 |
 |
| Korean J Intern Med > Volume 31(6); 2016 > Article |
|
To the Editor,
Hyperosmolar hyperglycemic state (HHS) is a life-threatening complication of diabetes mellitus, and it is relatively common in elderly patients with type 2 diabetes. A variety of neurological deficits may occur in patients with HHS, including changes in mental status such as drowsiness, lethargy, delirium, or coma. Seizures, sensory deficits, and visual disturbances also sometimes occur in patients with HHS. However, aphasia, as a neurological deficit, is not commonly associated with hyperglycemia. We report the case of man who presented with symptoms of motor aphasia including inability to speak fluently, concurrent with marked hyperglycemia.
A middle-aged man presented with motor-dominant aphasia lasting for 5 days, as well as a history of headache for the past month. He had type 2 diabetes that was previously well-controlled with antidiabetic medications, and he also had hypertension, diabetic retinopathy, and stage 4 chronic kidney disease. There was no history of strokes, seizures, or trauma. The patient relocated to China for work as an engineer 2 months prior to hospital admission and had recently experienced a common cold. He had been missing meals and not taking his antidiabetic medications since arriving in China because he disliked Chinese food, except fruit. His height and weight were 167 cm and 63 kg, respectively. However, after recurrent nausea and vomiting for several weeks, the patient had lost 10 kg of body weight. At the time of his first visit to a hospital in China, his blood glucose level was more than 400 mg/dL (22.2 mmol/L).
The patient presented at Emergency Department of Seoul National University Bundang Hospital upon his return to Korea, exhibiting difficulty finding the correct words to express himself, frequent pauses in speech production, and normal comprehension of spoken language. However, he mistook written Chinese characters for Korean phonetic symbols. Additionally, the patient experienced intermittent headache with a sensation of squeezing that lasted for 2 to 3 hours each day. Headaches were located in the left temporal area.
The patient was dehydrated, but alert and oriented at the time of physical examination. Verbal fluency and repetition were impaired, but comprehension of spoken language, reading, writing, and naming skills were intact. Other focal neurologic signs such as motor weakness, sensory abnormalities, or involuntary movements were absent.
The patientŌĆÖs blood pressure was 170/99 mmHg and body temperature was normal. Laboratory data indicated a blood glucose level of 471 mg/dL (26.2 mmol/L) and serum osmolality of 312 mOsmol/L. Glycated hemoglobin was 15.8% (149.2 mmol/mol), and venous blood gases indicated a serum pH of 7.388. Serum ketone was 1+, and urine ketone was 1+. Other electrolyte abnormalities included sodium 128 mEq/L, potassium 4.1 mEq/L, chloride 93 mEq/L, bicarbonate 26.8 mEq/L, and urea 42 mEq/L. Serum creatinine was 2.94 mg/dL.
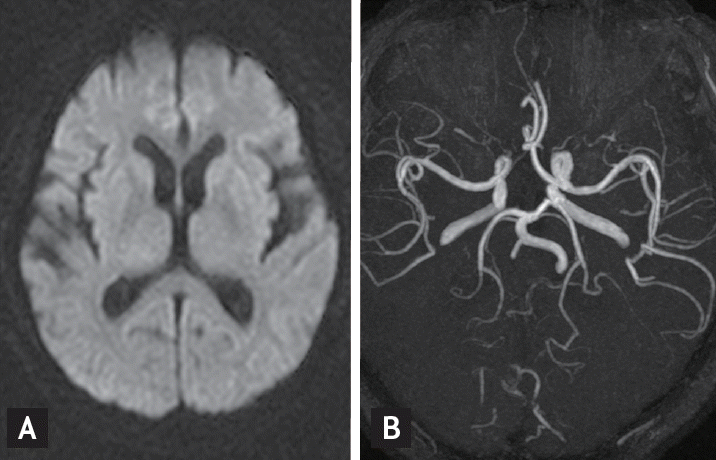
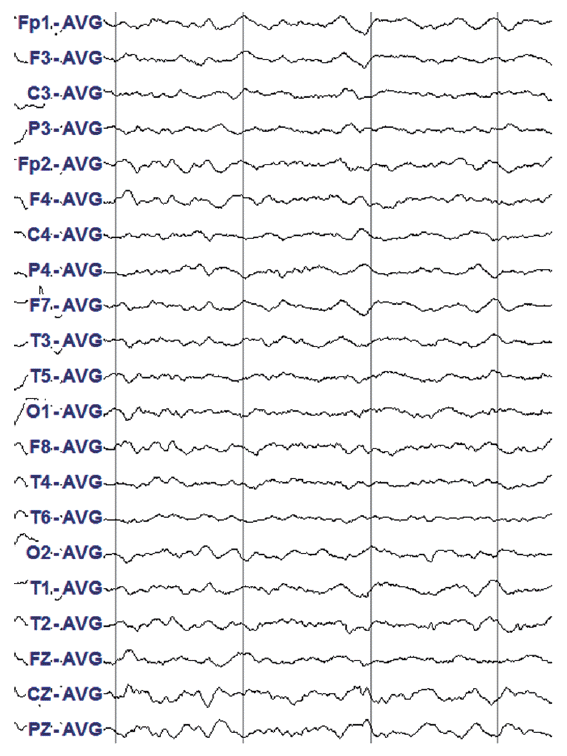
Brain magnetic resonance imaging (MRI), including diffusion-weighted images and magnetic resonance angiography, was performed in order to exclude structural lesions such as acute stroke or malignancy (Fig. 1). Electroencephalogram (EEG) demonstrated slow background activity and intermittent irregular delta slow activities in the whole hemisphere. Diffuse cerebral dysfunction was suspected, and the patient experienced metabolic encephalopathy. No evidence of epileptiform discharge was observed (Fig. 2).
Intensive intravenous insulin therapy was administered at an initial dose of 0.15 U/kg/hr, which was decreased to 0.1 U/kg/hr until the blood glucose level had fallen to 250 mg/dL. Massive fluid replacement was performed using isotonic saline to relieve dehydration. Verbal fluency was slightly impaired at this point in treatment, but it gradually recovered and repetition abilities normalized after 2 days in the hospital. The headaches also improved after glycemic control was achieved. The patientŌĆÖs blood glucose level was between 110 and 250 mg/dL after 6 days in the hospital. He could speak fluently after his hyperglycemia was treated and was discharged from the hospital after 11 days. Electrolyte imbalances improved when the patient was discharged, and serum creatinine decreased to a baseline level. Aphasia did not re-occur during the 18-month follow-up period.
A wide range of neurological abnormalities occur with HHS, including lethargy, confusion, delirium, or coma. Hemiparesis and chorea have also been observed in patients with HHS, and localized sensory or motor weakness may occur. In the present case, motor symptoms presented as diabetic striatopathy, which is a rare involuntary movement disorder that occurs when serum osmolality reaches 320 to 330 mOsmol/L, and which may recover after normalization of fluid deficits. The hypothesized mechanism for chorea in HHS is depletion of gamma-aminobutyric acid via an anaerobic pathway [1].
Aphasia sometimes occurs in cerebrovascular, epileptic, neurodegenerative, or traumatic conditions. The most common cause of aphasia is cerebrovascular disease, and aphasia is reported in 20% to 40% of patients with stroke [2]. Additionally, seizures may occur in approximately 25% of patients with HHS [2]. Table 1 presents several case reports of aphasic status epilepticus with hyperglycemia.
Aphasia is classified by the location of the brain lesion and the resulting symptoms. BrocaŌĆÖs aphasia is characterized by loss of the ability to speak, but with relatively well-preserved comprehension. The anterior region of the brain, including the inferior frontal gyrus in the dominant hemisphere, is associated with expressive aphasia [3]. Patients with WernickeŌĆÖs aphasia speak meaningless words fluently, but they do not have deficits in understanding language. WernickeŌĆÖs aphasia usually results from a lesion in the posterior area of the superior temporal gyrus of the dominant hemisphere [3]. In global aphasia, both repetition and comprehension are severely impaired, and the common sites of damage are in the brain areas surrounding the middle cerebral artery [3]. In the case of our patient, auditory comprehension was intact but he could not speak well, including demonstrating decreased repetition ability. This manifestation is similar to transcortical motor aphasia resulting from lesions in the left frontal cortices and BrocaŌĆÖs area. Hyperglycemia produces a global decrease in cerebral perfusion, resulting from impairment of cerebral autoregulation [1]. Therefore, a possible mechanism for expressive aphasia is an ischemic injury of the cerebrum under hyperglycemic conditions.
The pathogenesis of hyperglycemia-related aphasia is unclear. Glucose-induced reactive oxygen species lead to lipid peroxidation, protein carbonylation, and DNA damage, thereby producing nuclear factor-╬║ B-mediated vascular inflammation [4]. Hyperglycemia is also associated with reduced cerebral perfusion, which is mediated through decreased nitric oxide production in patients with diabetes. In a previous experimental study, high blood sugar levels may have aggravated blood-brain barrier injury after ischemia, thereby facilitating bradykinin-mediated brain edema in rats with intracerebral hemorrhage [5]. In addition to reducing serum glucose levels, insulin has anti-oxidant and anti-inflammatory effects [4]. Furthermore, intracellular shrinkage results from osmotic diuresis and water migration during HHS. Vascular injuries may therefore be aggravated by increased blood viscosity under hyperglycemic conditions.
Treatment of HHS consists of correcting the fluid deficits and electrolyte imbalances, as well as reducing serum glucose and plasma osmolality. In our patientŌĆÖs case, motor aphasia and headache improved dramatically within 2 days following hydration and glycemic control, and there were no residual neurological deficits. We did not prescribe any anti-epileptic medications, which is in contrast to previously described cases (Table 1), because our patientŌĆÖs MRI and EEG reports were normal.
Given that recovery from headaches occurred rapidly after glycemic control was achieved, headaches may be an early symptom of hyperglycemia. Early diagnosis and management of precipitating factors are also important strategies for treating HHS. If glycemic control had been delayed, it is possible that the patientŌĆÖs aphasia would not have recovered without sequelae.
In summary, this is the report of a Korean patient who experienced HHS and aphasia without seizure or other neurologic abnormalities. The patientŌĆÖs HHS-induced expressive aphasia was fully resolved, and he fully recovered without a need for anti-epileptic drugs after glycemic control was achieved. We recommend that diabetic patients with abrupt aphasia should have their blood sugar levels examined to confirm the diagnosis, and appropriate management should then be determined.
Figure┬Ā1.
Axial diffusion weighted image (A), magnetic resonance angiography (B) of patient showed no suspicious lesion of acute stroke and arterial stenosis.
Figure┬Ā2.
Electroencephalogram showed slow background activity and intermittent irregular delta slow activities in the whole hemisphere. Diffuse cerebral dysfunction was suspected which could be seen on metabolic encephalopathy. No evidence of epileptiform discharge was observed.
Table┬Ā1.
Baseline characteristics, imaging and EEG finding of aphasic status epilepticus patients in previous case reports
REFERENCES
1. Lai PH, Tien RD, Chang MH, et al. Chorea-ballismus with nonketotic hyperglycemia in primary diabetes mellitus. AJNR Am J Neuroradiol 1996;17:1057ŌĆō1064.


2. Huang LC, Ruge D, Tsai CL, et al. Isolated aphasic status epilepticus as initial presentation of nonketotic hyperglycemia. Clin EEG Neurosci 2014;45:126ŌĆō128.


-
METRICS

- Related articles
-
Body mass index at the crossroads of osteoporosis and type 2 diabetes2020 November;35(6)
Current status and therapeutic considerations of hypertension in the elderly2019 July;34(4)
The importance of treating mild hyperglycemia in pregnant women with diabetes2018 November;33(6)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


