 |
 |
| Korean J Intern Med > Volume 22(3); 2007 > Article |
|
Abstract
Background
The airway muscles from allergen-sensitized animals in vitro show a heightened response to histamine, but not to carbachol. This study investigated whether the airway responsiveness to histamine in vivo is comparable to that of methacholine in human subjects with varying degrees of atopy.
Methods
One-hundred-and-sixty-eight consecutive adult asthma patients or volunteers underwent bronchoprovocation tests to both histamine and methacholine after determining their blood eosinophil counts, serum total IgE levels and skin test reactivity to 10 common aeroallergens.
Results
The responsiveness to histamine was significantly related to that to methacholine (r=0.609, p<0.001), but many individuals with a negative methacholine test response showed a positive response to histamine. The histamine-bronchial reactivity index (BRindex) was significantly higher than the methacholine-BRindex in subjects with a positive response to none (n=69, p<0.01) or only one (n=42, p<0.001) of histamine and methacholine, while there was no significant difference in the subjects with positive responses to both of them (n=57). The histamine-BRindex was significantly higher than the methacholine-BRindex in the subjects with mild histamine hyperresponsiveness (n=58, 1.28±0.01 vs. 1.20±0.02, respectively, p<0.001). Both histamine and methacholine responsiveness was significantly related to the atopy markers. However, the histamine-BRindex/methacholine-BRindex ratio of the atopics was not significantly different from that of the non-atopics.
Conclusions
The airway responsiveness to histamine is comparable to that of methacholine in the subjects with positive responses to both histamine and methacholine, but the airway responsiveness to histamine is greater than that to methacholine in those subjects with mild airway hyperresponsiveness, regardless of atopy.
Atopic allergy, including asthma, is characterized not only by the enhanced production of allergen-specific IgE antibodies, but also by increased sensitivity of the target organs1). Some people can be sensitized to allergens, but not be allergic2), i.e., they lack increased sensitivity of the target organs; however, many individuals with respiratory allergy have an increased airway response specifically to allergens that they are sensitized to or they are nonspecifically sensitized to various stimuli such as histamine and methacholine. Airway responsiveness to histamine or methacholine is widely measured in general practice for diagnosing asthma3).
Although many patients with asthma have airway hyperresponsiveness (AHR) to various pharmacological or physical bronchoconstricting stimuli, it has been recognized that they often respond to one stimulus, but not to all other stimuli4). Antonissen et al.5, 6) demonstrated a heightened response to histamine, but not to a cholinergic agonist, in the airway muscles from allergen-sensitized animals. Histamine is the typical mediator of the allergic response, and leukotrienes, another allergic mediator, increase the number of histamine receptors7). Therefore, atopic individuals might have an increased specific sensitivity to an allergic mediator. However, Juniper, et al.8) have showed that the histamine-AHR is comparable to the methacholine-AHR in asthma patients, so both tests are used interchangeably in general practice to diagnose asthma. This study investigated whether the airway responsiveness to histamine is comparable to that of methacholine in subjects with varying degrees of atopy.
One-hundred-and-sixty-eight consecutive adult asthma patients and healthy volunteers whose ages were less than 50 years were recruited from Chonnam National University Hospital. The enrolled patients were mostly young adult men who required a medical certificate for asthma in order to be exempted from obligatory military service, and medical students and hospital employees, including doctors and nurses, were also enrolled. The participants were classified as 3 groups, based on their responses to methacholine and histamine bronchoprovocation tests. Sixty-nine subjects had negative responses to both tests (the both negative group); 42 subjects had a positive response to one test only (the sole positive group), and the other 57 subjects had positive responses to both tests (the both positive group). The Institutional Review Board of Chonnam National University Hospital approved the study. All the patients were informed of the experimental procedures and they provided written informed consent for participation.
On the first day of the study, baseline spirometry, allergy skin-prick tests and laboratory tests for the complete blood counts and measuring the total serum IgE were performed after taking a history and conducting a physical examination. The airway responsiveness to methacholine or histamine (selected randomly) was then measured. The test was performed again the next day, at the same time of day, with the other agent.
The total IgE levels were determined using nephelometry (normal < 100 IU/mL; Behring Diagnostics GmbH, Germany). Allergy skin-prick tests were carried out using ten common aeroallergen extracts (Allergopharma, Reinbek, Germany): Dermatophagoides farinae, D. pteronyssinus, cockroach (Periplaneta americana), cat, dog, Aspergillus, hazel, birch, timothy and ragweed; histamine (1 mg/mL) and saline (0.9%) solutions were used as the positive and negative controls, respectively. The longest and perpendicular diameters of each wheal were measured using vernier calipers (Absolute Digimatic; Mitutoyo, Japan) 15 minutes after a prick with a 26-gauge needle, and the arithmetic mean of the recorded measurements was used as the representative value. Reactivity was graded according to the ratio of the size of the allergen-induced wheal to the size of the wheal elicited by the histamine solution (the A/H ratio), and this was categorized as follows: 1+: 25-49%, 2+: 50~99%, 3+: 100~199%, 4+: ≥200%. The sums of the grades for the ten allergens and for the two house dust mite extracts were defined as the atopy score and the house dust mite score, respectively. The skin test reactivity of ≥3+ was regarded as indicative of a clinically significant positive response10), and the subjects having at least one significant positive response were deemed as atopic.
The bronchial challenge tests were followed a standardized tidal breathing method11). The forced expiratory volume in one second (FEV1) was measured in triplicate before the test and in duplicate (at 30 and 90 sec) after each inhalation period with using a spirometer (Spiro Analyzer ST-250, Fukuda Sangyo, Tokyo, Japan). The selected predictive equation for FEV1 was that recommended by the Intermountain Thoracic Society12). Isotonic saline, followed by a methacholine (Sigma-Aldrich, St. Louis, MO) solution or a histamine (Sigma-Aldrich) solution, was aerosolized at room temperature in a DeVilbiss 646 nebulizer (DeVilbiss, Somerset, PA; output 0.13 mL/min). In view of the chemical stability of methacholine and histamine solutions, they were prepared fresh on the morning of each challenge day. The dilution increments used in the first year of this study were 0.075, 0.15, 0.31, 0.62, 1.25, 2.5, 5.0, 10 and 25 mg/mL. Since many of the subjects could not tolerate the highest histamine dose, this highest dose was then reduced from 25 to 16 mg/mL in the second year. The aerosols were inhaled by tidal breathing over a 2 minute period at 5-min intervals, through the mouth with the nose clipped. The challenge test was discontinued if the FEV1 dropped by 20% or more from the post-saline FEV1, or if the maximum concentration of agonist was administered. The provocation concentration of methacholine or histamine resulting in a 20% fall in the FEV1 (PC20) was calculated by linear interpolation of the log-dose-response curve. A positive test response was defined as PC20 <16 mg/mL. Since the actual PC20 values were unobtainable for the subjects with a negative test response, the bronchial reactivity index (BRindex), as another index of AHR, was calculated using the following equation: log10 (10 + the maximal % fall in FEV1/log10 (the dose in mg/dL of the stimulus required to produce it))13).
The variables were summarized as the mean±SEM. All calculations of IgE and PC20 were performed after log transformation. Student's t-test, one way analysis of variance (ANOVA), and Chi-Square tests were used to determine the significance of inter-group differences. The paired Student's t-test was used for intra-group comparisons. Associations between variables were examined using Pearson's correlation coefficient. A value of p<0.05 was considered statistically significant.
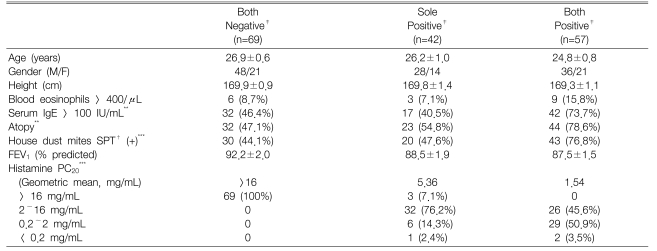
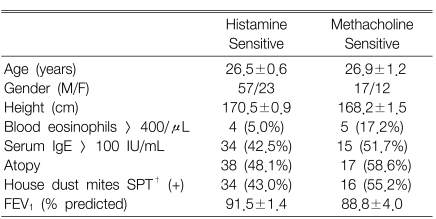
The clinical characteristics of the subjects are presented in Table 1. The age, gender, height and baseline FEV1 values did not differ significantly among the groups. However, the histamine PC20 and atopy markers, except the blood eosinophil counts, i.e., the serum total IgE levels, the atopy score and house dust mite score, were significantly different among the groups. The sole positive group had a mild AHR to histamine (PC20: 2~16 mg/mL) in 83.3% of the subjects as compared to the both positive group that had a mild AHR to histamine in 45.6% of the subjects (p<0.001).
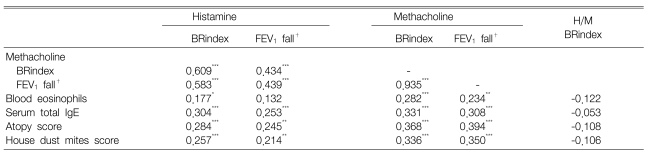
The BRindex and maximal % fall in FEV1 in response to histamine were significantly related to the responses to methacholine (BRindex: r=0.609, maximal FEV1 fall: r=0.439; all p<0.001) (Figure 1, Table 2). However, many values were distributed in the upper areas of the lines of identity; in other words, the histamine-BRindex and FEV1 fall were higher in 113 (67.3%) and 109 (64.9%) subjects, respectively, and lower only in 53 (31.5%) and 50 (29.8%) subjects, respectively, than the methacholine-BRindex and FEV1 fall, respectively. The BRindex and FEV1 fall of the response to histamine were significantly higher than those to methacholine (1.24±0.01 vs. 1.18±0.01 and 20.6±0.9 vs. 15.5±0.8%, respectively, all p<0.001).
Thirty-eight individuals with a negative methacholine test response showed a positive response to histamine, whereas only four persons with a negative histamine test response showed a positive response to methacholine. The BRindex of the response to histamine was significantly higher than that to methacholine in the both negative group (1.12±0.01 vs. 1.09±0.01, p<0.01) and the sole positive group (1.29±0.02 vs. 1.14±0.01, p<0.001), while there was no significant difference in the both positive group (1.37±0.02 vs. 1.36±0.02, p>0.05) (Figure 2). Similarly, the maximal % fall in the FEV1 for the response to histamine was significantly higher than that to methacholine in the both negative group (10.8±0.8 vs. 8.3±0.8%, p<0.01) and the sole positive group (27.4±1.8 vs. 12.7±1.0%, p<0.001), while there was no significant difference in the both positive group (27.3±1.0 vs. 26.3±1.1%, p>0.05). In addition, the BRindex and FEV1 fall for the response to histamine were significantly higher than those to methacholine in the subjects with mild histamine AHR (n=58, 1.28±0.01 vs. 1.20±0.02 and 26.8±1.1 vs. 17.5±1.3%, all p<0.001).
Both histamine and methacholine responsiveness were significantly related to the blood eosinophil counts, the serum total IgE levels, the atopy score and the skin responsiveness to the house dust mite extracts (Table 2). However, the histamine-/methacholine-BRindex ratio was not significantly related to the atopy markers. Since the airway responsiveness to histamine was significantly higher than that to methacholine in the both negative group and the sole positive group, but not in the both positive group, the measurements of the atopic markers were compared between the histamine-sensitive and methacholine-sensitive groups as classified according to the histamine-BRindex/methacholine-BRindex ratio in the both negative group and the sole positive group (Table 3). There was no significant difference between the groups. In addition, the histamine-BRindex/methacholine-BRindex ratio in the atopics (n=55) was not significantly different from that in the non-atopics (n=45) whose atopy scores were zero (1.07±0.02 in the atopics vs. 1.08±0.02 in the non-atopics, p=0.622). The subjects with mild histamine AHR also showed a similar result [1.07±0.02 in the atopics (n=32) vs. 1.09±0.03 in the non-atopics (n=20), p=0.451].
The histamine-BRindex/methacholine-BRindex ratio was significant higher in the subjects with histamine-AHR or methacholine-AHR than in those subjects without it, regardless of atopy (Figure 3). Among the subjects without AHR, the ratio in the atopics was not higher than that in the others; the subjects with AHR also showed a similar result. Many of the subjects complained of side effects following high doses of inhaled histamine.
In this study, the histamine-AHR was significantly related to, but significantly greater than the methacholine-AHR in the both negative group and the sole positive group, whereas it did not differ in the both positive group. Juniper et al.7) investigated patients with asthma and found that the histamine-AHR was comparable to the methacholine-AHR, which is consistent with our results of the both positive group. However, the greater AHR to histamine versus methacholine found in the subjects with mild histamine AHR suggests that further work is necessary for correctly interpreting the histamine-AHR test results of these groups. Woolcock et al.14) also reported greater sensitivity to histamine versus methacholine in COPD patients, whereas there was no difference in asthma patients. Cockcroft15) suggested a higher sensitivity to histamine versus methacholine even in asthma patients. It seems unlikely that the differences in sensitivity in our study resulted from any difference in the chemical stability of the histamine and methacholine solutions because the solutions were prepared fresh on the morning of each challenge day.
In addition, this study showed that AHR was related to atopy. It has been shown that the methacholine-AHR is significantly related to eosinophilia16), the total IgE level17) and the skin test reactivity to allergens18) in asthma patients, which is consistent with our results. Then, because histamine is the typical mediator of the atopic allergic reaction and the number of histamine receptors is increased by leukotrienes6), it can be speculated that atopic individuals may be specifically sensitive to histamine. Not only the animal studies by Antonissen et al.4, 5) but also the in vitro studies using human bronchial tissues19, 20) have shown that exposure to allergen or an allergic mediator increases the response to histamine, but not the response to cholinergic agents. Moreover, Spector and Farr21) reported that patients with asthma were more sensitive to histamine than to methacholine, and atopic asthmatics could not tolerate high concentrations of histamine. Cockcroft et al.22) found that the allergen-induced increase in reactivity to histamine tended to be greater than that to methacholine, and it tended to persist longer.
However, for the patients with positive responses to both histamine and methacholine in this study, their histamine-AHR was not significantly different from their methacholine-AHR. Nonspecific AHR in asthma occurs via a variety of mechanisms, including genetic linkage, airway inflammation, smooth muscle alteration and so on. Therefore, even if the histamine-specific hypersensitivity in atopics would contribute to the overall AHR, it may be trivial in full-blown asthma, resulting in minimal difference between the histamine-AHR and the methacholine-AHR. Alternatively, due to the airway obstruction in asthma, the inhaled histamine may not readily reach its receptors, which are primarily localized in small airways23), and this is unlike the methacholine receptors that are predominately localized in the central airways24), and so this obscures the atopy-related histamine-specific AHR.
Even in the subjects with mild histamine AHR in this study, atopy was not associated with an increased reactivity to histamine over methacholine. This discrepancy between our study and the previous studies4, 5, 19-22) may be related to the difference in disease activity between them. Most of the subjects in this study had no symptoms of atopic diseases on the study day, and only about 1/10 of the subjects had blood eosinophilia. The presence of AHR may suggest that the underlying airway disease is somewhat active, for which the leukotrienes that increase histamine receptor numbers are working without regard to atopy. Moreover, chronic exposure to histamine in active allergic diseases may decrease the response to histamine through the mechanism of tachyphylaxis25). Longitudinal studies are needed to examine whether histamine reactivity is predominantly increased during exacerbation of atopic diseases.
Inhaled histamine more frequently produces side effects such as throat irritation, flushing and headache than does inhaled methacholine7). Many of our subjects complained of side effects when given high doses of inhaled histamine. Unfortunately, we did not record their side effects in detail and so we could not determine whether the atopic individuals had more side effects than the non-atopic individuals. Further, a positive bronchial challenge test does not always mean that a patient has asthma because AHR has been described in patients with allergic rhinitis and also in those patients with airflow limitation caused by conditions other than asthma3). Therefore, further investigations involving taking a through history of allergy symptoms and conducting peak expiratory flow monitoring for a considerable period of time are required.
Notes
This study was financially supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea(AO50174)) and the Chonnam National University Hospital Research Institute (grant number: CUHRI-U-200448).
References
1. Demoly P, Piette V, Bousquet J. In: Adkinson NF, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FE, eds. In vivo methods for study of allergy. Middleton's allergy principle & practice 2003. 6th ed. Philadelphia: Mosby, 631–643.

2. Wasserman SI. In: Goldman L, Ausiello D, eds. Approach to the patient with allergic or immunologic disease. Cecil textbook of medicine 2004. 22nd ed. Philadelphia: Saunders, 1590–1593.

3. Global strategy for asthma management and prevention. Global Initiative for Asthma revised 2006. 19. Avail from: www.ginasthma.org.
4. O'Byrne PM. In: Middleton E, Reed CE, Ellis EF, Adkinson NF, Yunginger JW, Busse WW, eds. Airway hyperresponsiveness. Middleton's allergy principle & practice 1998. 5th ed. Philadelphia: Mosby, 859–866.

5. Antonissen LA, Mitchell RW, Kroeger EA, Kepron W, Tse KS, Stephens NL. Mechanical alterations of airway smooth muscle in a canine asthmatic model. J Appl Physiol 1979. 46:681–687PMID : 457546.

6. Antonissen LA, Mitchell RW, Kroeger EA, Kepron W, Stephens NL, Bergen J. Histamine pharmacology in airway smooth muscle from a canine model of asthma. J Pharmacol Exp Ther 1980. 213:150–155PMID : 7359363.

7. Pynaert G, Grooten J, van Deventer SJ, Peppelenbosch MP. Cysteinyl leukotrienes mediate histamine hypersensitivity ex vivo by increasing histamine receptor numbers. Mol Med 1999. 5:685–692PMID : 10602777.


8. Juniper EF, Frith PA, Dunnett C, Cockcroft DW, Hargreave FE. Reproducibility and comparison of responses to inhaled histamine and methacholine. Thorax 1978. 33:705–710PMID : 746496.



9. Weiss ST, Speizer FE. In: Weiss EB, Stein M, eds. Epidemiology and natural history. Bronchial asthma mechanisms and therapeutics 1993. 3rd ed. Toronto: Little Brown & Co, 15–25.
10. Adinoff AD, Rosloniec DM, McCall LL, Nelson HS. Immediate skin test reactivity to food and drug administration approved standardized extracts. J Allergy Clin Immunol 1990. 86:766–774.


11. Sterk PJ, Fabbri LM, Quanjer PH, Cockcroft DW, O'Byrne PM, Anderson SD, Juniper EF, Malo JL. Airway responsiveness: standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Eur Respir J Suppl 1993. 16:53–83PMID : 8499055.


12. Morris AH, Kanner RE, Crapo RO, Gardner RM. Clinical pulmonary function testing: a manual of uniform laboratory procedures 1984. 2nd ed. Salt Lake City: Intermountain Thoracic Society, 101.
13. Burrows B, Sears MR, Flannery EM, Herbison GP, Holdaway MD. Relationships of bronchial responsiveness assessed by methacholine to serum IgE, lung function, symptoms, and diagnoses in 11-year-old New Zealand children. J Allergy Clin Immunol 1992. 90:376–385PMID : 1527320.


14. Woolcock AJ, Anderson SD, Peat JK, Du Toit JI, Zhang YG, Smith CM, Salome CM. Characteristics of bronchial hyperresponsiveness in chronic obstructive pulmonary disease and in asthma. Am Rev Respir Dis 1991. 143:1438–1443PMID : 2048834.


15. Cockcroft DW. In: Barnes PJ, Grunstein MM, Leff AR, Woolcock AJ, eds. Airway responsiveness. Asthma 1997. Philadelphia: Lippincott Raven, 1253–1266.
16. Spector SL, Farr RS. A comparison of methacholine and histamine inhalations in asthmatics. J Allergy Clin Immunol 1975. 56:308–316PMID : 1176722.


17. Cockcroft DW, Ruffin RE, Dolovich J, Hargreave FE. Allergen-induced increase in non-allergic bronchial reactivity. Clin Allergy 1977. 7:503–513PMID : 589783.


18. Johnson PR, Ammit AJ, Carlin SM, Armour CL, Caughey GH, Black JL. Mast cell tryptase potentiates histamine-induced contraction in human sensitized bronchus. Eur Respir J 1997. 10:38–43PMID : 9032489.


19. Black JL, Marthan R, Armour CL, Johnson PR. Sensitization alters contractile responses and calcium influx in human airway smooth muscle. J Allergy Clin Immunol 1989. 84:440–447PMID : 2477428.


20. Durham SR, Kay AB. Eosinophils, bronchial hyperreactivity and late-phase asthmatic reactions. Clin Allergy 1985. 15:411–418PMID : 4053332.


21. Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med 1991. 325:1067–1071PMID : 1891008.


22. Sears MR, Burrows B, Herbison GP, Holdaway MD, Flannery EM. Atopy in childhood: II. relationship to airway responsiveness, hay fever and asthma. Clin Exp Allergy 1993. 23:949–956PMID : 10779283.


23. White J, Eiser NM. The role of histamine and its receptors in the pathogenesis of asthma. Br J Dis Chest 1983. 77:215–226PMID : 6137233.


Figure 1
Relationships of the bronchial reactivity index (BRindex), which was calculated using a formula: log10 (10 + the maximal % fall in FEV1/log10 (the dose in mg/dL of the stimulus required to produce it)), and maximal % fall in the forced expiratory volume in one second (FEV1) after a bronchial challenge between histamine and methacholine. The dashed line is the line of identity.
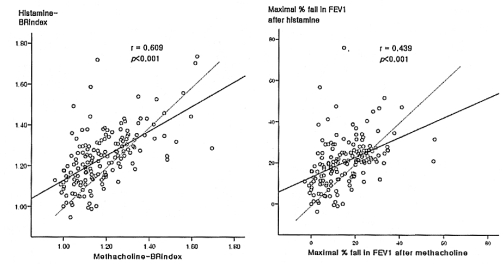
Figure 2
Comparisons of the bronchial reactivity index (BRindex) and maximal % fall in the forced expiratory volume in one second (FEV1) after a bronchial challenge between histamine and methacholine in the subjects with negative responses to both, a positive response to one, or positive responses to both histamine and methacholine tests.
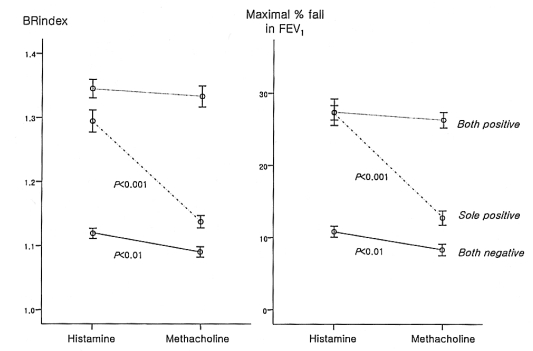
Figure 3
Histamine (H)/methacholine (M)- bronchial reactivity index (BRindex) ratios in the study groups.
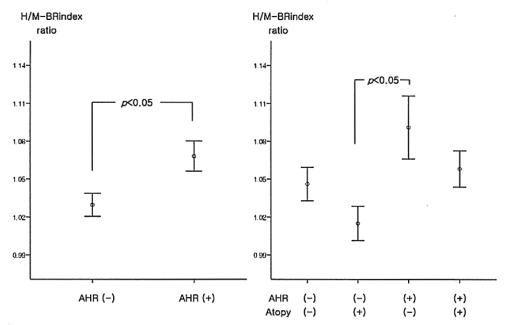
Table 1
Clinical characteristics of the subjects grouped according to their responsiveness to histamine and methacholine
-
METRICS

-
- 4 Crossref
- 0 Scopus
- 12,103 View
- 80 Download
- Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


