INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disorder involving various organ systems, including the pulmonary system. Transthoracic echocardiography (TTE)-suspected pulmonary hypertension (PH) is a fairly frequent pulmonary manifestation of SLE. Since Perez and Kramer [
1] first reported four PH cases in SLE in 1981, many reports of PH in SLE have documented its clinical manifestations, associated factors, and predictors of mortality. The prevalence of PH in SLE is estimated to be 1.8% to 14% [
1,
2,
3,
4,
5,
6,
7,
8,
9]. Furthermore, PH in SLE has been reported to be associated with poor prognosis by several studies [
3,
5,
9,
10]. In one study, PH was the third leading cause of mortality among SLE patients [
11], while another report demonstrated that PH was the most frequent cause of mortality among SLE-related deaths [
12]. These results demonstrated the importance of PH in SLE in relation to survival.
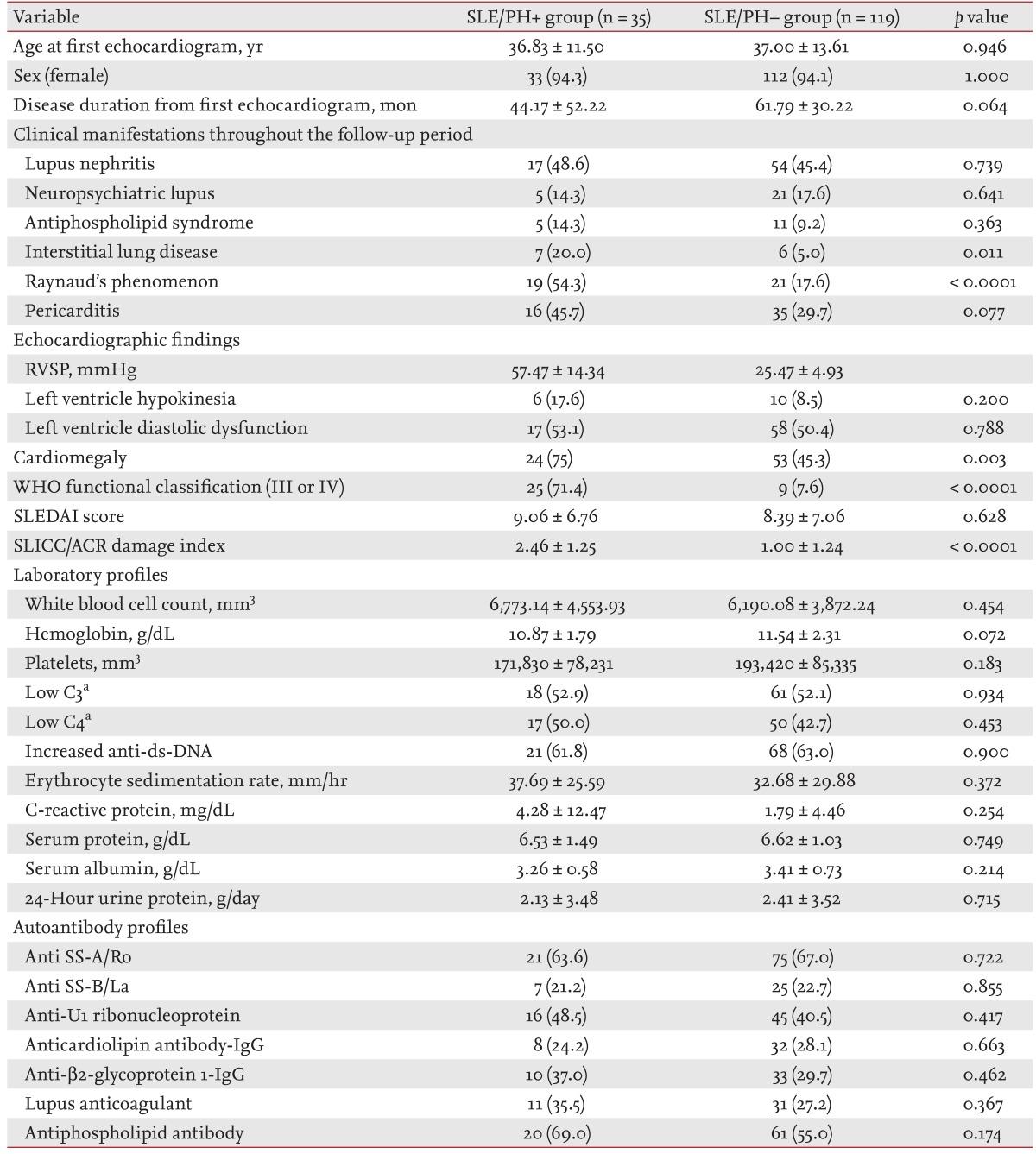
To date, several factors have been reported to be associated with SLE-related PH. Several studies reported that Raynaud's phenomenon (RP), antiphospholipid antibody (aPL), anti-U1 ribonucleoprotein (anti-U1RNP), and serositis may contribute to the development of PH or be associated with its pathogenesis in SLE [
2,
3,
5,
9,
13,
14]. However, the associations between these factors and PH in SLE remain unclear, because of the small sample sizes of these studies, with the majority drawing conclusions from a case series.
We investigated whether PH is an independent risk factor for mortality in SLE. Furthermore, we investigated the clinical characteristics and risk factors for PH in SLE patients. Finally, we determined the predictors of mortality in SLE patients with PH.
DISCUSSION
PH is a progressive disease that can accompany SLE. Since Perez and Kramer [
1] first recognized PH as a complication of SLE, further studies involving Doppler echocardiography have demonstrated that PH is not infrequently associated with SLE. The reported prevalence of PH in SLE patients ranged from 1.8% to 14% [
1,
2,
3,
4,
5,
6,
7,
8,
9], because the cutoff value for PH diagnosis from echocardiography differed among the studies. Nevertheless, the results suggested that SLE/PH+ patients showed a poorer prognosis than SLE/PH- patients [
3,
5,
9,
10]. However, it was unclear whether PH was an independent prognostic factor in SLE. Chung et al. [
19] demonstrated that SLE with PH had a poorer prognosis than with idiopathic pulmonary artery hypertension (iPAH). Although the prognosis of iPAH was poor, the use of novel therapeutics, including endothelin-1 antagonists, has improved survival rates in iPAH [
20]. To improve outcomes in SLE/PH+, it is important to determine SLE-specific risk factors that can be treated in addition to administering iPAH medications.
Several studies have investigated the factors associated with PH development in SLE. Simonson et al. [
2] reported a higher frequency of RP in SLE with than without PH. Subsequently, several further factors, including anticardiolipin (aCL) antibody, anti-U1RNP, and serositis, were proposed to be associated with SLE/PH+ [
3,
5,
9]. In contrast, a further study reported that aCL was not associated with PH in SLE [
8]. A systematic review of SLE/PH+ in China demonstrated an association between PH development and RP, serous effusion (pericardial effusion and pleural effusion), serositis, aCL, anti-╬▓2-glycoprotein I, and anti-U1RNP [
14]. In the present study, only the frequency of RP was significantly higher in SLE/PH+ patients than in SLE/PH- patients. Although higher incidences of the presence of aPL, anti-U1RNP, and serositis were noted in SLE/PH+ patients, these differences between the groups were not significant. The discrepancy between our findings and those of other studies may be due at least in part to selection bias. We selected control patients who were found not to have PH on echocardiography. However, echocardiograms were obtained only in patients who had experienced cardiopulmonary-system-related symptoms or in the presence of radiologically abnormal findings. Therefore, patients who had undergone echocardiography may have had more active disease than those who did not require examination. This was supported by the fact that SLE patients with PH in the present study were more positive for aPL than the 1,010 SLE patients who were enrolled regardless of PH in our previous study (69.0% vs. 36.0%) [
12]. The association of RP and aPL with PH requires further investigation, with a focus on its role in SLE/PH+ pathogenesis.
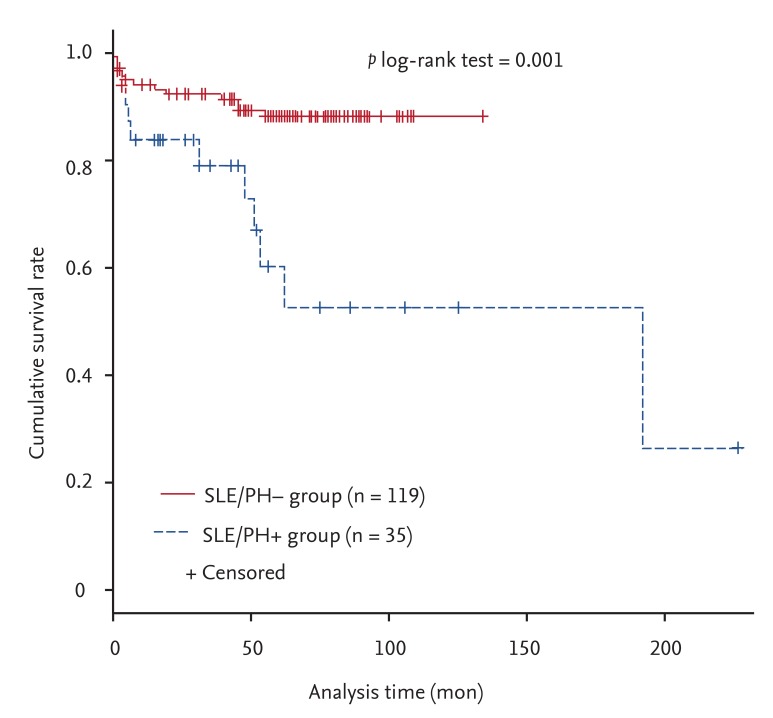
In the present study, PH was demonstrated to be an independent risk factor for mortality in SLE with an adjusted HR of 3.10 (95% CI, 1.10 to 8.69;
p = 0.032). Furthermore, the 1, 3, and 5-year survival rates in SLE/PH+ patients of 83.7%, 79.0%, and 60.2%, respectively, were lower than the 94.0%, 92.2%, and 88.1%, respectively, in SLE/PH- patients. We previously reported that the overall 5-year survival rate in SLE patients was 97.8% [
12]. In the univariate Cox proportional hazard analysis in the present study, lupus nephritis was the only significant predictor for mortality in SLE/PH+ patients. In contrast, multivariate analysis demonstrated that RP was the only factor significantly associated with an increased survival rate. As with iPAH, the level of the RVSP was not associated with mortality [
18]. Echocardiography is a non-invasive method for detecting PH that can also identify secondary causes of PH. However, the RVSP estimated using echocardiography could not predict the prognosis of SLE/PH+. In contrast to a previous study, which suggested that pericardial effusion found on echocardiography was correlated with a poor prognosis in iPAH [
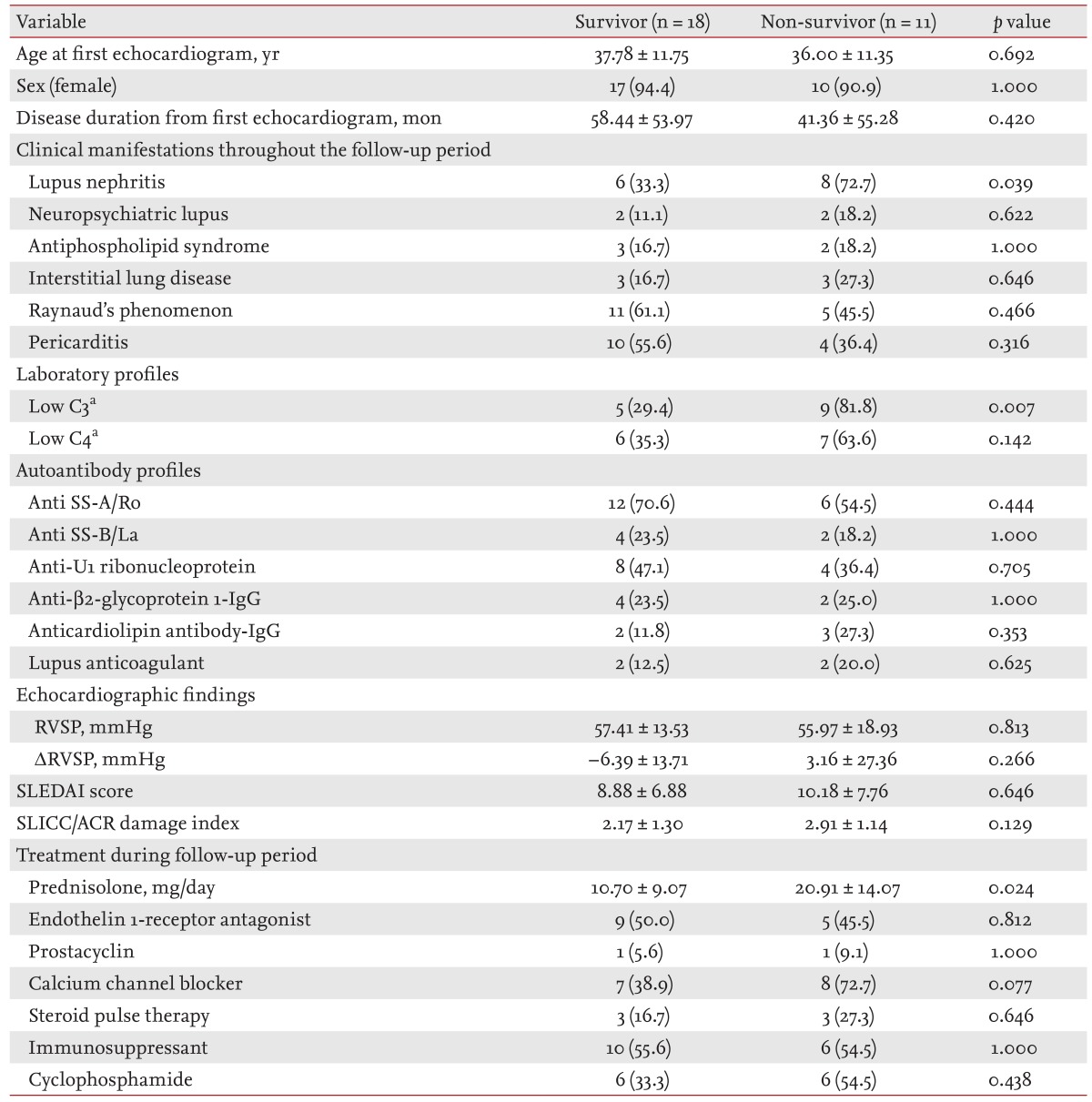
18], our results demonstrated that pericardial effusion was not associated with mortality in SLE/PH+ patients. Chow et al. [
21] systematically reviewed the available literature to identify prognostic factors in SLE/PH+ patients, and found several such possible factors, including RP, thrombocytopenia, and aCL. However, none of these factors were associated with decreased survival in SLE/PH+ patients in the present study. Although the frequency of the presence of aCL was higher in the SLE/PH+ patients who died, the difference was not significant. Moreover, the presence of RP in SLE/PH+ patients was associated with an increased survival rate. The discrepancies between the present study and previous studies may be because of the aforementioned selection bias and the small sample size in previous studies. SLE patients with RP had a greater possibility of undergoing TTE at an earlier stage than SLE patients without RP because of the clinical impression of PH. Therefore, we suggest that SLE patients with RP had a better prognosis because of the early treatment initiation. In addition, because calcium-channel blockers administered to RP patients are helpful in PH patients with vasoreactivity, these patients may have shown a better prognosis because of the early treatment. A prospective cohort study with a larger number of subjects is needed to clarify this issue.
With respect to the therapeutic approach, none of the medications conferred a survival benefit in SLE/PH+ patients. Interestingly, the daily prednisolone dosage was higher in the patients who had died, which may indicate that patients with a more active disease died despite treatment. However, there were no differences in the SLEDAI score or the SLICC damage index between the survivors and non-survivors. The role of prednisolone in SLE/PH+ needs further investigation. The most common medication used in SLE/PH+ patients was an immunosuppressant followed by an endothelin-1 receptor antagonist. In the present study, the 1, 3, and 5-year survival rates of SLE/PH+ patients were better than those reported by Chung et al. [
19], of 50.5%, 44.9%, and 16.8%, respectively. The improved survival rate in SLE/PH+ patients in the present study may have been due to various medications, including immunosuppressants and novel iPAH-targeted medications.
The prevalence of SLE/PH+ in the present report was approximately 1.4%. Li and Tam [
5] proposed that the prevalence of SLE/PH+ might differ dependent on ethnicity. Estimated prevalence rates in Asians have been reported to be 4.2% to 6.2%, while many studies involving Caucasian cohorts have reported prevalences of up to 4.2% to 14% [
2,
3,
4,
8,
16,
22,
23]. Moreover, by defining PH using the same cutoff value (i.e., RVSP > 30 mmHg), prevalences of 4.2% to 14% in Caucasian patients, 4.2% to 5.9% in an Asian population, and 1.8% in a Turkish study were reported [
2,
4,
5,
6,
9,
20]. These differences in the prevalence of PH among SLE patients from various ethnicities suggest that the genetic background may contribute to PH development in SLE.
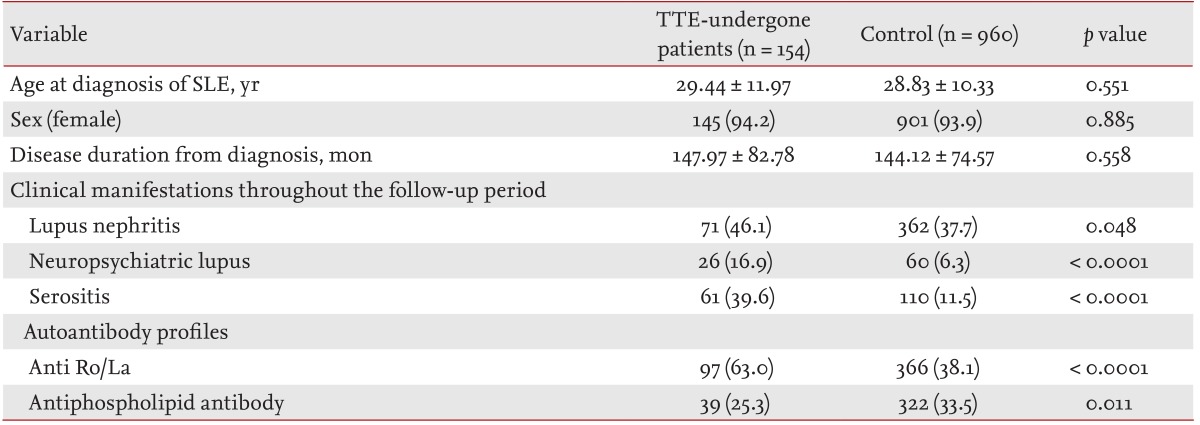
This study had certain limitations. First, the control group, which comprised SLE patients without PH, was selected from SLE patients who had undergone echocardiography (i.e., patients who presented with cardiopulmonary symptoms or abnormal radiographic findings). Therefore, it is very likely that patients with a more severe disease than general SLE patients were included as disease controls. However, a comparison of the survival rates between the two groups showed statistically significant differences, while the difference in the survival rate between the SLE/PH+ group in the present study and our historical cohort [
12] was even greater, suggesting that the difference between the two groups reported here was valid. A further limitation was the diagnosis of PH being defined by echocardiographic findings. Therefore, mild asymptomatic SLE/PH+ patients were not enrolled in this study. Moreover, the RVSP may have overestimated the pulmonary artery pressure determined during RHC.
In conclusion, we demonstrated that PH is an independent prognostic factor in SLE. Additionally, SLE patients with RP have a higher risk of developing PH. Conversely, RP was associated with a better prognosis for survival in SLE/PH+ patients.








 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


