 |
 |
| Korean J Intern Med > Volume 30(5); 2015 > Article |
|
Abstract
Currently, the most effective treatment for end-stage liver fibrosis is liver transplantation; however, transplantation is limited by a shortage of donor organs, surgical complications, immunological rejection, and high medical costs. Recently, mesenchymal stem cell (MSC) therapy has been suggested as an effective alternate approach for the treatment of hepatic diseases. MSCs have the potential to differentiate into hepatocytes, and therapeutic value exists in their immune-modulatory properties and secretion of trophic factors, such as growth factors and cytokines. In addition, MSCs can suppress inflammatory responses, reduce hepatocyte apoptosis, increase hepatocyte regeneration, regress liver fibrosis and enhance liver functionality. Despite these advantages, issues remain; MSCs also have fibrogenic potential and the capacity to promote tumor cell growth and oncogenicity. This paper summarizes the properties of MSCs for regenerative medicine and their therapeutic mechanisms and clinical application in the treatment of liver fibrosis. We also present several outstanding risks, including their fibrogenic potential and their capacity to promote pre-existing tumor cell growth and oncogenicity.
Although the liver has a considerable inherent regenerative capacity [1], sustained and chronic injury results in the onset of liver fibrosis. Stimuli such as viral hepatitis, alcohol, drugs, metabolic diseases, and autoimmune attack by hepatic cells trigger hepatocyte apoptosis, the impairment of the endothelial barrier, the recruitment of inflammatory cells and the activation of hepatic stellate cells (HSCs) [2-9]. Liver fibrosis is the result of an imbalance in extracellular matrix (ECM) synthesis and degradation mediated by portal fibroblasts, bone marrow-derived fibroblasts, mesenchymal cells, and activated HSCs [10]. Currently, liver transplantation is the only effective treatment for end-stage liver fibrosis [11].
Recently, stem cell transplantation has been suggested as an effective alternative therapy for hepatic diseases [12]. Alison et al. [13] and Theise et al. [14] have reported the presence of Y chromosome-positive hepatocytes in autopsied women who had received therapeutic bone marrow transplantations from male donors, suggesting the existence of pluripotent stem cells among their bone marrow cells. Moreover, stem cells, including embryonic, induced pluripotent, hematopoietic and mesenchymal stem cells (MSCs), can be differentiated into hepatocyte-like cells both in vitro and in vivo [15-17]. Of these stem cell types, MSCs have several advantages, such as easy acquisition, strong proliferative capacities and ex vivo expansion. In addition, MSCs have immune-modulatory properties and are able to migrate to damaged tissues. MSCs also secrete trophic factors, including growth factors and cytokines, which promote the regeneration of impaired tissues, including the liver.
In this review, we summarize (1) the properties of MSCs for regenerative medicine, (2) the therapeutic mechanisms of MSCs in the treatment of liver fibrosis, and (3) the clinical application of MSCs for the treatment of liver fibrosis. We also present several outstanding risks associated with their use, including their fibrogenic, tumor cell growth promotion and oncogenic potentials.
MSCs are a promising source for cell-based tissue engineering and regenerative medicine. MSC transplantation is considered safe and has been widely tested in clinical trials of cardiovascular, neurological and immunological diseases with encouraging results. The properties of MSCs can be represented by their basic characteristics as stem cells and their therapeutic potentials as drugs. With regard to their basic characteristics, MSCs have the potential for self-renewal and differentiation into multiple types of cells. Sufficient numbers of these MSCs can be expanded without the loss of their potential for clinical application. In addition, MSCs can move toward areas of injury in response to signals of cellular damage, which are known as homing signals. This migration property of MSCs is important in regenerative medicine because various injection routes can be used depending on the damaged tissue or organ. MSCs can be transplanted into the liver by intravenous, intraperitoneal, intrahepatic, intrasplenic, or portal-venous injection, although the reported effectiveness has differed slightly based on the injection route and research group. MSCs are characterized by low expression of human leukocyte antigen (HLA) class I molecules and the absence of major histocompatibility complex (MHC) class II antigens, Fas ligand and the co-stimulatory molecules B7-1, mB7-2, CD40, and CD40L. These reduced immunogenic expression profiles cause MSCs to have immuno-tolerant phenotypes, allowing them to be used in allogeneic transplantation [18,19].
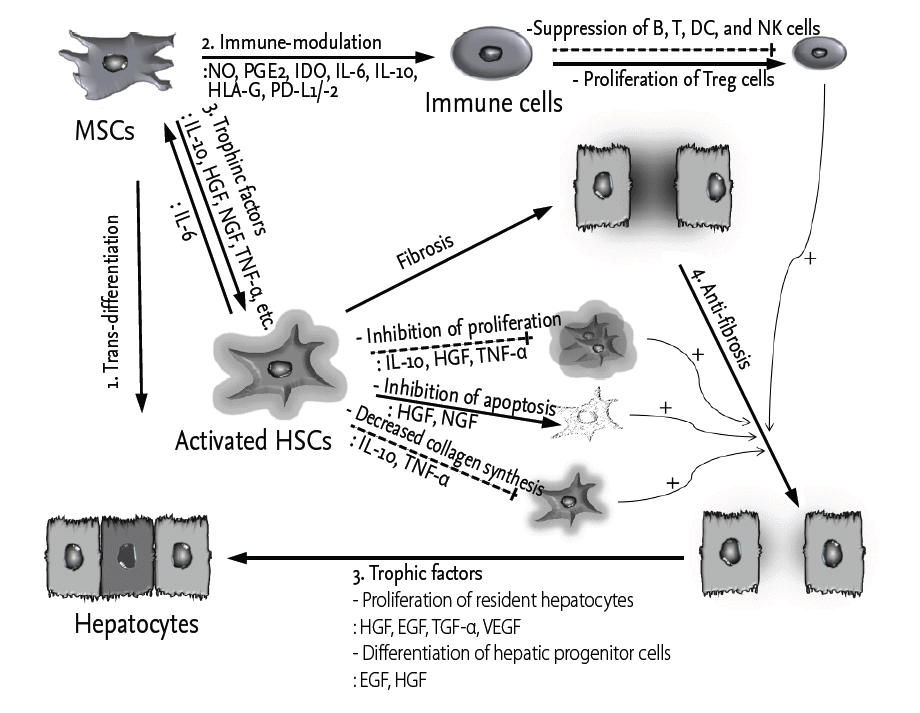
The therapeutic properties of MSCs that are relevant to liver fibrosis are related to their capacities for hepatocyte-like differentiation and their immune-modulatory, trophic factor secretory, anti-fibrotic, and anti-oxidant activities (Fig. 1). MSCs can be differentiated into multiple cell lineages, including hepatocytes, both in vivo and in vitro. They play immune-modulatory roles both in the adaptive and innate immune systems by suppressing T- [20], B- [21,22], dendritic [23,24], and nature killer (NK) cells [25] or promoting the generation of regulatory T (Treg) cells via a mechanism involving interleukin 10 (IL-10) [26,27]. MSCs express various trophic factors (growth factors and cytokines) that stimulate resident cells and matrix remodeling to promote the differentiation of native progenitor cells and the recovery of injured cells. In fibrotic tissue, MSCs can down-regulate myofibroblasts and lead to anti-fibrotic activity. In addition, MSCs display cytoprotective effects by inducing anti-oxidant response elements (AREs) in carbon tetrachloride (CCl4)- and thioacetamide (TAA)-induced liver injury [28,29].
Hepatocyte-like cells differentiated from MSCs are promising sources of liver regeneration. MSCs can be differentiated into hepatocyte-like cells by treatment with hepatocyte growth factor (HGF), fibroblast growth factor (FGF)-2/-4, epidermal growth factor (EGF), oncostatin M, leukemia inhibitory factor, dexamethasone, insulin-transferrin-selenium, and/or nicotinamide [30]. Hepatocyte-like cell differentiation can also be induced by co-culture with liver cells [31] and pellet culture [32]. Moreover, human bone marrow-derived MSCs can be differentiated into hepatocyte-like cells without fusion in allyl alcohol-treated rat livers [33]. However, the trans-differentiation of MSCs into hepatocytes has been rarely observed (less than 1% of the total liver mass) in animal models in relation to the amounts injected [34].
MSCs can express various soluble factors, such as nitric oxide, prostaglandin E2 (PGE2), indoleamine 2,3-dioxygenase (IDO), IL-6, IL-10, and HLA-G. These soluble factors regulate the proliferation and functions of a variety of immune cells and induce Treg cells [35]. In particular, PGE2 increases the anti-inflammatory cytokine IL-10 and decreases tumor necrosis factor ╬▒ (TNF-╬▒), interferon ╬│ (IFN-╬│), and IL-12 in dendritic cells (DCs). PGE2 also reduces IFN-╬│ and IL-4 in Th1 and Th2 cells and stimulates the proliferation of Treg cells [35]. Moreover, IDO and HLA-G suppress the proliferation of effector T cells, inhibit the maturation of DCs, inhibit the proliferation and immunoglobulin G secretion of B cells and reduce the cytotoxicity of NK cells [36,37]. In addition to the secretion of soluble factors from MSCs, these cells can suppress the activation of immune cells through direct cell-cell contact. MSCs can inhibit T-cell proliferation by inducing the apoptosis of effector T cells by promoting the association of programmed death-1 (PD-1) with its ligands PD-L1 and PD-L2, and MSCs are capable of rendering T cells anergic by down-regulating the expression of the co-stimulatory molecules CD80 and CD86 on antigen-presenting cells [38-40]. Unbalanced immune cell populations or immune cell infiltration of the liver can disrupt its immune-privileged state, resulting in liver injury or fibrosis. Therefore, the immune-modulatory potential of MSCs plays an important role in the treatment of liver fibrosis.
Accumulating evidence has revealed that various trophic factors secreted by MSCs play key therapeutic roles in regenerative medicine. MSCs express trophic factors, such as growth factors, cytokines, and chemokines, which are known not only to reduce the inflammation, apoptosis and fibrosis of damaged tissues but also to stimulate angiogenesis and tissue cell regeneration [41]. Moreover, after MSCs move to damaged sites for repair, they are stimulated by local factors, such as inflammatory cytokines, ligands of Toll-like receptors and hypoxic conditions. These stimuli lead to the production of a large amount of growth factors that perform multiple functions to achieve tissue regeneration [42,43]. Hepatocytes in fibrotic livers reach replicative senescence after many rounds of injury and repair; however, trophic factors secreted by MSCs can lead to the survival of living and dying hepatocytes via anti-apoptotic (stromal cell-derived factor 1, HGF, insulin-like growth factor 1 [IGF-1], and vascular endothelial growth factor [VEGF]), mitogenic (EGF, HGF, nerve growth factor [NGF], and transforming growth factor ╬▒ [TGF-╬▒]), and angiogenic effects (VEGF) [44-46]. Anti-apoptotic events that correlate with reduced inflammation have been observed in fibrotic tissues following MSC transplantation in association with alterations in HGF and IGF-1 expression [47,48]. HGF, EGF, and TGF-╬▒, which are potent mitogens, are primarily associated with hepatocyte proliferation [49-52], and VEGF enhances angiogenesis, which is responsible for liver regeneration. In addition to hepatocytes, hepatic progenitor cells, which are located in the canals of Hering, can be differentiated into hepatocytes or biliary lineage cells following treatment with EGF or HGF, respectively [53]. Trophic factors, such as IL-10, HGF, NGF, TGF-╬▓, and TNF-╬▒, regulate the proliferation of activated HSCs and decrease collagen synthesis in liver fibrosis.
Liver fibrosis, which is the precursor to cirrhosis, is the result of the deposition of ECM proteins and is mediated primarily by activated HSCs. Following liver injury, HSCs undergo a phenotypic switch from quiescent, vitamin A-storing cells to proliferative, ╬▒-smooth muscle actin (SMA)-positive, myofibroblast-like cells with increased collagen synthesis. Interestingly, the anti-fibrotic activities of MSCs have been reported in various fibrotic animal models in the heart, liver, kidneys, lungs, peritoneum, pancreas, skin, and rectum [54]. MSCs suppress the pathophysiological process that is mediated by chronic inflammation, and this immunosuppressive mechanism contributes to a modification of the microenvironment; the result is diminished tissue fibrosis, increased resident stem cell proliferation and eventually tissue regeneration.
Moreover, MSCs are able to reduce the proliferation of activated HSCs and collagen synthesis through indirect or direct cell-cell contact. In indirect contact mode, trophic factors (i.e., IL-10, HGF, TGF-╬▓3, and TNF-╬▒) that are secreted by MSCs inhibit the proliferation of HSCs and decrease collagen synthesis [55,56], while HGF and NGF promote the apoptosis of HSCs [55,57]. MSCs that are directly co-cultured with HSCs significantly suppress the proliferation and ╬▒-SMA expression of HSCs through cell-cell contact, and this activity is partially mediated by Notch pathway activation [58]. Furthermore, MSCs can regulate the expression of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs). In several fibrosis models, MSCs have been shown to increase the expression of MMPs (i.e., MMP-2, -9, -13, and -14) [59-61] or to decrease TIMP-1 expression [60,62], and these alterations are generally associated with fibrosis resolution.
Reactive oxygen species (ROS) trigger oxidative stress, which induces liver diseases such as liver fibrosis, cirrhosis, viral hepatitis, hepatocellular carcinoma (HCC), and others [63-67]. CCl4 and TAA are toxins used worldwide to generate experimental liver injury [29,68,69]. These toxins stimulate ROS production, which results in hepatocyte damage through lipid peroxidation and the alkylation of proteins, nucleic acids, and lipids [29,70-72]. MSCs have been shown to overcome CCL4- and TAA-induced oxidative stress in vitro and to reduce liver injury through anti-oxidant activities in vivo [28,29]. The up-regulation of ROS in CCl4-treated liver cells has been reported to be attenuated by co-culturing with MSCs via an increase in superoxide dismutase activity and the induction of AREs, which represents a cytoprotective response in the injured liver [29]. Additionally, MSCs protect hepatocytes by reducing ROS damage that is induced by TAA both in vivo and in vitro [28].
Clinical trials using MSCs have been designed to investigate their therapeutic potentials for the treatment of cirrhosis (Table 1). In a phase 1 trial, autologous bone marrow-derived MSCs were infused through the peripheral veins of four patients with decompensated cirrhosis. There were no side effects reported in these patients during follow-up, and the Mayo End-Stage Liver Disease score was improved in half of the patients. Furthermore, the qualities of life of all four patients improved by the end of follow-up [73]. In a phase 1-2 trial, Kharaziha et al. [74] showed an improvement in liver function in cirrhosis patients who were injected with 30 to 50 million autologous MSCs via the peripheral or portal veins. In phase 2 trials [75-80], Jang and colleagues [79] showed the beneficial effects of autologous bone marrow MSC transplantation for the treatment of alcoholic cirrhosis. MSCs (5 ├Ś 107 cells) were injected into the hepatic artery twice at weeks 4 and 8. According to the Laennec fibrosis system, histological improvement was observed in 6 of 11 patients (54.5%). The Child-Pugh score was improved in ten patients (90.9%), and the levels of TGF-╬▓1, type 1 collagen, and ╬▒-SMA significantly decreased after MSC therapy [79]. Similar results were obtained by Amer et al. [75], Amin et al. [76], Zhang et al. [77], El-Ansary et al. [78], Peng et al. [80] (Table 1). Autologous bone marrow-derived hepatocyte-like cells also improved the liver function of 20 patients with endstage liver failure when these cells were transplanted via intrasplenic and intrahepatic routes [75]. In addition to the improvement of liver function by MSCs, the incidence of HCC or mortality in patients with hepatitis B-related liver failure showed no significant difference between autologous MSCs-injected and control groups after 192 weeks of follow-up [80]. In addition to autologous bone marrow-derived MSCs, allogeneic MSCs from umbilical cords have been used to improve liver function in decompensated cirrhosis patients [77].
Although MSCs have been widely used in clinical and pre-clinical studies of liver fibrosis, several issues must be carefully considered, including their fibrogenic potentials and capacities for tumor cell growth promotion and oncogenicity. Depending on the MSC injection route and liver disease status, MSCs can differentiate into myofibroblasts rather than hepatocytes [81,82]. Engraftment of human MSCs is very low in normal and acutely injured livers compared to chronically injured livers. Moreover, a significant number of human MSCs exhibit a myofibroblast-like morphology during acute liver injury [81]. Baertschiger et al. [82] observed that stable engraftment of MSCs in the liver is not achieved following intrasplenic injection; however, after intrahepatic injection, MSCs permanently remain in the liver but primarily differentiate into myofibroblasts. Another risk of MSC transplantation has been identified with regard to the susceptibility of these cells to malignant transformation and the promotion of pre-existing tumor growth. As mentioned above, MSCs can secrete various growth factors (i.e., FGFs, EGF, TGF-╬▓, HGF, and VEGF) that promote tumor cell growth and neo-vascularization [83]. Although the malignant transformation of human MSCs has not been reported in clinical trials, the risk of the introduction of genetic mutations during the ex vivo expansion of MSCs must be carefully considered prior to transplantation. Despite these risks, the therapeutic effects of MSCs in liver fibrosis have been verified in pre-clinical and clinical studies. Thus, these cells can be expected to become a new treatment for liver fibrosis in the near future.
To develop MSC therapy for liver fibrosis, larger clinical studies must be conducted to obtain meaningful insights into the safety and clinical efficacy of MSC infusion [84,85]. Accumulating evidence has revealed that autologous MSC infusion is safe because autologous MSCs do not induce an immune reaction; in particular, autologous MSCs expand the Treg population and reduce the T cell population [86]. Reports of the efficacy of MSCs are controversial and depend on the research group; however, we expect that issues regarding their efficacy will be resolved in the near future by a large multicenter randomized clinical trial that is currently being conducted in Korea. Moreover, multicenter international clinical studies on the safety and efficacy of MSC treatments for liver fibrosis can help clinicians reach a consensus on the treatment of liver fibrosis, which will ultimately improve the prognosis of patients.
Several important considerations must still be addressed to support stem cell therapies for liver fibrosis treatment. First, the delivery route of MSCs into the liver has not been standardized but is important for optimizing their therapeutic effects and engraftment. Second, the number of injections of MSCs and their concentrations must be optimized to improve therapeutic effects. Third, the survival duration of engrafted MSCs is important for achieving sustained efficacy. In many pre-clinical animal studies, human MSCs have been observed by immunohistochemical analysis using human-specific markers [87-89]; however, more sophisticated techniques to identify and follow the fates of injected MSCs will be required for clinical translation. For instance, MSCs can be labeled with superparamagnetic iron oxide nanoparticles or reporter genes, causing them to be traceable using advanced imaging technologies [90-95]. Because nanoparticles or reporter genes can modify the properties of MSCs, biomarkers specific to injected MSCs that do not cause cell damage must be developed, even if developing such tools takes a long time.
MSCs are potentially relevant therapeutic agents for the treatment of liver diseases because of their potential to differentiate into hepatocytes as well as their immune-modulatory properties and ability to secrete trophic factors. Nevertheless, MSC therapy needs to be further evaluated in large randomized and controlled clinical trials with longer follow-up periods. In addition, further studies are needed to solve various issues, including those involving the fibrogenic potential of MSCs and their ability to promote pre-existing tumor cell growth.
Acknowledgments
This work was supported by the Yonsei University Future-leading Research Initiative of 2014.
Figure┬Ā1.
Potential roles of mesenchymal stem cells (MSCs) in liver fibrosis. Liver fibrosis is initiated by hepatic injury and the subsequent imbalance of extracellular matrix (ECM) synthesis and degradation mediated by activated hepatic stellate cells (HSCs). Potential protective mechanisms of MSCs include the following: (1) trans-differentiation into hepatocyte-like cells; (2) suppression of immune reactions; (3) secretion of trophic factors to suppress activated HSCs and increase the proliferation of both resident hepatocytes and hepatic progenitor cells; and (4) anti-fibrosis resulting from the regulation of activated HSCs and immune cells. Solid lines and dashed lines indicate stimulatory and inhibitory modifications, respectively. The + sign represents tentative stimulatory effects. The shadows represent ECM that is secreted from the HSCs. NO, nitric oxide; PGE2, prostaglandin E2; IDO, indoleamine 2,3-dioxygenase; IL, interleukin; HLA-G, human leukocyte antigen G; PD, programmed death; DC, dendritic cell; NK, nature killer; Treg, regulatory T; HGF, hepatocyte growth factor; NGF, nerve growth factor; TNF-╬▒, tumor necrosis factor ╬▒; EGF, epidermal growth factor; TGF-╬▒, transforming growth factor ╬▒; VEGF, vascular endothelial growth factor.
Table┬Ā1.
Completed clinical trials using MSC transplantation to treat chronic liver diseasesa
| Liver disease | No. of patients | Source | Cell type/hepatocyte-like cells | Delivery route | Main results | Country | Source |
|---|---|---|---|---|---|---|---|
| Decompensated liver cirrhosis | 4 | Iliac crest | Autologous MSCs/no | Cubital vein of the arm | Improvements in MELD score and serum creatinine level (6ŌĆō12 mon after transplantation) | Iran | Mohamadnejad et al. [73] (2007) |
| Liver cirrhosis (4 HBV, 1 HCV, 1 alcoholic, and 2 cryptogenic) | 8 | Iliac crest | Autologous MSCs/partial | Peripheral and portal veins | Improvement in MELD score (24 wk after transplantation) | Sweden | Kharaziha et al. [74] (2009) |
| End-stage liver failure due to chronic HCV infection | 20 | Iliac crest | Autologous MSCs/yes | Intrasplenic (10) and intrahepatic (10) | Improvements in child and MELD scores (noted at 2 wk and maintained for 6 mon after transplantation) | Egypt | Amer et al. [75] (2011) |
| Liver failure due to chronic HBV infection | 53 | Iliac crest | Autologous MSCs/no | Proper hepatic artery | Improvement in MELD score (2ŌĆō3 wk after transplantation) | China | Peng et al. [80] (2011) |
| HCV-related liver cirrhosis | 15 | Iliac crest | Autologous MSCs/both | Peripheral vein | Improvements in MELD score and serum albumin level (3 and 6 mon after transplantation) | Egypt | El-Ansary et al. [78] (2012) |
| Chronic HBV infection | 31 | Umbilical cord | Allogeneic MSCs/no | Peripheral vein | Improvements in MELD score, ascites, hyaluronic acid, procolla gen type III and type IV collagen (up to 48 wk after transplantation) | China | Zhang et al. [77] (2012) |
| Post-HCV liver cirrhosis | 20 | Iliac crest | Autologous MSCs/no | Intrasplenic injection | Improvements in MELD score and serum albumin level (24 wk after transplantation) | Egypt | Amin et al. [76] (2013) |
| Alcoholic liver cirrhosis | 11 | Iliac crest | Autologous MSCs/no | Right hepatic artery | Improvements in MELD score and liver histology (12 wk after transplantation) | Korea | Jang et al. [79] (2014) |
MSC, mesenchymal stem cell; MELD, Mayo End-Stage Liver Disease; HBV, hepatitis B virus; HCV, hepatitis C virus.
a The data found by a search on www.clinicaltrials.gov (October 2014) with the terms ŌĆ£mesenchymal stem cellsŌĆØ and ŌĆ£liver cirrhosis.ŌĆØ
REFERENCES
4. Snowdon VK, Fallowfield JA. Models and mechanisms of fibrosis resolution. Alcohol Clin Exp Res 2011;35:794ŌĆō799.


5. Ghatak S, Biswas A, Dhali GK, Chowdhury A, Boyer JL, Santra A. Oxidative stress and hepatic stellate cell activation are key events in arsenic induced liver fibrosis in mice. Toxicol Appl Pharmacol 2011;251:59ŌĆō69.



6. Domitrovic R, Jakovac H. Effects of standardized bilberry fruit extract (Mirtoselect┬«) on resolution of CCl4-induced liver fibrosis in mice. Food Chem Toxicol 2011;49:848ŌĆō854.


7. Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology 2008;134:1641ŌĆō1654.



8. Hong WK, Kim MY, Baik SK, et al. The usefulness of non-invasive liver stiffness measurements in predicting clinically significant portal hypertension in cirrhotic patients: Korean data. Clin Mol Hepatol 2013;19:370ŌĆō375.



9. Moon KM, Kim G, Baik SK, et al. Ultrasonographic scoring system score versus liver stiffness measurement in prediction of cirrhosis. Clin Mol Hepatol 2013;19:389ŌĆō398.



10. Lichtinghagen R, Michels D, Haberkorn CI, et al. Matrix metalloproteinase (MMP)-2, MMP-7, and tissue inhibitor of metalloproteinase-1 are closely related to the fibroproliferative process in the liver during chronic hepatitis C. J Hepatol 2001;34:239ŌĆō247.


11. Fallowfield JA, Iredale JP. Targeted treatments for cirrhosis. Expert Opin Ther Targets 2004;8:423ŌĆō435.


12. Zhang Z, Wang FS. Stem cell therapies for liver failure and cirrhosis. J Hepatol 2013;59:183ŌĆō185.


13. Alison MR, Poulsom R, Jeffery R, et al. Hepatocytes from non-hepatic adult stem cells. Nature 2000;406:257.


14. Theise ND, Nimmakayalu M, Gardner R, et al. Liver from bone marrow in humans. Hepatology 2000;32:11ŌĆō16.


15. Si-Tayeb K, Noto FK, Nagaoka M, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 2010;51:297ŌĆō305.



16. Kia R, Sison RL, Heslop J, et al. Stem cell-derived hepatocytes as a predictive model for drug-induced liver injury: are we there yet? Br J Clin Pharmacol 2013;75:885ŌĆō896.



17. Shu SN, Wei L, Wang JH, Zhan YT, Chen HS, Wang Y. Hepatic differentiation capability of rat bone marrow-derived mesenchymal stem cells and hematopoietic stem cells. World J Gastroenterol 2004;10:2818ŌĆō2822.



18. Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med 2012;18:128ŌĆō134.


19. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007;25:2739ŌĆō2749.


20. Sundin M, Ringden O, Sundberg B, Nava S, Gotherstrom C, Le Blanc K. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica 2007;92:1208ŌĆō1215.


21. Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006;107:367ŌĆō372.


22. Asari S, Itakura S, Ferreri K, et al. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol 2009;37:604ŌĆō615.



23. Zhang W, Ge W, Li C, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev 2004;13:263ŌĆō271.


24. Zhang B, Liu R, Shi D, et al. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood 2009;113:46ŌĆō57.


25. Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006;107:1484ŌĆō1490.


26. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815ŌĆō1822.


27. English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol 2009;156:149ŌĆō160.



28. Quintanilha LF, Takami T, Hirose Y, et al. Canine mesenchymal stem cells show antioxidant properties against thioacetamide-induced liver injury in vitro and in vivo. Hepatol Res 2014;44:E206ŌĆōE217.


29. Cho KA, Woo SY, Seoh JY, Han HS, Ryu KH. Mesenchymal stem cells restore CCl4-induced liver injury by an antioxidative process. Cell Biol Int 2012;36:1267ŌĆō1274.


30. Schwartz RE, Reyes M, Koodie L, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest 2002;109:1291ŌĆō1302.



31. Lange C, Bassler P, Lioznov MV, et al. Liver-specific gene expression in mesenchymal stem cells is induced by liver cells. World J Gastroenterol 2005;11:4497ŌĆō4504.



32. Ong SY, Dai H, Leong KW. Inducing hepatic differentiation of human mesenchymal stem cells in pellet culture. Biomaterials 2006;27:4087ŌĆō4097.


33. Sato Y, Araki H, Kato J, et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood 2005;106:756ŌĆō763.


34. Dai LJ, Li HY, Guan LX, Ritchie G, Zhou JX. The therapeutic potential of bone marrow-derived mesenchymal stem cells on hepatic cirrhosis. Stem Cell Res 2009;2:16ŌĆō25.


35. Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion 2014;54:1418ŌĆō1437.



36. Volarevic V, Al-Qahtani A, Arsenijevic N, Pajovic S, Lukic ML. Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity 2010;43:255ŌĆō263.


37. Parekkadan B, van Poll D, Suganuma K, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One 2007;2:e941.



38. Augello A, Tasso R, Negrini SM, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol 2005;35:1482ŌĆō1490.


39. Haddad R, Saldanha-Araujo F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: what do we know so far? Biomed Res Int 2014;2014:216806.




40. Najar M, Raicevic G, Fayyad-Kazan H, et al. Immune-related antigens, surface molecules and regulatory factors in human-derived mesenchymal stromal cells: the expression and impact of inflammatory priming. Stem Cell Rev 2012;8:1188ŌĆō1198.

41. Kupcova Skalnikova H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie 2013;95:2196ŌĆō2211.


42. Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by TNF-╬▒lpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol 2008;294:C675ŌĆōC682.


43. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98:1076ŌĆō1084.


44. Sakaida I, Terai S, Yamamoto N, et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology 2004;40:1304ŌĆō1311.


45. Wang L, Wang X, Wang L, et al. Hepatic vascular endothelial growth factor regulates recruitment of rat liver sinusoidal endothelial cell progenitor cells. Gastroenterology 2012;143:1555.e2ŌĆō1563.e2.



46. Kim SU, Oh HJ, Wanless IR, Lee S, Han KH, Park YN. The Laennec staging system for histological sub-classification of cirrhosis is useful for stratification of prognosis in patients with liver cirrhosis. J Hepatol 2012;57:556ŌĆō563.


47. Mohammadi Gorji S, Karimpor Malekshah AA, Hashemi-Soteh MB, Rafiei A, Parivar K, Aghdami N. Effect of mesenchymal stem cells on doxorubicin-induced fibrosis. Cell J 2012;14:142ŌĆō151.


48. Zhang D, Jiang M, Miao D. Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse. PLoS One 2011;6:e16789.



49. Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 2010;176:2ŌĆō13.



50. Marsden ER, Hu Z, Fujio K, Nakatsukasa H, Thorgeirsson SS, Evarts RP. Expression of acidic fibroblast growth factor in regenerating liver and during hepatic differentiation. Lab Invest 1992;67:427ŌĆō433.

51. Webber EM, Godowski PJ, Fausto N. In vivo response of hepatocytes to growth factors requires an initial priming stimulus. Hepatology 1994;19:489ŌĆō497.


52. Nozawa K, Kurumiya Y, Yamamoto A, Isobe Y, Suzuki M, Yoshida S. Up-regulation of telomerase in primary cultured rat hepatocytes. J Biochem 1999;126:361ŌĆō367.


53. Li WL, Su J, Yao YC, et al. Isolation and characterization of bipotent liver progenitor cells from adult mouse. Stem Cells 2006;24:322ŌĆō332.


54. Usunier B, Benderitter M, Tamarat R, Chapel A. Management of fibrosis: the mesenchymal stromal cells breakthrough. Stem Cells Int 2014;2014:340257.




55. Parekkadan B, van Poll D, Megeed Z, et al. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem Biophys Res Commun 2007;363:247ŌĆō252.



56. Wang J, Bian C, Liao L, et al. Inhibition of hepatic stellate cells proliferation by mesenchymal stem cells and the possible mechanisms. Hepatol Res 2009;39:1219ŌĆō1228.


57. Lin N, Hu K, Chen S, et al. Nerve growth factor-mediated paracrine regulation of hepatic stellate cells by multipotent mesenchymal stromal cells. Life Sci 2009;85:291ŌĆō295.


58. Chen S, Xu L, Lin N, Pan W, Hu K, Xu R. Activation of Notch1 signaling by marrow-derived mesenchymal stem cells through cell-cell contact inhibits proliferation of hepatic stellate cells. Life Sci 2011;89:975ŌĆō981.


59. Rabani V, Shahsavani M, Gharavi M, Piryaei A, Azhdari Z, Baharvand H. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biol Int 2010;34:601ŌĆō605.


60. Semedo P, Correa-Costa M, Antonio Cenedeze M, et al. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells 2009;27:3063ŌĆō3073.


61. Wu Y, Huang S, Enhe J, et al. Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice. Int Wound J 2014;11:701ŌĆō710.


62. Ali G, Mohsin S, Khan M, et al. Nitric oxide augments mesenchymal stem cell ability to repair liver fibrosis. J Transl Med 2012;10:75.



63. Clement S, Pascarella S, Negro F. Hepatitis C virus infection: molecular pathways to steatosis, insulin resistance and oxidative stress. Viruses 2009;1:126ŌĆō143.



64. Tanikawa K, Torimura T. Studies on oxidative stress in liver diseases: important future trends in liver research. Med Mol Morphol 2006;39:22ŌĆō27.


65. Ivanov AV, Smirnova OA, Ivanova ON, Masalova OV, Kochetkov SN, Isaguliants MG. Hepatitis C virus proteins activate NRF2/ARE pathway by distinct ROS-dependent and independent mechanisms in HUH7 cells. PLoS One 2011;6:e24957.

66. Zhu R, Wang Y, Zhang L, Guo Q. Oxidative stress and liver disease. Hepatol Res 2012;42:741ŌĆō749.


67. Cash WJ, McCance DR, Young IS, et al. Primary biliary cirrhosis is associated with oxidative stress and endothelial dysfunction but not increased cardiovascular risk. Hepatol Res 2010;40:1098ŌĆō1106.


68. Li X, Benjamin IS, Alexander B. Reproducible production of thioacetamide-induced macronodular cirrhosis in the rat with no mortality. J Hepatol 2002;36:488ŌĆō493.


69. Ledda-Columbano GM, Coni P, Curto M, et al. Induction of two different modes of cell death, apoptosis and necrosis, in rat liver after a single dose of thioacetamide. Am J Pathol 1991;139:1099ŌĆō1109.


70. Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol 2001;35:297ŌĆō306.


72. Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol 2003;33:105ŌĆō136.


73. Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med 2007;10:459ŌĆō466.

74. Kharaziha P, Hellstrom PM, Noorinayer B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol 2009;21:1199ŌĆō1205.


75. Amer ME, El-Sayed SZ, El-Kheir WA, et al. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol 2011;23:936ŌĆō941.


76. Amin MA, Sabry D, Rashed LA, et al. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin Transplant 2013;27:607ŌĆō612.


77. Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol 2012;27 Suppl 2:112ŌĆō120.


78. El-Ansary M, Abdel-Aziz I, Mogawer S, et al. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev 2012;8:972ŌĆō981.

79. Jang YO, Kim YJ, Baik SK, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int 2014;34:33ŌĆō41.


80. Peng L, Xie DY, Lin BL, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology 2011;54:820ŌĆō828.


81. di Bonzo LV, Ferrero I, Cravanzola C, et al. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut 2008;57:223ŌĆō231.


82. Baertschiger RM, Serre-Beinier V, Morel P, et al. Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One 2009;4:e6657.


83. Zhu W, Xu W, Jiang R, et al. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol 2006;80:267ŌĆō274.


84. Gladman M, Cudkowicz M, Zinman L. Enhancing clinical trials in neurodegenerative disorders: lessons from amyotrophic lateral sclerosis. Curr Opin Neurol 2012;25:735ŌĆō742.


85. Healy BC, Schoenfeld D. Comparison of analysis approaches for phase III clinical trials in amyotrophic lateral sclerosis. Muscle Nerve 2012;46:506ŌĆō511.


86. Mudrabettu C, Kumar V, Rakha A, et al. Safety and efficacy of autologous mesenchymal stromal cells transplantation in patients undergoing living donor kidney transplantation: a pilot study. Nephrology (Carlton) 2015;20:25ŌĆō33.


87. Xu L, Ryugo DK, Pongstaporn T, Johe K, Koliatsos VE. Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. J Comp Neurol 2009;514:297ŌĆō309.



88. Yan J, Xu L, Welsh AM, et al. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med 2007;4:e39.



89. Raore B, Federici T, Taub J, et al. Cervical multilevel intraspinal stem cell therapy: assessment of surgical risks in Gottingen minipigs. Spine (Phila Pa 1976) 2011;36:E164ŌĆōE171.


90. Wang F, Dennis JE, Awadallah A, et al. Transcriptional profiling of human mesenchymal stem cells transduced with reporter genes for imaging. Physiol Genomics 2009;37:23ŌĆō34.



91. Yaghoubi SS, Campbell DO, Radu CG, Czernin J. Positron emission tomography reporter genes and reporter probes: gene and cell therapy applications. Theranostics 2012;2:374ŌĆō391.



92. Zhang SJ, Wu JC. Comparison of imaging techniques for tracking cardiac stem cell therapy. J Nucl Med 2007;48:1916ŌĆō1919.



93. Chen J, Wang F, Zhang Y, et al. In vivo tracking of superparamagnetic iron oxide nanoparticle labeled chondrocytes in large animal model. Ann Biomed Eng 2012;40:2568ŌĆō2578.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



