 |
 |
| Korean J Intern Med > Volume 22(3); 2007 > Article |
|
Abstract
Background
Second-line chemotherapy offers advanced non-small cell lung cancer (NSCLC) patients a small, but significant increase in survival. Docetaxel is usually administered as a 3-week schedule, yet there is significant toxicity with this therapy. Therefore, a weekly schedule has been explored in several previous trials. In this retrospective study, we compared the efficacy and safety of a weekly schedule and a 3-week schedule of docetaxel monotherapy in a second-line setting.
Methods
Docetaxel was administered as 75 mg/m2 on day 1 every 3 weeks or as 37.5 mg/m2 on day 1 and 8 every 3 weeks until disease progression or severe toxicity developed.
Results
From October 2003 to March 2006, a total of 37 patients received docetaxel monotherapy and 36 patients could be evaluated. A total of 135 cycles were administered and then evaluated. The median overall survival was 13.3 months (95% confidence interval: 6.3~20.3) for the weekly schedule and 10.7 months (95% confidence interval: 8.3~13.0) for the 3-week schedule (p=0.41). The median time to progression was 3.0 months (95% confidence interval: 1.9~4.0) and 2.8 months (95% confidence interval: 1.0~4.6), respectively (p=0.41). The response rate was 16.7% for the weekly schedule and 21.1% for the 3-week schedule. The major form of hematologic toxicity was grade 3-4 neutropenia (3-week: 38.9%, weekly: 9.5%). The non-hematologic toxicities were similar between the two schedules. There were no treatment-related deaths.
A meta-analysis of the published data has shown a modest, but significant survival benefit for platinum-based chemotherapy and supportive care as compared to best supportive care alone for patients suffering with non-small cell lung cancer (NSCLC)1). Platinum-based chemotherapy has been regarded as a standard treatment for patients with advanced NSCLC. However, the importance of second-line treatment has been highlighted in research because most NSCLC patients fail to respond to a front-line regimen. Several reports have suggested that docetaxel2, 3) or pemetrexed4) are optimal regimens as second-line treatment.
The administration of docetaxel (Taxotere®; Aventis Pharmaceuticals Inc.; Bridgewater, NJ), every 3 weeks at doses of 75~100 mg/m2 in phase II trials5, 6) has resulted in a response rate of 16%~22% and a survival period of 6.9~9.7 months. Two phase III trials (the TAX3172) and TAX 3203)) displayed the superiority of docetaxel at 75 mg/m2 for disease/symptom control, survival and the quality of life, and this was despite the relatively low response rate of 5.5~6.7% when compared to the best supportive care or an alternative single-agent such as vinorelbine or ifosfamide. In spite of these promising results, there were severe docetaxel-induced toxicities. Grade 3-4, severe neutropenia occurred in 85.7% of the patients in the 100 mg/m2 arm and in 67.3% of the patients in the 75 mg/m2 arm of the TAX 317 trial2). In the TAX 320 trial3), the frequency of neutropenia was significantly greater in the docetaxel group (54%) as compared to the vinorelbine/ifosfamide group (31%). Considering the recent steep increases in the incidence of elderly patients or patients with a poor performance status (PS) who fail with first-line chemotherapy7), it seems that a 3-week schedule of docetaxel would be difficult to applied to these specific populations in a clinical setting.
A weekly schedule may enable the delivery of repeated effective doses with minimal toxicity, which would lead to achieving increased drug dose intensity and maximized efficacy. Weekly schedules with the newer chemotherapeutic agents, such as paclitaxel8, 9) and docetaxel10-13), have recently been intensively tried in advanced NSCLC patients. The phase II and III trials that have compared a weekly schedule with a 3-week schedule have demonstrated that a weekly schedule could significantly reduce grade 3-4 neutropenia by 2~20% with similar therapeutic efficacy10-13).
We retrospectively compared the efficacy and toxicity of weekly docetaxel to those of a standard 3-week regimen as second-line chemotherapy for patients suffering with advanced NSCLC.
The eligible patients for this study were treated from October 2003 to March 2006 at Kangnam St. Mary's Hospital, and they were required to have histological or cytological proof of having stage IIIB or IV NSCLC, and they had least one bidimensionally measurable lesion outside the previously irradiated area and they had received only one prior platinum-containing chemotherapy regimen. The patients were also required to have a minimum ECOG performance status of (PS) Ōēż2 and adequate physiological organ functions: 1) hepatic function (a normal bilirubin level, a normal asparate aminotransferase level and a normal alanine aminotransferase level Ōēż1.5 the upper limit of normal (ULN) and an alkaline phosphatase level Ōēż5 ULN), 2) renal function (a serum creatinine level Ōēż1.5 ULN) and 3) hematological function (an absolute neutrophil count Ōēź 2├Ś109/L, a platelet count Ōēź100├Ś109/L and a hemoglobin level 10 g/dL).
Docetaxel was administered on a 3-week or weekly schedule. Three-week schedules were conducted from October 2003 to September 2004 and then weekly schedules were conducted from October 2004 to March 2006. For the 3-week docetaxel administration regimen, docetaxel 75 mg/m2 was given intravenously on day 1 of a 21-day cycle, whereas during the weekly regimen, docetaxel 37.5 mg/m2 was administered intravenously on days 1 and 8 of a 21-day cycle. Docetaxel was administered intravenously as a 1-h infusion, whereas premedications (dexamethasone 8 mg twice daily (orally) the day before chemotherapy and immediately after docetaxel infusion, and ranitidine 50 mg, diphenhydramine 50 mg and 5-hydroxytryptamine 3 receptor antagonist) were administered at the same dosage for each schedule. Docetaxel was discontinued if a patient experienced disease progression, irreversible grade 3/4 toxicity or wanted to discontinue the treatment. Chemotherapy was postponed until attaining recovery of the bone marrow function. The investigators determined to continue the consecutive chemotherapy when the patients experienced an objective decline in their performance status.
The patients were evaluated using computed tomography for determining the anti-tumor efficacy according to the WHO criteria at every 2 cycles. A complete response (CR) was defined as the disappearance of all tumor lesions. A partial response (PR) was defined as an estimated Ōēź50% decrease in tumor size with no detectable new lesions. Progressive disease (PD) was defined as the appearance of any new tumor lesions or a Ōēź25% increase in the size of the existing tumor lesions. When the responses did not qualify as CR, PR or PD, then they were reported as stable disease (SD). Toxicity was assessed on days 1 and 8 of a 21-day cycle for the weekly regimen and on day 1 of a 21-day cycle for the 3-week regimen. The toxicity was graded using the NCI CTC version 2.0.
The overall survival (OS) duration was defined as the time between the date of starting treatment and the date of the last follow-up or death. Time to progression (TTP) was defined as the time between the date of starting treatment and the date of progressive disease. The response duration was calculated from the day of the observed response until the day of documented progression. The disease control rate was defined as the proportion of patients with a partial or complete response to an investigational treatment plus those patients for whom their disease had stabilized. The durations of the OS and TTP were estimated using the Kaplan-Meier method and the differences between the Kaplan-Meier curves were evaluated by the log-rank test.
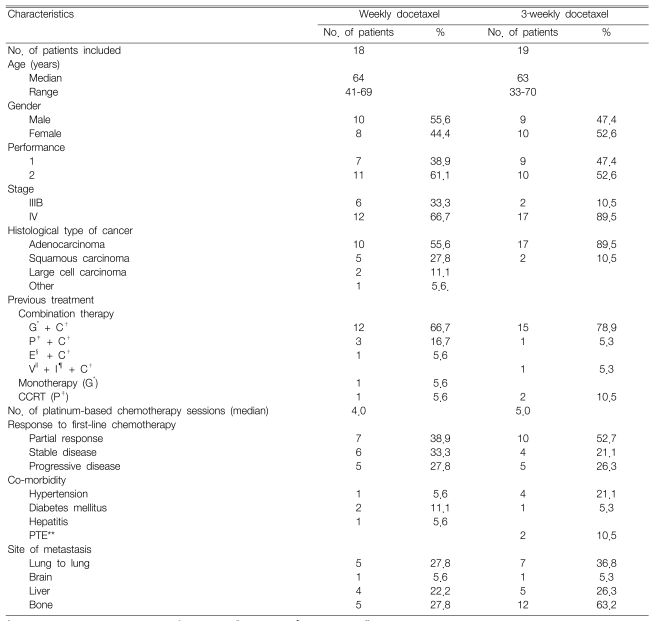
Our retrospective study included 37 patients who received docetaxel monotherapy as a second-line treatment between Oct 1, 2003 and March 31, 2006. One patient on the weekly schedule was excluded due to loss of follow-up. The patients' characteristics are summarized in Table 1. Eighteen patients received weekly administration of docetaxel, whereas 19 patients received a 3-week administration schedule of docetaxel. The median patient age was 64 (range: 41~69) for the weekly schedule and 63 (range: 33~70) for the 3-week schedule. The patients' gender ratio was similar for both the weekly and 3-week schedules. The patients with an ECOG PS of 2 or those with stage IV disease were examined more frequently during the 3-week schedule. The histopathology most frequently revealed adenocarcinoma, which was more common for the 3-week schedule (89.5%) than for the weekly schedule (55.6%). Thirty-three patients (89.2%) received platinum-based chemotherapy as a first-line treatment, and a combination of gemcitabine/cisplatin was the most common chemotherapy. Seven patients (38.9%) from the weekly schedule and 10 patients (52.7%) from the 3-week schedule responded to first-line treatment. Of the 37 patients in this study, nine had chronic diseases, five had hypertension and three had diabetes mellitus. The median time from diagnosis to initiation of docetaxel was 7.1 months (range: 1.9~74.1). A total of 135 chemotherapy cycles were administered with the median number of cycles/patient being 3.0 (range: 1~9).
A response evaluation was assessed for a total of 37 patients. The objective response rate was 16.7% (95% confidence interval: 0~36.0) for the weekly schedule and 21.1% for the 3-week schedule. Disease control was achieved in 10 patients (55.6%) for the weekly schedule and in 12 patients (63.2%) for the 3-week schedule. The length of response was a median of 2.2 months (range: 0.7~3.9) for the weekly schedule and 2.6 months (range: 0.2~7.6) for the 3-week schedule (Table 2). The median overall TTP was 2.8 months (95% confidence interval: 2.3~3.3) with a median TTP of 3.0 months (95% confidence interval: 1.9~4.0) for the weekly schedule and 2.8 months (95% confidence interval, 1.0~4.6) for the 3-week schedule. No significant differences were observed between the two different treatment schedules (p=0.41) (Figure 1). The median OS time was 12.0 months (95% confidence interval: 5.9~18.1) for all the patients with a median OS of 13.3 months (95% confidence interval: 6.3~20.3) for the weekly schedule and a median OS of 10.7 months (95% confidence interval: 8.3~13.0) for the 3-week schedule. The 2.6 months difference in the OS between the 2 groups was not statistically significant (p=0.41) (Figure 1). Out of the 24 patients (weekly schedule: 3 males and 6 females, 3-week schedule: 4 males and 7 females)who had been on a third-line therapy after docetaxel therapy, 20 received an EGFR tyrosine kinase inhibitor (TKI), and the remaining four were treated with gemcitabine/vinorelbine combination chemotherapy.
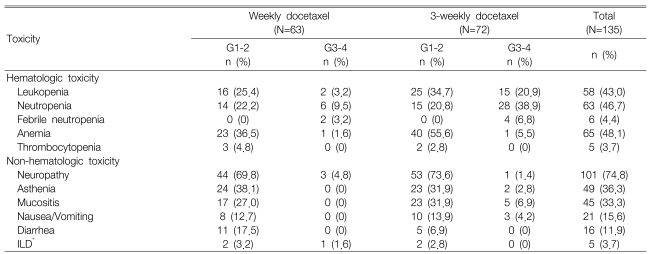
Toxicity was quantifiable in all 135 cycles and the treatment-linked severe toxicities including grade 3 or more neutropenia (25.2%), which occurred much more frequently in the 3-week schedule (28 cycles, 38.9%) as compared with the weekly schedule (6 cycles, 9.5%). Febrile neutropenia was observed during 2 cycles for the weekly schedule and this was observed during 4 cycles for the 3-week schedule. Grade 3 or more anemia occurred in only 2 cycles and no severe thrombocytopenia was observed. The non-hematologic toxicities were generally mild and they primarily included peripheral neuropathy (101 cycles, 74.8%), asthenia (49 cycles, 36.3%) and oral mucositis (45 cycles, 33.3%). Moreover, grade 3 or greater peripheral neuropathy occurred during three cycles (4.8%) of the weekly schedule and during one cycle (1.4%) of the 3-week schedule. Interstitial lung disease (ILD) was found in five cases, and one of these cases displayed grade 3 peripheral neuropathy (Table 3). ILD was diagnosed by high-resolution computed tomography and also with excluding the infectious causes. Administration of chemotherapy was postponed in 19 cycles. However, there was no justification to reduce the chemotherapy doses. The administered docetaxel dose intensity (D.I.) for the weekly schedule was 96% of the planned D.I. (25 mg/m2/week) and it was 95.6% for the 3-week schedule. Moreover, there was no treatment-related mortality; during the median follow-up of 8.7 months (range: 1.8~3.6) for 37 patients, 23 patients died of cancer progression and one patient died of a cerebrovascular event.
Platinum-based regimens have been the mainstay of lung cancer chemotherapy since a meta-analysis in 1995 demonstrated their statistically significant survival benefit over the best supportive care alone for treating advanced NSCLC1). Those advanced NSCLC patients who exhibit disease progression or local relapse after first-line chemotherapy may be candidates for second-line treatment. Two drug combination regimens have shown increased toxicities without any survival benefits, and the increased toxicity has sometimes led to toxicity-related deaths. Consequently, single-agent chemotherapy has been actively attempted, and this has become the preferred treatment option in a second-line setting14). Of the newer cytotoxic drugs that are used for treating NSCLC, gemcitabine, vinorelbine, taxane and pemetrexed monotherapy have been widely explored for treating NSCLC patients who failed their front-line treatment. Docetaxel monotherapy in the previous phase II studies has demonstrated promising results with a response rate of 16~22% and a median survival of 6.9~9.7 months5, 6). The phase III trials have shown statistically greater differences of the median survival time (5.7~7.5 months), the 1-yr survival rate (32~37%) and the quality of life in comparison to the control groups (the best supportive care, or vinorelbine or ifosfamide monotherapy), and this is despite the low response rates (less than 10%)2, 3). Vinorelbine3), paclitaxel9) and gemcitabine15) monotherapy have shown response rates of 20%, 0.8% and 14%, respectively, and a median survival duration of 5.5 months, 5.6 months and 3.7 months, respectively. However, these drugs have not been accepted as standard second-line chemotherapy for advanced NSCLC patients because of the poor clinical outcome compared to docetaxel monotherapy3, 9). A large-scale randomized phase III trial has recently compared pemetrexed and docetaxel as second-line monotherapy treatments, and the results have indicated similar efficacy and significantly improved safety4).
The TAX320 study was a 3-arm, phase III randomized trial that compared docetaxel (100 mg/m2 to start and then 75 mg/m2 every 3 weeks) and a "standard" arm of either vinorelbine or ifosfamide3). This trial established the superiority of docetaxel, at the intermediate dose of 75 mg/m2, for the response rate, the time to progression and the overall survival of the study patients. Shim et al. reported that 75 mg/m2 of docetaxel monotherapy every 3 week was also effective in a Korean population with a response rate of 18.2% and a median survival time of 11 months16).
Despite these promising results, docetaxel-associated toxicity is known to be a significant factor during therapy. In the TAX 320 trial3), the occurrence of grade 3 or more neutropenia was more frequent (54%) for the docetaxel 75 mg/m2 group compared to that of the vinorelbine or ifosfamide group (31%). In the effort to reduce the hematologic toxicities of the 3-week administration schedule, four randomized trials were conducted with using different dosages and schedules of the standard treatment. In a phase II trial conducted by Geravis et al10), similar efficacy was observed between a weekly schedule and 3-week schedule of docetaxel monotherapy with response rates of 3.2% and 4.8%, respectively, a median survival duration of 5.8 months and 5.5 months, respectively, a 1-yr survival rate of 6% and 18%, respectively. Moreover, several phase III trials have shown a response rate of 5.5~10.5% and 2.7~12.6%, respectively, and a median survival duration of 6.1~9.2 months and 5.8~6.7 months, respectively, for the weekly and 3-week regimens of docetaxel monotherapy11-13). In our study, there were no significantly differences in efficacy between the weekly and 3-week regimens with response rates of 16.7% and 21.1%, respectively. (p=0.57), median TTPs of 3 months and 2.8 months, respectively (p=0.41) and median OSs of 13.3 months and 9.3 months, respectively (p=0.41), for the weekly and 3-week regimens. Although the 3-week regimen group included more female patients and more adenocarcinoma patients, the proportion of female adenocarcinoma patients (54.5%) who received EGFR TKI as a third-line treatment was similar to that of the weekly regimen group (44.4%). In addition, several pharmacokinetics (PK) studies have provided data that the PK parameters, including the half life, clearance and area under the concentration-time curve (AUC) were similar between the weekly and 3-week schedule17, 18). Baker et al. have reported that the plasma concentration of docetaxel was maintained as 0.5 nM for 21 days in the 3-week docetaxel group and the plasma concentration was measured in the weekly group as 1.0 nM for the whole 7 day period after its administration17). It has been suggested that the anti-tumor efficacy of taxanes come about both by inhibiting the mobility of microtubules and the anti-angiogenesis effect at a relatively lower intracellular concentration19, 20). These data support the clinical results of weekly docetaxel monotherapy.
In a randomized phase III trial12), Scheutte et al. reported that a weekly docetaxel regimen was unlike the 3-week regimen with respect to the toxicity profile. The incidence of grade 3 or more neutropenia and anemia were 4.8% and 1.0%, respectively, in the weekly arm compared to 20.6% and 5.9%, respectively, in the 3-week arm. In our study, the incidence of grade 3 or more neutropenia in the 3-week arm and the weekly arm concurred with these previous findings (38.9% and 9.5%, respectively, in our study). Because polysorbate 80, which is a vehicle for docetaxel, competes with docetaxel itself for binding plasma ╬▒-1-acid glycoprotein, a higher dose of docetaxel may give rise to an increased plasma concentration of the unbound form21). Past studies have shown that the pharmacokinetics of the unbound forms, rather than that of the total docetaxel, are more closely correlated with the incidence and severity of neutropenia due to docetaxel administration22). Although the reason for the different toxicity profile has not been clearly determined, the above facts could be one of the possible explanations. In contrast to the clinical trials that used docetaxel in a first-line setting, the most common non-hematologic toxicity in our study was peripheral neuropathy, and this was seen in 74.8% of the patients. This finding might be associated with an accumulation of peripheral nerve injury by platinum because most of the patients had received platinum-based chemotherapy as a first-line treatment. Other toxicity symptoms included asthenia, oral mucositis and gastrointestinal symptoms. No difference was observed for the non-hematological toxicities between the two groups, except for peripheral neuropathy. In addition, severe peripheral neuropathy over grade 3 occurred more frequently in the patients of the weekly regimen (4.8%) as compared to that of the patients of the 3-week regimen (1.4%). In the CALGB 9840 study that compared paclitaxel 80 mg/m2 weekly (n=234) with 175 mg/m2 every 3 weeks (n=230) for treating advanced breast cancer patients, a severe sensory type of peripheral neuropathy (over grade 3) occurred in 19% of the patients of the weekly group and in 12% of the patients in the 3-weeks group (p=0.001)23). However, there was no significant difference of peripheral neuropathy between the schedules in the phase III trials of NSCLC that compared weekly docetaxel with 3-week docetaxel11, 13). With regard to pulmonary toxicity, ILD was found in five cases (3 weekly cases and 2 3-week cases). All the cases developed ILD 1 month after therapy had begun. In one case, a lung biopsy finding showed bronchiolitis obliterans/organizing pneumonia; infiltration of mononuclear cells into the alveolar septa and interstitial fibrosis was observed in 2 cases. Even though serious ILD of more than grade 3 was observed in one weekly regimen case, this patient recovered after steroid treatment. The co-morbid diseases afflicting the study patients included diabetes mellitus with nephropathy, COPD and decreased pulmonary function after previous lobectomy. Three cases received gemcitabine/cisplatin, one case received etoposide/cisplatin and another case received paclitaxel-based concurrent chemoradiotherapy as first-line chemotherapy. We cannot make conclusions about the relationship between the administration schedule and the non-hematologic toxicities (e.g., peripheral neuropathy, ILD, etc.) with such a small sample size.
Our study is limited by a small patient sample size, and their quality of life or their satisfaction with the treatment could not be evaluated because of the study's retrospective nature. Despite these limitations, this study affirmed that the weekly regimen of docetaxel monotherapy has an equally favorable toxicity profile compared to the 3-week schedule. This study supports that a weekly schedule of docetaxel monotherapy is a reasonable alternative for those NSCLC patients who fail with first-line platinum-based chemotherapy.
References
1. Non Small Cell Lung Cancer Collaborative Group. Chemotherapy in non small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomized clinical trials. BMJ 1995. 311:899ŌĆō909PMID : 7580546.



2. Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Brukes R, Coughlin S, Kim Y, Berille J. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000. 18:2095ŌĆō2103PMID : 10811675.


3. Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, Miller V, Lee JS, Moore M, Gandara D, Karp D, Vokes E, Kris M, Kim Y, Gamza F, Hammershaimb L. The TAX 320 Non-Small Cell Lung Cancer Study Group. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum containing chemotherapy regimens. J Clin Oncol 2000. 18:2354ŌĆō2362PMID : 10856094.


4. Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T, Lim HL, Desch C, Szondy K, Geravis R, Sharharyar , Manegold C, Paul S, Paoletti P, Einhorn L, Bunn PA Jr. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004. 22:1589ŌĆō1597PMID : 15117980.


5. Fossella FV, Lee JS, Shin DM, Calayag M, Huber M, Perez-Soler R, Murphy WK, Lippman S, Benner S, Glisson B. Phase II study of docetaxel for advanced or metastatic platinum refractory non-small-cell lung cancer. J Clin Oncol 1995. 13:645ŌĆō651PMID : 7884425.



6. Gandara DR, Vokes E, Green M, Bonomi P, Devore R, Comis R, Carbone D, Karp D, Belani C. Activity of docetaxel in platinum-treated non-small-cell lung cancer: results of a phase II multicenter trial. J Clin Oncol 2000. 18:131ŌĆō135PMID : 10623703.


7. Gridelli C, Perrone F, Monfardini S. Lung cancer in the elderly. Eur J Cancer 1997. 33:2313ŌĆō2314PMID : 9616273.


8. Inoue A, Usui K, Ishimoto O, Matsubara N, Tanaka M, Kanbe M, Gomi K, Koinumaru S, Saijo Y, Nukiwa T. A phase II study of weekly paclitaxel combined with carboplatin for elderly patients with advanced non-small cell lung cancer. Lung Cancer 2006. 52:83ŌĆō87PMID : 16483687.


9. Esteban E, Gonzalez de Sande L, Fernandez Y, Corral N, Fra J, Muniz I, Vieitez JM, Palcio I, Fernandez JL, Estrada E, Lacave AJ. Prospective randomized phase II study of docetaxel versus paclitaxel administered weekly in patients with non-small-cell lung cancer previously treated with platinum based chemotherapy. Ann Oncol 2003. 14:1640ŌĆō1647PMID : 14581272.


10. Gervais R, Ducolone A, Breton JL, Braun D, Lebeau B, Vaylet F, Debieuvre D, Pujol JL, Tredaniel J, Coulet P, Quoix E. Phase II randomized trial comparing docetaxel given every 3 weeks with weekly schedule as second line therapy in patients with advanced non small cell lung cancer (NSCLC). Ann Oncol 2005. 16:90ŌĆō96PMID : 15598944.


11. Gridelli C, Gallo C, Di Maio M, Barletta E, Illiano A, Maione P, Salvagni S, Poantedosi FV, Palazzolo G, Caffo O, Ceribelli A, Falcone A, Mazzanti P, Brancaccio L, Capuano MA, Isa L, Babera S, Perrone F. A randomized clinical trial of two docetaxel regimens (weekly vs. 3 week) in the second line treatment of non-small-cell lung cancer. Br J Cancer 2004. 91:1996ŌĆō2004PMID : 15558071.



12. Schuette W, Nagel S, Blankenburg T, Lautenschlaeger C, Hans K, Schmidt EW, Dittrich I, Schweisfurth H, von Weikersthal LF, Raghavachar A, Reissig A, Serke M. Phase III study of second line chemotherapy for advanced non-small-cell lung cancer with weekly compared with 3 weekly docetaxel. J Clin Oncol 2005. 23:8389ŌĆō8395PMID : 16293869.


13. Camps C, Massuti B, Jimenez A, Maestu I, Gomez RG, Isla D, Gonzalez JL, Amenar D, Blasco A, Rosell R, Carrato A, Vinolas N, Batista N, Giron CG, Galan A, Lopez M, Blanco R, Provencio M, Diz P, Felip E. Randomized phase III study of 3 weekly versus weekly docetaxel in pretreated advanced non small cell lung cancer. Ann Oncol 2006. 17:467ŌĆō472PMID : 16371411.


14. Barlesi F, Jacot W, Astoul P, Pujol JL. Second line treatment for advanced non-small cell lung cancer: a systematic review. Lung Cancer 2006. 51:159ŌĆō172PMID : 16360238.


15. Gridelli C, Perrone F, Gallo C, Rossi A, Barletta E, Barzelloni ML, Creazzola S, Gatani T, Fiore F, Guida C, Scognamiglio F. Single-agent gemcitabine as second-line treatment in patients with advanced non small cell lung cancer (NSCLC): a phase II trial. Anticancer Res 1999. 19:4535ŌĆō4538PMID : 10650806.

16. Shim BY, Jim CH, Song SH, Ahn MI, Hong EJ, Kim SW, Kim S, Jo MS, Cho DG, Cho KD, Yoo J, Kim HK. The safety and efficacy of second-line single docetaxel (75 mg/m2) therapy in advanced non-small cell lung cancer patients who were previously treated with platinum based chemotherapy. Cancer Res Treat 2005. 37:339ŌĆō343.




17. Baker SD, Zhao M, Lee CK, Verweij J, Zabelina Y, Brahmer JR, Wolff AC, Sparreboom A, Carduci MA. Comparative pharmacokinetics of weekly and every-three-weeks docetaxel. Clin Cancer Res 2004. 10:1976ŌĆō1983PMID : 15041715.


18. Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, Kaye SB, Verweij J, Fossella FV, Valero V, Rigas JR, Seidman AD, Chevallier B, Fumoleau P, Burris HA, Ravdin PM, Sheiner LB. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol 1998. 16:187ŌĆō196PMID : 9440742.


19. Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J, Sledge GW Jr. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res 2001. 61:3369ŌĆō3372PMID : 11309294.

20. Giannakakou P, Robey R, Fojo T, Blagosklonny MV. Low concentrations of paclitaxel induce cell type-dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: molecular determinants of paclitaxel-induced cytotoxicity. Oncogene 2001. 20:3806ŌĆō3813PMID : 11439344.


21. Sparreboom A, Zhao M, Brahmer JR, Verweij J, Baker SD. Determination of the docetaxel vehicle, polysorbate 80, in patient samples by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2002. 773:183ŌĆō190.


22. Loos WJ, Baker SD, Verweij J, Boonstra JG, Sparreboom A. Clinical pharmacokinetics of unbound docetaxel: role of polysorbate 80 and serum proteins. Clin Pharmacol Ther 2003. 74:364ŌĆō371PMID : 14534523.


23. Seidman AD, Berry D, Cirrincione C, Harris L, Dressler L, Muss H, Naughton M, Norton L, Winer E, Hudis C. CALGB 9840: phase III study of weekly (W) paclitaxel (P) via 1 hour(h) infusion versus standard (S) 3h infusion every third week in the treatment of metastatic breast cancer (MBC), with trastuzumab (T) for HER2 positive MBC and randomized for T in HER2 normal MBC. Proc Am Soc Clin Oncol 2004. 22:6s. (suppl 14; abstr 512).

-
METRICS

- Related articles
-
S-1 Monotherapy as a Neoadjuvant Treatment for Locally Advanced Gastric Cancer2008 March;23(1)





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


