To the Editor,
Ovarian hyperstimulation syndrome (OHSS) is a potentially life-threatening condition that classically occurs in adult women undergoing the excessive administration of gonadotropins as an assisted reproductive technique to induce ovulation [1]. In the absence of stimulation from exogenous gonadotropins, rare cases of spontaneous OHSS have been reported in women with severe primary hypothyroidism, polycystic ovary syndrome while pregnant, gonadotroph pituitary adenoma, and normal pregnancy [2-4]. In women with primary hypothyroidism, extremely elevated levels of thyroid stimulating hormone (TSH) can stimulate the ovaries because TSH and gonadotropins share homologous structural features [3]. Herein, we describe a patient with profound primary hypothyroidism who presented with growth retardation and pituitary enlargement. To our knowledge, this is the first report in Korea in which spontaneous OHSS has been described in a non-pregnant woman with primary hypothyroidism.
A 14-year-old female presented at our clinic with growth retardation and incidentally detected pituitary enlargement that was observed on a brain magnetic resonance imaging (MRI) scan (Fig. 1A). The MRI scan was performed due to the parentsŌĆÖ concern regarding the patientŌĆÖs poor academic performance. The patient had no significant medical history, was not taking any medications, weighed 45 kg, and was 127 cm in height; according to the Korean growth curve for young females (2 to 20 years of age), her height was below the third percentile. The patientŌĆÖs menarche was at 10 years of age, after which she experienced irregular menses with periods of amenorrhea that sometimes lasted for 4 to 5 months. The clinical signs and symptoms of hypothyroidism were not conspicuous, but she exhibited very slow responses to questions, and her face was round and puffy. There was no enlargement of the thyroid, and a formal IQ test was not performed.
The routine biochemical tests did not reveal any abnormalities and her serum-free thyroxine and total triiodothyronine levels were 0.118 ng/dL (reference range, 0.6 to 1.7) and < 0.195 ng/mL (reference range, 0.8 to 1.71), respectively. However, the patientŌĆÖs TSH level was markedly elevated (> 1,000 ╬╝U/mL; reference range, 0.4 to 4.8), and no thyroid autoantibodies were detected. Her serum level of luteinizing hormone (LH) was undetectable but her serum level of follicle stimulating hormone (FSH; 7.31 mIU/mL) was within a normal range; the reference ranges for FSH and LH in the follicular phase are 1.1 to 9.6 and 0.8 to 25.8 mIU/mL, respectively. The levels of her other pituitary hormones were as follows: growth hormone (0.55 ng/mL), insulin-like growth factor 1 (104.67 ng/mL), prolactin (46.10 ng/mL), adrenocorticotropic hormone (16 pg/mL), and cortisol (10.0 ╬╝g/mL).
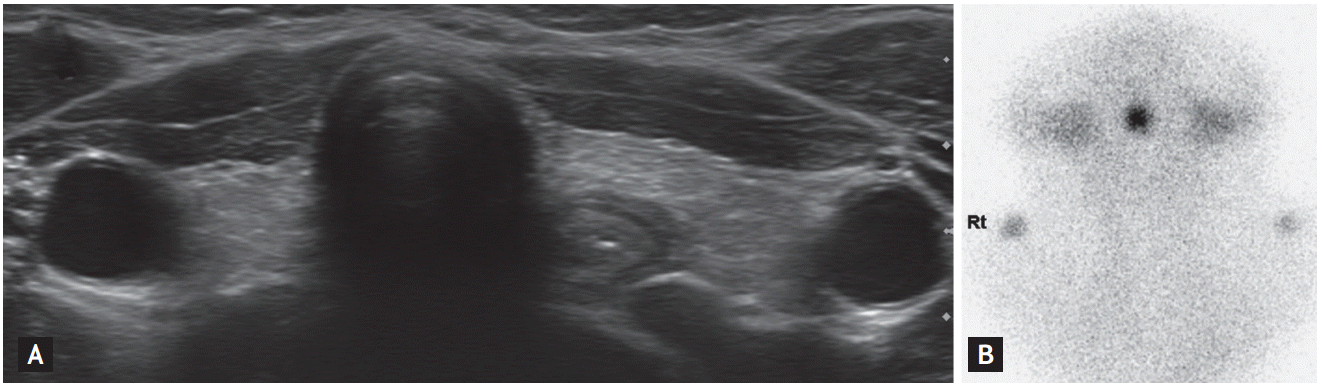
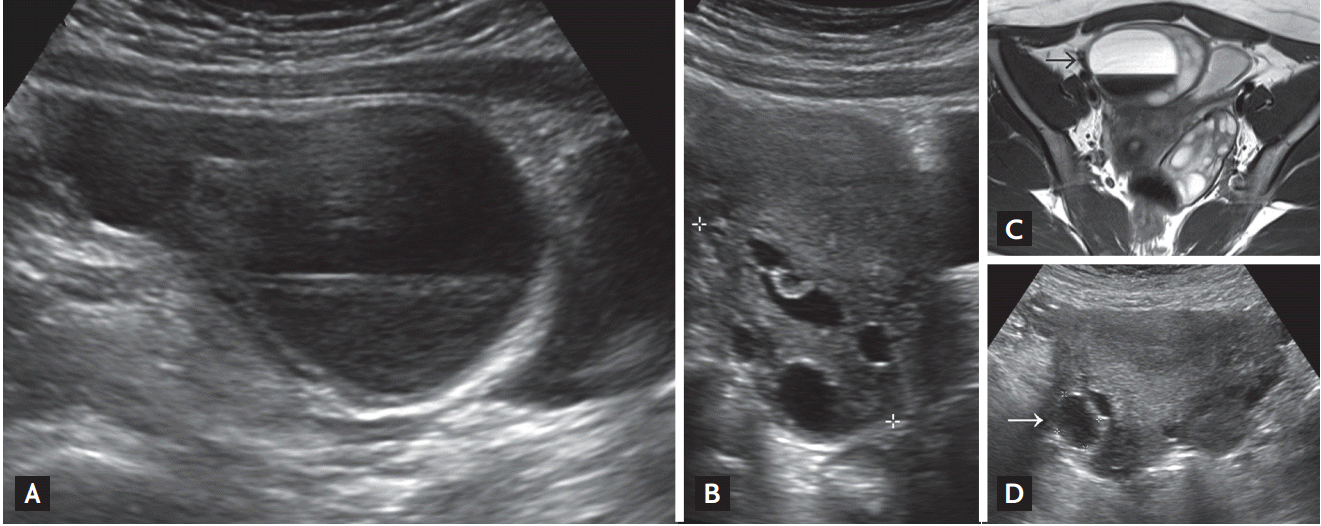
A neck ultrasonography (US) scan did not show any thyroid tissue in a eutopic position and revealed that the thyroid bed was replaced with fatty tissue (Fig. 2A). However, a subsequent thyroid scan showed an ectopic lingual thyroid gland (Fig. 2B). An abdominal US was performed because the patient complained of mild abdominal discomfort and nausea. She had an enlargement of both ovaries (right, 8.9 ├Ś 4.6 cm; left, 5.9 ├Ś 8.2 cm) with multiple cystic changes, and an abdominal US scan unexpectedly revealed a large hemorrhagic cyst in the right ovary (the largest diameter was 67 mm) (Fig. 3A and 3B). A pelvic MRI scan showed the hemorrhagic cyst in the right ovary to be 5.2 ├Ś 4.3 ├Ś 5.2 cm in size and that both ovaries and the uterus were enlarged compared with a control group of a similar age (Fig. 3C). The patientŌĆÖs serum level of cancer antigen 125 was normal (25.87 U/mL; range, 0 to 35), and her total human chorionic gonadotropin level was undetectable.
The patient was diagnosed with primary hypothyroidism (which stemmed from the ectopic lingual thyroid), bilateral ovarian cystic enlargement (which was similar to mild OHSS), and reactive pituitary hyperplasia. Subsequently, levothyroxine (LT4; 100 ╬╝g/day) therapy was initiated, and an abdominal US scan revealed significant reductions in the sizes of both ovaries 1 month after the beginning of LT4 treatment. At this time, the patientŌĆÖs serum TSH level was 0.841 ┬ĄU/mL, and it has since remained within a normal range. At 3 months after LT4 treatment was initiated, both ovaries had returned to a normal size and appearance with a complete regression of the ovarian cyst (Fig. 3D). At 6 months, the patientŌĆÖs menstrual cycle had normalized and a follow-up sellar MRI scan revealed a marked reduction in the size of the pituitary gland (Fig. 1B). The patient underwent a 7.8 cm growth spurt over 12 months following the achievement of euthyroidism.
The present report describes the case of a young female patient with primary hypothyroidism complicated by reactive thyrotroph hyperplasia and spontaneous OHSS that mimicked ovarian tumors. LT4 replacement reduced the ovarian volume and resulted in a regression of the ovarian cysts which enabled the differentiation of OHSS from the presence of primary ovarian tumors without costly or invasive diagnostic procedures.
Using serial US scans, the regression of the ovarian cysts and a reduction in ovarian volume was observed 3 months after the initiation of LT4 replacement. Other studies investigating non-pregnant hypothyroid patients found a considerable regression of cysts after 3 to 4 months of treatment for the hypothyroidism with 100 ┬Ąg doses of LT4 [3]. It is noteworthy that the kinetics of the declining serum levels of TSH are closely related to the regression of the ovarian cysts; this temporal correlation supports the suggestion that the elevated TSH mimicked endogenous gonadotropins and caused ovarian hyperstimulation.
Primary hypothyroidism and OHSS are likely linked by the stimulation of the ovaries following extreme elevations in the level of TSH because gonadotropins and TSH share the same ╬▒ subunit. Additionally, TSH has weak FSH- and LH-like action at the FSH and LH receptors, which can cause gonadal stimulation. This hypothesis would explain why the present patient had relatively low FSH and LH levels. In the present case and in most previously reported cases, TSH-induced OHSS occurred in relatively young females; thus, it is possible that immature ovaries are more vulnerable to excessive levels of TSH [3].
The diffuse enlargement of the pituitary gland in the present patient was thought to be due to compensatory thyrotroph hyperplasia. In primary hypothyroidism, an attenuation of negative feedback signals from peripheral thyroid hormones induces thyrotroph and lactotroph cell hyperplasia and results in excessive levels of TSH and prolactin [5]. In the present case, the patientŌĆÖs compensatory pituitary hyperplasia might have been mistakenly diagnosed as a pituitary macroadenoma, but the marked regression of the pituitary enlargement after LT4 replacement allowed for a differential diagnosis.
The present report describes the resolution of ovarian cysts and the normalization of ovarian size in a young female patient with a lingual thyroid following LT4 administration. To avoid costly interventions, a diagnosis of OHSS should be considered when there is bilateral ovarian enlargement in the clinical context of profound primary hypothyroidism.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





