INTRODUCTION
Over the last several decades, the incidence of lymphoproliferative malignancies has rapidly increased in Caucasian cohorts in many advanced countries [
1,
2], and these malignancies consist mostly of non-Hodgkin lymphoma (NHL) [
1-
4]. Although the epidemiology based on immunologic aspects of NHL is not clearly understood, immune dysregulation is speculated to play an important role in lymphomatogenesis on the basis of data showing an increased risk of certain lymphomas in patients following organ transplants or viral infections, in those with primary or acquired immunodeficiencies, and particularly in patients with autoimmune diseases [
5]. Like the immunologic pathophysiology of NHL, autoimmune diseases also comprise a variety of situations based on deregulation of many components of the immune response [
6]. Based on those aspects, it has been considered that autoimmune diseases and lymphoproliferative malignancies have a certain relationship, and some studies have reported that autoimmune diseases are implicated in the increased incidence of NHL [
4,
6-
9]. Indeed, a few nation-wide population-based retrospective studies have shown that certain specific autoimmune diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and Sjogren syndrome have strong relationships with an increased risk of NHL [
4,
6]. In this way, the relationship between autoimmunity and lymphomatogenesis has been represented through animal models and human studies in recent years. However, the exact reasons for the relationship between these disease processes are still not fully known [
3]. Several possible explanations for the connection include the role of long-lasting chronic hyper-immune stimulation, treatment with immunosuppressive agents such as methotrexate (MTX), and other shared genetic signals [
7,
10]. Although large cohort studies regarding the association between autoimmune disease and lymphomas have been reported in Western countries [
6,
11-
13], studies in Asian cohorts have been limited and only a few cases have been reported [
4,
8,
11,
12]. Autoimmune disease-related NHL tends to be disregarded because of its minimal incidence and because of the heterogeneous background of lymphoproliferative disease itself in Asian countries. Because several subtypes of NHL differ between Caucasian and Asian subjects in terms of incidence and pathophysiology, we assumed that those ethnic and demographic variations could make a difference in the characteristics, incidence, and clinical manifestations of autoimmune disease-related NHL between Western and Eastern populations as well. Therefore, we retrospectively evaluated the clinicopathological features, clinical characteristics, prognostic factors, and treatment outcomes in 11 Korean patients diagnosed with autoimmune disease-related NHL at a single center.
DISCUSSION
As treatment methods for the autoimmune disease have been improved and rising incidence of autoimmune disease, increase in the number of long-term survivors with persistent immunologic dysfunction has been attended by increasing in autoimmune disease-related lymphoma (especially for autoimmune disease-related NHL), it has become an increasingly important clinical issue. Although this study has a few limitations including the retrospective analysis with a small cohort in a single center, the somewhat short observation period for the analysis of OS and PFS, and the heterogeneity of each NHL subtype, the study did reveal important different characteristics of autoimmune disease-related NHL and of different treatment strategies (including auto-PBSCT) compared to previous reported data. The current study revealed several clinical findings.
The first, NHL is diagnosed at advanced ages more commonly in patients with RA than in non-RA patients (median age, 63 years vs. 49 years, respectively;
p = 0.042), but the duration of autoimmune disease at the time of diagnosis of NHL was not significantly different between the two groups (median duration, 80 months vs. 105 months,
p = 0.164). Considering age at diagnosis with NHL in patients with autoimmune disease, a few studies showed that the length of time between the onset of the autoimmune disease and the onset of NHL was a more important factor than age at diagnosis [
18], but our study showed that that duration was not significant (
p = 0.164) and that RA-related NHL patients were significantly older (
p = 0.042). This means that the average age of RA onset was older than that for other autoimmune diseases.
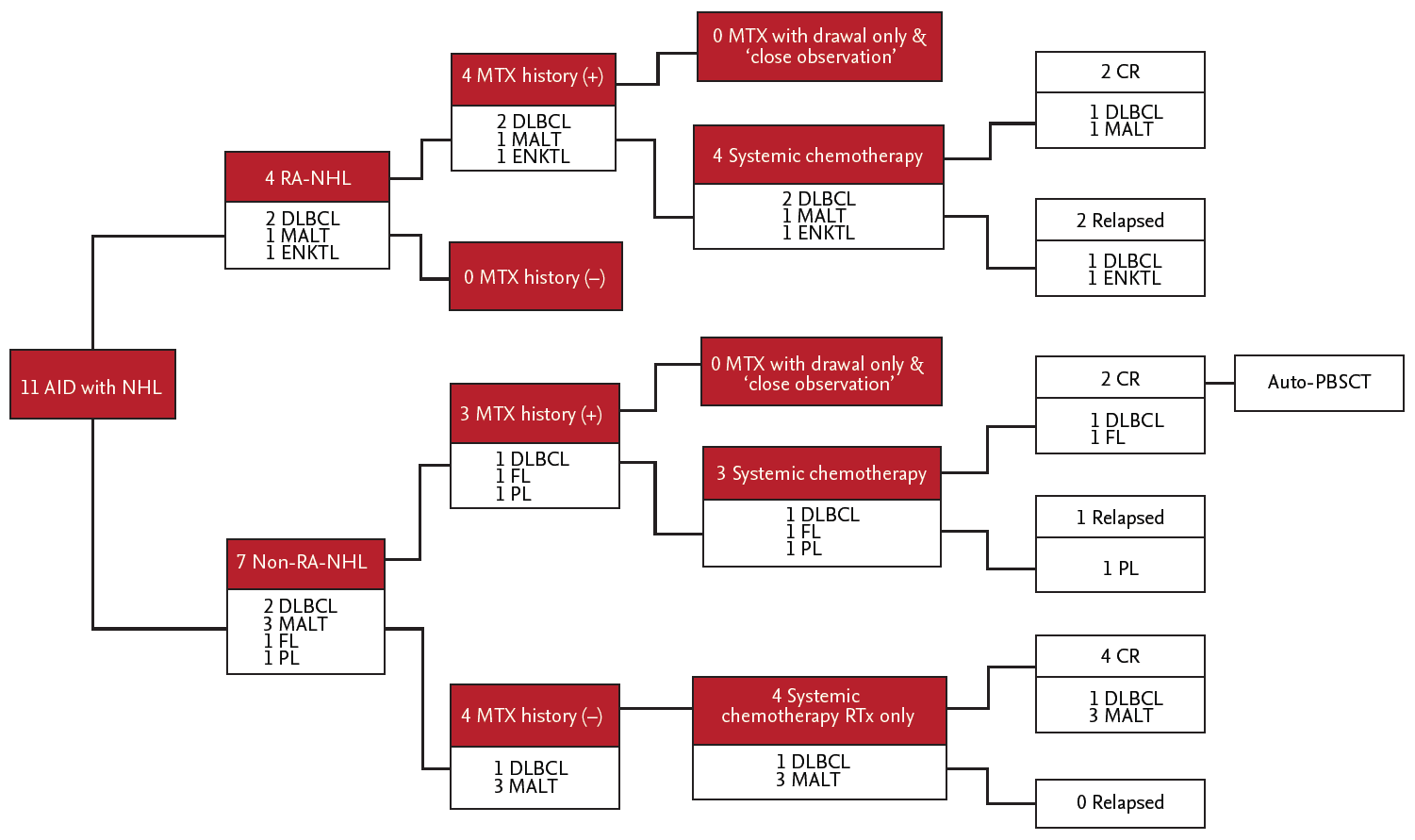
The second, subgroup of autoimmune disease-NHL have 36% (four of 11 patients) in DLBCL. Also, MALT lymphoma accounts for 36% (four of 11 patients). This point is presumed that multistep courses induced by chronic antigenic stimulation, which conduced to the mass of polyclonal B-lymphocytes in mucosal sites and the formation of secondary MALT.
The third, no incremental increase in the lymphoma risk was observed in patients treated with MTX (
p = 0.21). EBV infection was also not associated with an increased lymphoma risk. Regarding anti-autoimmune disease medications, it has been known since 1993 that MTX and EBV infection have correlations in a certain subgroup of NHL, based on the fact that withdrawal of MTX alone resulted in the regression of lymphoma [
19]. In contrast, it is difficult to draw a conclusion that they are strongly related to the incidence of NHL. The pathogenic role of MTX in autoimmune-related NHL was not fully clear. Furthermore, Moder et al. [
20] reported that there did not appear to be a relationship between either peak or cumulative dosage or the duration of MTX therapy and the incidence of lymphoproliferative disease, and the histological subtypes of hematologic malignancy seen in MTX-treated patients did not differ from those of patients treated with other DMARDs. In addition, whether RA patients treated with MTX have an increased risk of developing lymphoma remains a matter of controversy [
18]. Our data show that treatment with MTX did not have any effect on the occurrence of lymphoma, which was considered to be related to a long-lasting inflammatory reaction of the autoimmune disease itself. In our data, although all three of the patients who experienced recurrence had a history of treatment with MTX, there was no significant difference in recurrence based on whether patients were treated with MTX (
p = 0.21). It was suggested that treatment with MTX did not have any effect on the occurrence of lymphoma, which might be related to a sustained inflammatory activity of the disease. In addition, total cumulative amounts of MTX had no statistical correlation with autoimmune disease and NHL (
p = 0.073).
The forth, all patients enrolled in the study were diagnosed with the aggressive subgroup of NHL, and all patients were directly treated with withdrawal of immunosuppressive agents followed by concurrent systemic chemotherapy or local therapy instead of employing a wait and watch approach. Generally, regarding the management for autoimmune-related NHL, several previously reported studies have demonstrated that close observation after MTX withdrawal without another treatment was one of the best options in certain patients, and according to the presence of EBV in lymphoma cells, withdrawal of MTX only may be suitable [
6,
11,
12,
18,
21]. However, we actively treated with systemic chemotherapy or localized radiation therapy in all 11 cases.
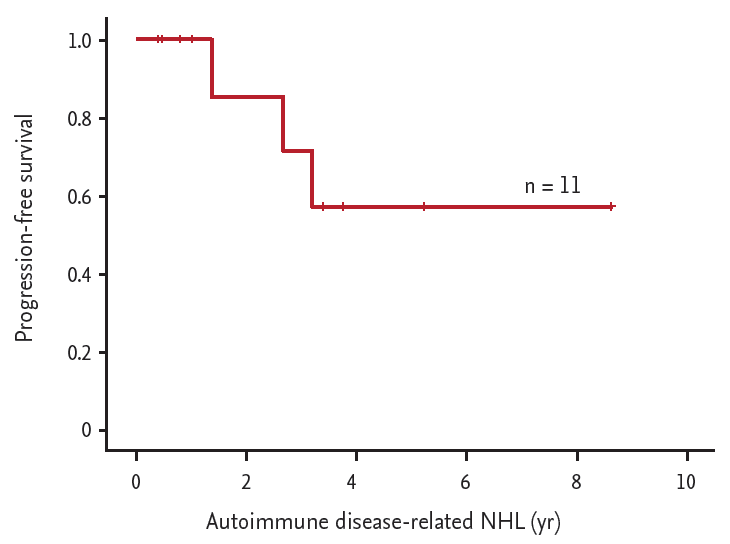
OS and PFS between RA related NHL and non-RA related NHL were not significant statistically. Previously reported data showed 2-year OS and PFS for autoimmune disease-related NHL were 80% to 86% and 68% to 75% [
4-
6]. However, OS and 2-year PFS in all autoimmune disease-related NHL were 100% and 87.5% (
Fig. 2), with no treatment-related mortality during the 2-year follow-up period of our study. Although each group have very different lymphoma subtype, and it is hard to make a definitive conclusion due to too small cohort numbers, considering those points, we suggest that early treatment strategies were more suitable than delayed treatment or watchful management, regardless of the presence of EBV infection.
In conclusion, our study suggests that patients diagnosed with RA-related NHL were older than patients with non-RA related NHL, regardless of MTX duration and total amount. However our results need to be re-evaluated in more large study population. In the presence of aggressive NHL, we prefer early and intensive treatment instead of delayed watchful management, such as withdrawal of immunomodulatory drugs (DMARDs) or the wait and watch approach.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



