL, diffuse large B-cell lymphoma; PTCL, peripheral T-cell lymphoma; LDH, lactate dehydrogenase; aaIPI, age-adjusted International Prognostic Index; LI, low-intermediate; HI, high-intermediate; BM, bone marrow; PR, partial response; CR, complete response; NS, not significant; WBC, white blood cell; HDC, high-dose cyclophosphamide; DHAP, dexamethasone, cytarabine, and cisplatin.
Table 4.
Table 5.
OS, overall survival; HR, hazards ratio; CI, confidence interval; NS, not significant; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; aaIPI, age-adjusted International Prognostic Index; LI, low-intermediate; HI, high-intermediate; BM, bone marrow; DLBCL, diffuse large B-cell lymphoma; PTCL, peripheral T-cell lymphoma; CR, complete response; PR, partial response; DHAP, dexamethasone, cytarabine, and cisplatin; HDC, high-dose cyclophosphamide.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



