 |
 |
| Korean J Intern Med > Volume 30(2); 2015 > Article |
|
Abstract
In women receiving evaluation for suspected ischemic symptoms, a "normal" diagnosis is five times more common than it is in men. These women are often labeled as having cardiac syndrome X, also known as microvascular angina (MVA). MVA is defined as angina pectoris caused by abnormalities of the small coronary arteries, and is characterized by effort chest pain and evidence of myocardial ischemia with a non-invasive stress test, although the coronary arteries can appear normal or near normal by angiography. MVA patients are often neglected due to the assumption of a good prognosis. However, MVA has important prognostic implications and a proper diagnosis is necessary in order to relieve the patients' symptoms and improve clinical outcomes. The coronary microvasculature cannot be directly imaged using coronary angiography, due to the small diameter of the vessels; therefore, the coronary microvascular must be assessed functionally. Treatment of MVA initially includes standard anti-ischemic drugs (╬▓-blockers, calcium antagonists, and nitrates), although control of symptoms is often insufficient. In this review, we discuss the pathophysiology, diagnosis, and treatment of MVA.
Clear gender differences exist between men and women in the context of cardiovascular disease. Although women appear to have a lower risk of coronary artery disease (CAD), the CAD process is delayed by 10 years rather than avoided [1].
The symptom "chest pain" has many causes, which may be of cardiac or non-cardiac origin. Coronary angiography is a tool used to determine whether chest pain can be attributed to myocardial ischemia and to diagnose CAD. However, when women with suspected ischemic chest pain undergo coronary angiography, a normal diagnosis is made five times more frequently in women than in men [2]. Microvascular angina (MVA), also known as cardiac syndrome X, is characterized by angina or chest pain, an abnormal stress test that indicates myocardial ischemia, and an absence of obstructive CAD (a luminal diameter reduction of > 50% or > 70% of the luminal area reduction) on angiography [3]. It is well documented that there is a preponderance of MVA in the female gender, and that it occurs predominantly in postmenopausal women [4]. MVA patients are often diagnosed as having "non-cardiac" chest pain, and are offered no treatment beyond reassurance [5]. In this review, we discuss the pathophysiology, diagnosis, and treatment of MVA.
Classic MVA is defined as a disease entity with (1) effort angina; (2) findings compatible with myocardial ischemia/coronary microvascular dysfunction upon diagnostic investigation; (3) the appearance of normal or near normal coronary arteries on angiography; and (4) absence of any other specific cardiac disease, such as variant angina, cardiomyopathy, or valvular disease [6]. Findings compatible with myocardial ischemia include: (1) diagnostic ST segment depression during spontaneous or stress-induced typical chest pain; (2) reversible perfusion defects on stress myocardial scintigraphy; (3) documentation of stress-related coronary blood flow abnormalities using more advanced diagnostic techniques, such as cardiac magnetic resonance (MR), positron emission tomography (PET) or Doppler ultrasound; (4) metabolic evidence of transient myocardial ischemia (cardiac PET or MR, invasive assessment).
Among patients suspected of having myocardial ischemia, and who are referred for clinically indicated coronary angiography, 41% of the women, as compared to only 8% of the men, showed insignificant epicardial coronary artery [2]. The prevalence of MVA is estimated to be up to 30% of stable angina patients with non-obstructive coronary arteries. Nineteen percent of women presenting with acute coronary syndrome, 30% of women presenting with unstable angina, 9.1% of women with non-ST-elevation myocardial infarction, and 10% of women with ST-elevation myocardial infarction were determined to have normal or non-obstructive CAD using coronary angiography [7,8,9,10]. Overall, 10% to 25% of women with ACS have a "normal" coronary angiography [11].
In order to receive a diagnosis of MVA, objective evidence of ischemia is required. It is important to note that myocardial ischemia is the result of an imbalance between myocardial oxygen supply and coronary oxygen demand, and that ischemia leads to micro-dysfunction of the myocardium. Thus, an "angina attack" in patients with MVA suggests an on-going ischemic condition in the myocardium.
The prognosis of MVA is not always benign as previously believed [4]. Patients with MVA have a 1.5-fold increase in mortality compared with those without any evidence of myocardial ischemia. In addition, more than 40% of patients are readmitted to the hospital for chest pain, and 30% undergo repeat coronary angiography [11]. They also have a worse quality of life, as compared to healthy counterparts [12,13]. Thus, many patients with chest pain, without significant obstruction on coronary angiography, are often inappropriately reassured and remain untreated [14].
Whenever the coronary blood supply cannot meet the myocardial oxygen demand, myocardial ischemia occurs along with chest pain. In the absence of anatomic stenosis of the epicardial arteries, dysfunction on the epicardial and microvascular levels may be responsible for decreased coronary blood supply.
With regard to the epicardial coronary artery, a temporary spasm of the coronary artery may account for the decreased blood supply. In this case, the diagnosis of Prinzmetal angina, or variant angina can be made.
In terms of the microvascular artery, abnormal vasodilation or spasm may be a pathophysiologic cause of the ischemia [15]. The Hagen-Poiseuille law states that vessel resistance (R) is directly proportional to the length (L) of the vessel and the viscosity (╬Ę) of the blood, and inversely proportional to the radius to the fourth power (r4). Consequently, a 2-fold increase in radius decreases resistance by 16-fold.
Therefore, small arterioles mostly determine the vascular resistance and, consequently, the flow of blood. Vasodilation results from the relaxation of vascular smooth muscles cells. The response may be intrinsic, due to local processes in the surrounding tissue, or extrinsic, due to hormones or the nervous system. A complex regulation of the endothelium, smooth muscle cells, and autonomous nervous system assures optimal blood flow via autocrine, paracrine, endocrine, and neurohumoral pathways in the microvasculature. Endothelial dysfunction, attributed to the impairment of endothelium-dependent vasodilation as a result of reduced nitric oxide (NO) release, is the most commonly proposed mechanism for MVA [16]. NO, the endothelium-derived relaxing factor, is the prominent vasodilator and exerts many biological actions via cyclic guanosine monophosphate (cGMP), which causes the relaxation of vascular smooth muscle cells. The endothelium plays a critical role in vascular homeostasis by secreting substances that not only acutely regulate vascular tone, platelet activity, and coagulation factors but also influence vascular inflammation, cell migration, and proliferation over the longer term [17].
MVA can occur both during exercise and at rest. During exercise, potent vasodilatory metabolites, such as adenosine, adenosine-diphosphate (ADP), adenosine-monophosphate (AMP), NO, etc., are produced and cause significant vasodilation through the relaxation of vascular smooth muscles. However, a blunted response to metabolic stimuli can cause an inappropriate increase in blood flow leading to ischemia, which is responsible for the exercise-induced MVA [4,18].
In contrast, spontaneous hypercontraction, or spasms, of the microvasculature, cause the chest pain episode in MVA patients [15].
In patients with MVA, the perception of pain is commonly increased. Reasons for the increase in pain perception include the release of potassium and adenosine, and abnormalities in the central modulation of pain perception [19]. MVA patients may have an ineffective thalamic gate that results in inadequate cortical activation by afferent stimuli from the heart, which results in the increased perception of pain. An autonomic nervous system imbalance with increased adrenergic activity and impaired parasympathetic tone could explain both the increased pain sensitivity as well as endothelial dysfunction [19,20,21].
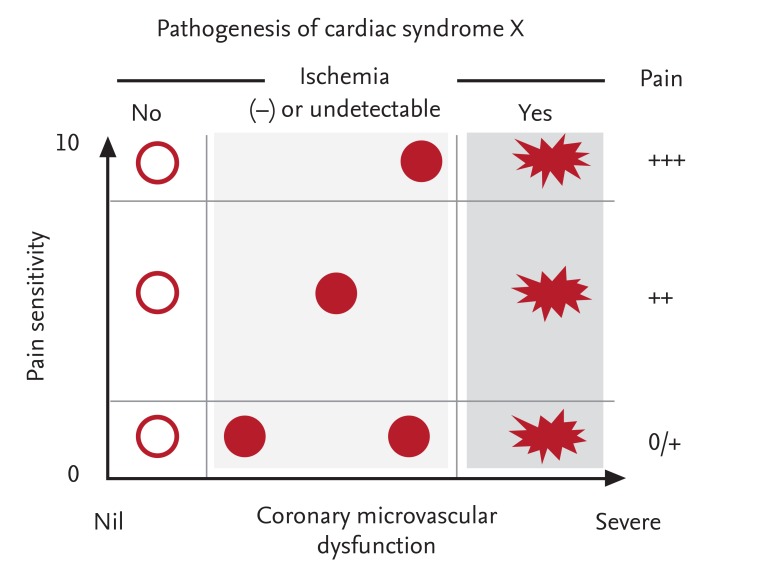
Interactions between chest pain and microvascular dysfunction are important in the pathogenesis of MVA and are likely to determine the clinical presentation of the patient (Fig. 1).
The symptoms of patients with MVA are often indistinguishable from those with obstructive CAD. Nonetheless, normal or non-obstructive CAD coronary angiography often leads to the misdiagnosis of "non-cardiac" chest pain; therefore, MVA patients remain untreated. Considering the prognostic and therapeutic implications of MVA, a correct diagnosis is mandatory to minimize the risk of false-negative results.
A recently proposed diagnostic algorithm for patients with effort angina and documented myocardial ischemia in a functional stress test suggests the use of a step-wise approach [14]. Patients should abstain from the use of medication that may affect the coronary vasomotor, such as a calcium-channel blocker (CCB), for at least 24 hours before invasive coronary angiography [22]. If an intermediate lesion is present (diameter stenosis between 50% to 70%), fractional functional reserve should be performed to evaluate the functional significance [23,24,26,26]. In the absence of functionally significant epicardial stenosis, epicardial vasoreactivity should be tested next.
Since spontaneous vasospasm may occur in response to various stimuli, the cold compressor test or hyperventilation test can also be utilized. However, non-pharmacologic tests have a relatively poor diagnostic performance; specifically, a low sensitivity, and a pharmacologic provocation test is currently widely accepted.
Ergonovine is an ergot alkaloid that was used to control postpartum uterine bleeding. In 1949, it was found to provoke angina, and in 1963, it was used as a diagnostic test for CAD. Ergonovine has structural homology to norepinephrine, and causes the contraction of vascular smooth muscle via the serotonin 1D receptor in vascular smooth muscle cells [27,28]. In a normal artery, there is a mild, generalized vasoconstriction (< 20% diameter narrowing). In order to reach a diagnosis of variant angina, (1) near total, and localized spasm; (2) reproducing the patient's typical symptoms; or (3) associated ST segment change are required. Since the ergonovine provocation test can trigger dangerous, life-threatening complications, due to refractory and multivessel spasms, some authors discourage the use of noninvasive ergonovine protocols and advocate the use of provocative tests with intracoronary administration where intracoronary nitroglycerin can promptly reverse the vasospasm [29].
After an epicardial coronary artery spasm is excluded, the coronary microvasculature must undergo a thorough evaluation. While the diameter of the epicardial artery ranges between 1 to 5 mm, the small resistance vessels have a diameter between 0.1 to 0.3 mm. Considering that the spatial resolution of invasive coronary angiography is 0.5 mm, the microvascular structure cannot be adequately visualized, and other methods to detect microvascular ischemia must be utilized. The best surrogate marker is the ischemic symptom when a patient complains during the provocation test, and the ischemic electrocardiogram change (either a ST-elevation of depression of 1 mm or greater, or tall picking T-wave in two consecutive leads).
Microvascular dysfunction can be further divided into endothelial-dependent or endothelial-independent dysfunction. In order to ascertain the presence of endothelial-dependent dysfunction, intracoronary administration of acetylcholine is considered to be the reference standard [3]. Acetylcholine can cause vasospasms of both epicardial and microvascular vessels. However, in the absence of epicardial vasospasm and the presence of signs and symptoms of myocardial ischemia, endothelial-dependent microvascular dysfunction can be diagnosed [22].
For the determination of endothelial-independent vasodilation, a direct vascular smooth muscle dilator must be used. Adenosine is released from myocardial cells under ischemic conditions and directly causes the dilation of vascular smooth muscle cells. An inadequate increase of coronary flow reserve during intracoronary adenosine administration is indicative of endothelial-independent microvascular dysfunction. An abnormal response of resistance vessels to the direct vasodilatory stimuli is the presumed pathophysiologic mechanism of effort angina in MVA patients.
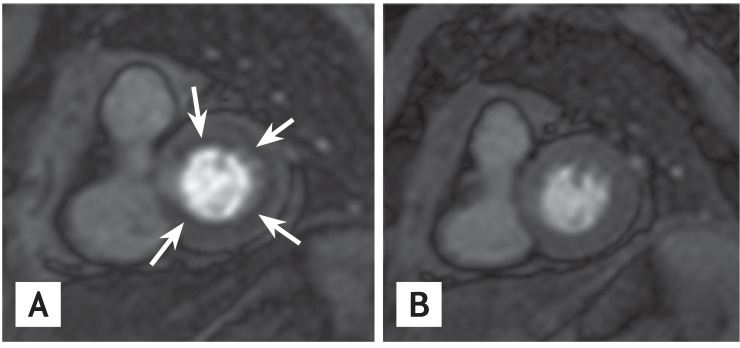
In recent years, non-invasive imaging methods, such as cardiac magnetic resonance imaging (MRI) and PET, have been utilized [30]. Perfusion imaging using gadolinium-enhanced stress cardiac MR allows the detection of subendocardial perfusion defects that are induced by adenosine through a coronary steal phenomenon in myocardial areas supplied by a dysfunctional microcirculation (Fig. 2) [31].
The goal of anti-anginal medication is to improve the coronary blood flow or reduce myocardial oxygen demand, or a combination of the two. This general principle is also valid for the treatment of MVA. However, there have been no large, randomized clinical trials that specifically investigated the effect of classical anti-anginal drugs on the improvement of symptoms and prognosis of MVA patients. We suggest beginning with classical anti-ischemic drugs, including ╬▓-blockers, CCBs, and nitrates. Nonetheless, only half of the patients respond to classical anti-anginal drugs. Alternative forms of the drugs are reserved for unresponsive patents.
╬▓-Blockers have negative chrono-, dromo-, and inotropic effects, and reduce myocardial oxygen consumption, especially under conditions of increased sympathetic activity. They also prolong the diastolic period and enhance the flow of coronary blood. Due to the potential to increase vasospasms, the use of ╬▓-blockers has been avoided in variant angina patients or MVA patients with endothelium-dependent microvascular dysfunction. Nevertheless, in recent years, third generation ╬▓-blockers, like carvedilol and nebivolol, possess additional endothelium-dependent vasodilating properties via the stimulation of NO release from the microvascular endothelium [32]. Clinical investigation will determine if future generations of ╬▓-blockers can safely be administered to MVA-patients.
CCBs are also a potent anti-anginal drug. They are a direct vasodilator and cause the relaxation of vascular smooth muscle cells, independently of endothelial function [33]. CCBs improve microvascular ischemia through the dilation of dysfunctional resistance coronary arteries [34]. They also reduce the myocardial oxygen requirement by lowering the systemic blood pressure, which leads to a decrease in the wall tension of the left ventricle. CCBs are effective in MVA patients [35,36,37,38]. Reflex tachycardia may attenuate the effect of CCBs, especially that of dihydropyridines [39]. Therefore, non-dihydripyridine CCBs may be the first-line option, and dihydropyridine (DHP)-CCBs may be used in combination with ╬▓-blockers.
Nitroglycerin predominantly dilates larger coronary arteries while exerting minimal effects on coronary resistance vessels with a size < 100 micrometers in diameter [40]. Isosorbidie-5-mononiatre was unable to reduce angina symptoms in patients with syndrome X [41]. Consequently, we do not recommend nitrates as a first-line treatment in MVA patients.
Ranolazine is a relatively new drug that exerts anti-ischemic effects through the inhibition of the inward late Na+ current in cardiomyocytes; this results in a reduction of intracellular Ca2+ inflow during ischemia, which leads to an improvement in myocardial relaxation and ventricular diastolic function. Additionally, it has been shown to improve endothelial function in patients with type 2 diabetes mellitus [42]. In several pilot studies including patients with stable MVA whose angina symptoms were inadequately controlled by classical anti-ischemic medications, ranolazine improved their chest pain [43] and coronary blood flow using cardiac MRI [44].
Ivabradine inhibits the If channel in the sinus node and selectively reduces the heart rate. It leads to decreased myocardial oxygen consumption and also increased the coronary blood flow by prolonging the diastolic period. Villano and colleagues [43] showed that ivabradine significantly improved angina symptoms in MVA patients.
Nicorandil is an adenosine triphosphate sensitive potassium channel opener that also has nitrate-like effects. It dilates both epicardial and resistance vessels and modulates the vasomotor responses of the vessels to sympathetic stimulation [45,46]. Nicorandil moderately improved exercise-induced myocardial ischemia in MVA patients [47], and improved myocardial perfusion [48].
Trimetazidine is a new class of drug that switches the cellular metabolism from free fatty acids to glucose oxidation, potentially leading to a better tolerance of myocardial ischemia. In placebo-controlled studies, the drug improved the exercise capacity and time of patients [49]; however, other studies observed no significant clinical effects [50]. Thus, the therapeutic potential of trimetazidine as a treatment for MVA patients currently remains controversial.
Impairment of endothelium-dependent vasodilation reduces NO release and is the most commonly proposed mechanism attributed to MVA [16]. NO exerts many of its biological actions via cGMP, which is rapidly degraded by cGMP phosphodiesterase (PDE). Thus, PDE inhibitors have the potential to improve endothelial function, which has been shown in both animal and clinical studies [16,51,52]. In dysfunctional endothelium, the release of NO by the endothelium is significantly compromised. However, the inhibition of cGMP breakdown by PDE-5 inhibitors may, at least in part, compensate for reduced NO-related cGMP production due to endothelial dysfunction, and restore the NO-dependent vasodilation.
Our group is currently investigating the role of PDE-5 inhibitors in women with MVA. The 'Understanding Chest Pain in Microvascular Disease Proven by Cardiac Magnetic Resonance Image: (UMPIRE)' trial is a multicenter, prospective, randomized, placebo controlled trial, designed to evaluate the effect of udenafil on myocardial ischemia and symptoms in female patients with MVA (Clinical Trials.gov: NCT01769482) [53]. In the event positive results are observed, a PDE-5 inhibitor may provide a valuable therapeutic option to reduce myocardial ischemia and improve cardiac function in female MVA patients.
MVA confers important prognostic implications. Due to the difficulty involved in visualizing the coronary microvasculature, a functional assessment of both the epicardial coronary arteries and the coronary microcirculation is necessary to diagnose MVA, and to avoid the clinical scenario in which MVA patients are diagnosed with "non-cardiac chest pain" and remain untreated. Due to the high rate of "non-responsiveness," both classical and novel anti-anginal medications should be carefully selected and customized to individual patients.
References
1. Mieres JH, Shaw LJ, Arai A, et al. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation 2005;111:682ŌĆō696PMID : 15687114.


2. Sullivan AK, Holdright DR, Wright CA, Sparrow JL, Cunningham D, Fox KM. Chest pain in women: clinical, investigative, and prognostic features. BMJ 1994;308:883ŌĆō886PMID : 8173366.



3. Task Force Members. Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949ŌĆō3003PMID : 23996286.


4. Kaski JC, Rosano GM, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA. Cardiac syndrome X: clinical characteristics and left ventricular function: long-term follow-up study. J Am Coll Cardiol 1995;25:807ŌĆō814PMID : 7884081.


5. Panza JA. Myocardial ischemia and the pains of the heart. N Engl J Med 2002;346:1934ŌĆō1935PMID : 12075053.


6. Lanza GA. Cardiac syndrome X: a critical overview and future perspectives. Heart 2007;93:159ŌĆō166PMID : 16399854.



7. Hochman JS, Tamis JE, Thompson TD, et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes: global use of strategies to open occluded coronary arteries in acute coronary syndromes IIb investigators. N Engl J Med 1999;341:226ŌĆō232PMID : 10413734.


8. Glaser R, Herrmann HC, Murphy SA, et al. Benefit of an early invasive management strategy in women with acute coronary syndromes. JAMA 2002;288:3124ŌĆō3129PMID : 12495392.


9. Krumholz HM, Douglas PS, Lauer MS, Pasternak RC. Selection of patients for coronary angiography and coronary revascularization early after myocardial infarction: is there evidence for a gender bias? Ann Intern Med 1992;116:785ŌĆō790PMID : 1567092.


10. Diver DJ, Bier JD, Ferreira PE, et al. Clinical and arteriographic characterization of patients with unstable angina without critical coronary arterial narrowing (from the TIMI-IIIA Trial). Am J Cardiol 1994;74:531ŌĆō537PMID : 8074033.


11. Bugiardini R, Bairey Merz CN. Angina with "normal" coronary arteries: a changing philosophy. JAMA 2005;293:477ŌĆō484PMID : 15671433.


12. Jespersen L, Hvelplund A, Abildstrom SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33:734ŌĆō744PMID : 21911339.


13. van de Hoef TP, Bax M, Damman P, et al. Impaired coronary autoregulation is associated with long-term fatal events in patients with stable coronary artery disease. Circ Cardiovasc Interv 2013;6:329ŌĆō335PMID : 23899871.


14. Radico F, Cicchitti V, Zimarino M, De Caterina R. Angina pectoris and myocardial ischemia in the absence of obstructive coronary artery disease: practical considerations for diagnostic tests. JACC Cardiovasc Interv 2014;7:453ŌĆō463PMID : 24746648.


15. Mohri M, Koyanagi M, Egashira K, et al. Angina pectoris caused by coronary microvascular spasm. Lancet 1998;351:1165ŌĆō1169PMID : 9643687.


16. Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation 2010;121:2317ŌĆō2325PMID : 20516386.


17. Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med 1998;105(1A):32SŌĆō39SPMID : 9707266.

18. Cannon RO 3rd, Epstein SE. "Microvascular angina" as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol 1988;61:1338ŌĆō1343PMID : 3287885.


19. Rosen SD, Paulesu E, Wise RJ, Camici PG. Central neural contribution to the perception of chest pain in cardiac syndrome X. Heart 2002;87:513ŌĆō519PMID : 12010930.



20. Lanza GA, Giordano A, Pristipino C, et al. Abnormal cardiac adrenergic nerve function in patients with syndrome X detected by [123I]metaiodobenzylguanidine myocardial scintigraphy. Circulation 1997;96:821ŌĆō826PMID : 9264488.


21. Gulli G, Cemin R, Pancera P, Menegatti G, Vassanelli C, Cevese A. Evidence of parasympathetic impairment in some patients with cardiac syndrome X. Cardiovasc Res 2001;52:208ŌĆō216PMID : 11684068.


22. Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC, Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries: the ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries). J Am Coll Cardiol 2012;59:655ŌĆō662PMID : 22322081.


23. Pijls NH, De Bruyne B, Peels K, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 1996;334:1703ŌĆō1708PMID : 8637515.


24. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213ŌĆō224PMID : 19144937.


25. De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991ŌĆō1001PMID : 22924638.


26. De Bruyne B, Fearon WF, Pijls NH, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med 2014;371:1208ŌĆō1217PMID : 25176289.


27. Henry PD, Yokoyama M. Supersensitivity of atherosclerotic rabbit aorta to ergonovine: mediation by a serotonergic mechanism. J Clin Invest 1980;66:306ŌĆō313PMID : 7400317.



28. Auch-Schwelk W, Paetsch I, Krackhardt F, Grafe M, Hetzer R, Fleck E. Modulation of contractions to ergonovine and methylergonovine by nitric oxide and thromboxane A2 in the human coronary artery. J Cardiovasc Pharmacol 2000;36:631ŌĆō639PMID : 11065224.


29. Hackett D, Larkin S, Chierchia S, Davies G, Kaski JC, Maseri A. Induction of coronary artery spasm by a direct local action of ergonovine. Circulation 1987;75:577ŌĆō582PMID : 3815770.


30. Kaufmann PA, Camici PG. Myocardial blood flow measurement by PET: technical aspects and clinical applications. J Nucl Med 2005;46:75ŌĆō88PMID : 15632037.

31. Yilmaz A, Athanasiadis A, Mahrholdt H, et al. Diagnostic value of perfusion cardiovascular magnetic resonance in patients with angina pectoris but normal coronary angiograms assessed by intracoronary acetylcholine testing. Heart 2010;96:372ŌĆō379PMID : 19934103.


32. Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, et al. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation 2003;107:2747ŌĆō2752PMID : 12742996.


33. Bertrand ME, Lablanche JM, Tilmant PY. Treatment of Prinzmetal's variant angina: role of medical treatment with nifedipine and surgical coronary revascularization combined with plexectomy. Am J Cardiol 1981;47:174ŌĆō178PMID : 7457402.


34. Lanza GA, Parrinello R, Figliozzi S. Management of microvascular angina pectoris. Am J Cardiovasc Drugs 2014;14:31ŌĆō40PMID : 24174173.


35. Cannon RO 3rd, Watson RM, Rosing DR, Epstein SE. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small-vessel coronary artery disease and abnormal vasodilator reserve. Am J Cardiol 1985;56:242ŌĆō246PMID : 4025160.


36. Ozcelik F, Altun A, Ozbay G. Antianginal and anti-ischemic effects of nisoldipine and ramipril in patients with syndrome X. Clin Cardiol 1999;22:361ŌĆō365PMID : 10326170.



37. Li L, Gu Y, Liu T, et al. A randomized, single-center double-blinded trial on the effects of diltiazem sustained-release capsules in patients with coronary slow flow phenomenon at 6-month follow-up. PLoS One 2012;7:e38851. PMID : 22761709.



38. Montorsi P, Cozzi S, Loaldi A, et al. Acute coronary vasomotor effects of nifedipine and therapeutic correlates in syndrome X. Am J Cardiol 1990;66:302ŌĆō307PMID : 2368675.


39. Lindqvist M, Kahan T, Melcher A, Ekholm M, Hjemdahl P. Long-term calcium antagonist treatment of human hypertension with mibefradil or amlodipine increases sympathetic nerve activity. J Hypertens 2007;25:169ŌĆō175PMID : 17143189.


40. Harrison DG, Bates JN. The nitrovasodilators: new ideas about old drugs. Circulation 1993;87:1461ŌĆō1467PMID : 8491000.


41. Lanza GA, Colonna G, Pasceri V, Maseri A. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. Am J Cardiol 1999;84:854ŌĆō856PMID : 10513787.


42. Lamendola P, Nerla R, Pitocco D, et al. Effect of ranolazine on arterial endothelial function in patients with type 2 diabetes mellitus. Atherosclerosis 2013;226:157ŌĆō160PMID : 23146293.


43. Villano A, Di Franco A, Nerla R, et al. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol 2013;112:8ŌĆō13PMID : 23558043.


44. Mehta PK, Goykhman P, Thomson LE, et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging 2011;4:514ŌĆō522PMID : 21565740.



45. Kaski JC, Valenzuela Garcia LF. Therapeutic options for the management of patients with cardiac syndrome X. Eur Heart J 2001;22:283ŌĆō293PMID : 11161946.


46. Hongo M, Takenaka H, Uchikawa S, Nakatsuka T, Watanabe N, Sekiguchi M. Coronary microvascular response to intracoronary administration of nicorandil. Am J Cardiol 1995;75:246ŌĆō250PMID : 7832132.


47. Chen JW, Lee WL, Hsu NW, et al. Effects of short-term treatment of nicorandil on exercise-induced myocardial ischemia and abnormal cardiac autonomic activity in microvascular angina. Am J Cardiol 1997;80:32ŌĆō38PMID : 9205016.


48. Yamabe H, Namura H, Yano T, et al. Effect of nicorandil on abnormal coronary flow reserve assessed by exercise 201Tl scintigraphy in patients with angina pectoris and nearly normal coronary arteriograms. Cardiovasc Drugs Ther 1995;9:755ŌĆō761PMID : 8850379.


49. Nalbantgil S, Altintiğ A, Yilmaz H, Nalbantgil II, Onder R. The effect of trimetazidine in the treatment of microvascular angina. Int J Angiol 1999;8:40ŌĆō43PMID : 9826407.


50. Leonardo F, Fragasso G, Rossetti E, et al. Comparison of trimetazidine with atenolol in patients with syndrome X: effects on diastolic function and exercise tolerance. Cardiologia 1999;44:1065ŌĆō1069PMID : 10687257.

51. Perez NG, Piaggio MR, Ennis IL, et al. Phosphodiesterase 5A inhibition induces Na+/H+ exchanger blockade and protection against myocardial infarction. Hypertension 2007;49:1095ŌĆō1103PMID : 17339532.





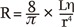
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



