 |
 |
| Korean J Intern Med > Volume 29(5); 2014 > Article |
|
Abstract
Background/Aims
SKI306X, a mixed extract of three herbs, Clematis mandshurica (CM), Prunella vulgaris (PV), and Trichosanthes kirilowii (TK), is chondroprotective in animal models of osteoarthritis (OA). The objectives of this study were to investigate its effect on interleukin (IL)-1╬▓-induced degradation of glycosaminoglycan (GAG) and the basis of its action in human OA cartilage, as well as to screen for the presence of inhibitors of matrix metalloproteinase (MMP)-13 and a disintegrin and metalloprotease with thrombospondin motifs (ADAMTS)-4 in SKI306X and its component herbs, as well as in fractions from SKI306X.
Methods
Human OA chondrocytes and cartilage explants were obtained during total knee replacements and incubated with IL-1╬▓ ┬▒ oncostatin M with or without SKI306X or its component herb extracts. GAG degradation was assayed in cartilage explants using a commercial kit. Expression of genes involved in cartilage destruction was measured by real-time polymerase chain reaction using chondrocyte RNA. SKI306X was fractionated by preparative liquid chromatography to test for the presence of inhibitors of MMP-13 and ADAMTS-4.
Results
SKI306X and PV inhibited IL-1╬▓-induced GAG release from cartilage explants, and SKI306X, CM, PV, and TK inhibited IL-1╬▓-induced MMP gene expression. Unexpectedly, SKI306X greatly stimulated IL-1╬▓ + oncostatin M-induced ADAMTS-4 gene expression, probably due to its TK component. Some fractions of SKI306X also inhibited ADAMTS-4 activity.
Osteoarthritis (OA) is one of the most common causes of disability in the elderly, characterized by an imbalance between the synthesis and degradation of articular cartilage matrix, especially collagen and proteoglycan [1,2]. Interleukin (IL)-1 is the main cytokine causing articular cartilage destruction by inducing chondrocytes to express matrix metalloproteinases (MMPs) and aggrecanase (a disintegrin and metalloprotease with thrombospondin motifs, ADAMTS) in a paracrine and autocrine fashion [3].
Although therapeutic strategies targeting cartilage degradation, such as anticytokine therapy mainly focusing on IL-1, have been emerging, most pharmacological treatments target the resulting pain [4]. Many medicinal plants and their ingredients have been tested for chondro-protective activity [5].
SKI306X (Joins tablet, SK Chemicals Co., Seoul, Korea) is made from three medicinal plants, Clematis mandshurica (CM), Prunella vulgaris (PV), and Trichosanthes kirilowii (TK), mixed in a weight ratio of 1:1:2, and used traditionally for inflammatory conditions such as various forms of arthritis [6]. SKI306X has chondro-protective and anti-inflammatory effects in in vitro and animal models of OA [6,7,8]. Furthermore, a clinical trial has demonstrated that SKI306X decreases joint pain and improves functional capacity in OA patients [9,10]. However, all published in vitro studies demonstrating the anti-OA effects of SKI306X have employed chondrocytes from bovine and rabbit articular cartilage [6,7,8].
The goal of this study was to examine the effect of SKI306X and its components on glycosaminoglycan (GAG) degradation in human OA cartilage explants and its impact on cytokine-induced expression of anabolic and catabolic genes involved in cartilage homeostasis. We also examined whether preparative liquid chromatography fractions of SKI306X and its herbal components contain inhibitor(s) of MMP-13 and ADAMTS-4.
Human IL-1╬▓ and oncostatin M (OSM) were purchased from R&D Systems (Minneapolis, MN, USA). SKI306X and its individual components, CM, PV, and TK were generously provided by the Life Science R&D Center of SK Chemicals (Seongnam, Korea).
Articular cartilage samples for preparing chondrocytes were obtained from OA patients undergoing total knee arthroplasty. The Hanyang University Institutional Review Board approved this study, and cartilage samples were obtained after written informed consent was granted. Cartilage samples were cut into small pieces (about 2 ├Ś 2 mm), washed in Dulbecco's Modified Eagle's Medium (DMEM), and digested with a mixture of 1 mg/mL collagenase and 1 mg/mL hyaluronidase for 3 hours. After filtering through mesh, cell suspensions were washed twice with DMEM and centrifuged at 250 ├Śg for 5 minutes. The resulting cells were cultured and passaged in DMEM supplemented with 10% fetal bovine serum (FBS) under normal culture conditions (37Ōäā, 5% CO2) until use (third or fourth passage).
Human femoral condylar articular cartilage obtained from OA patients undergoing knee joint replacement surgery was prepared as described previously, with minor modifications [11]. Briefly, the cartilage was chopped into ~1-mm3 pieces with scissors, and 50 to 60 mg of cartilage in medium containing 5% FBS were incubated in each well of 24-well plates for 24 hours for stabilization. Following 4 day of culture, the supernatant was collected for GAG assays.
Human OA chondrocytes from three patients were starved in medium with 0.5% FBS overnight, and treated with IL-1 (10 ng/mL) and SKI306X or its herbal components (50, 100, 200, and 400 ┬Ąg/mL) for 24 hours. Cell viability was measured by MTT assay.
Human OA cartilage explants from three patients were incubated with IL-1╬▓ (10 ng/mL), IL-1Ra (500 ┬Ąg/mL), and IL-1╬▓ + SKI306X (200 ┬Ąg/mL), CM, PV, or TK (50 ┬Ąg/mL, respectively). Proteoglycan loss from cartilage explants was determined by measuring the release of sulfated GAG into culture supernatants using a commercially available kit (Blyscan, Biocolor, Belfast, Northern Ireland).
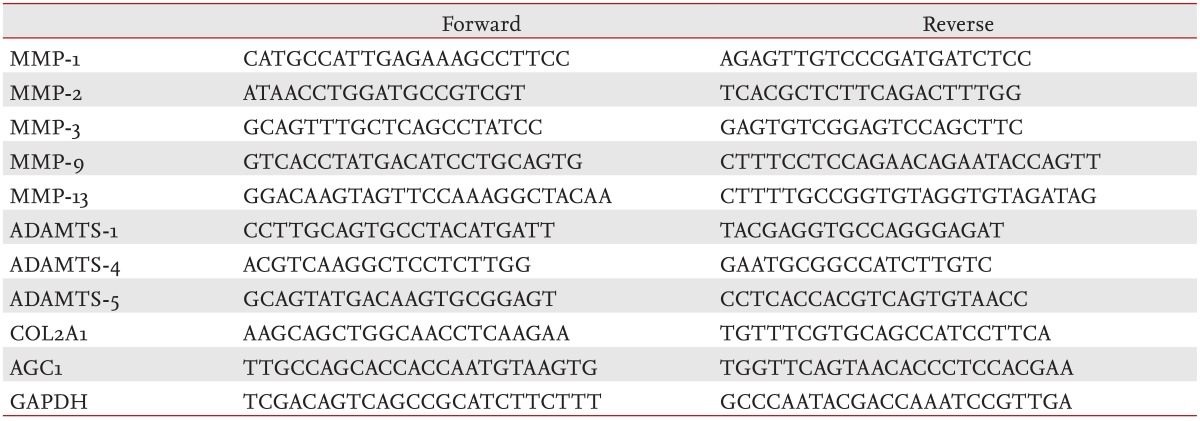
After starvation overnight and treatment with IL-1╬▓ (10 ng/mL) ┬▒ SKI306X (200 ┬Ąg/mL) or its herbal components (50 ┬Ąg/mL) for 24 hours, total RNA was isolated from cultured chondrocytes of 10 strains using RNAzolB (Tel Test Inc., Friendswood, TX, USA) and quantified by spectrophotometer. For assessment of ADAMTS expression, the IL-1╬▓ treatment step was modified; the IL-1╬▓ concentration was reduced to 0.02 ng/mL and OSM (10 ng/mL) was also added. RNA was converted to cDNA with reverse transcriptase (Promega, Madison, WI, USA) and used as a template for real-time polymerase chain reaction (PCR). Real-time PCR was performed using a LightCycler (Bio-Rad Lab Inc., Hercules, CA, USA) and specific primers for glyceraldehyde-3-phosphate dehydrogenase, type II collagen (COL2A1), aggrecan (AGC1), tissue inhibitors of metalloproteinases (TIMPs), MMPs (-1, -2, -3, -9, and -13), and ADAMTS-1, -4, and -5 (Table 1).
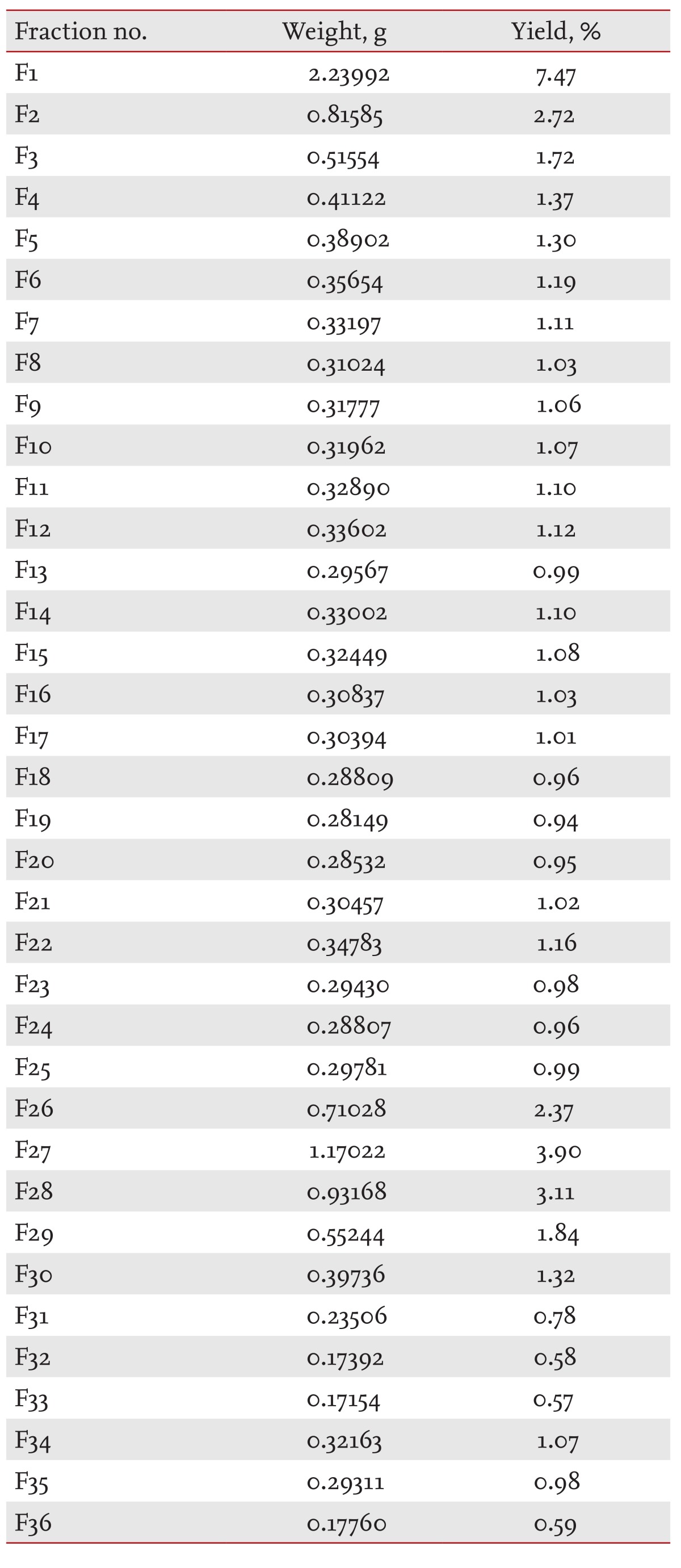
SKI306X was fractionated by medium-pressure liquid chromatography (MPLC, Biotage LLC system, Charlotte, NC, USA) using a prepacked C18 reverse-phase column (120-g KP-C18-HS SNAP Flash Cartridge, Biotage, Uppsala, Sweden). The mobile phase comprised water and acetonitrile (AN) in a gradient of ratios: 5% AN at 0 to 10 minutes, 5% to 35% AN at 10 to 100 minutes and 35% to 100% AN at 100 to 120 minutes. The flow rate was set at 50.0 mL minutes-1 and the detection wavelength was 210 nm.
SKI306X (3 g) was suspended in the mobile phase (3 mL) of the initial MPLC conditions and loaded on a column. Thirty-six 170-mL fractions were collected in each of 10 independent runs. Each fraction was collected and evaporated in vacuo.
We screened for inhibitors of MMP-13 and ADAMTS-4 by detecting MMP-13 and ADAMTS-4 activity using fluorescence resonance energy transfer (FRET) peptides (SensoLyte MMP-13 and Aggrecanase-1 Assay Kit, AnaSpec, San Jose, CA, USA) as substrate in the presence of SKI306X, its herbal components, or 1 of the 36 fractions of SKI306X obtained by HPLC. Various index materials (vanillic acid, rosmarinic acid, protocatechuic acid, 4-hydroxybenzoic acid, oleanolic acid, caffeic acid, isoferulic acid, and ferulic acid) known to be present in SKI306X (all at 300 ┬Ąg/mL), the MMP-13 inhibitor 4-aminophenylmercuric acetate and the aggrecanase-1 inhibitor TAPI-0 served as controls (Peptides International, Louisville, KY, USA).
Statistical analyses were performed using SigmaPlot version 11.2 (Systat Software Inc., San Jose, CA, USA). Data were expressed as means ┬▒ SDs. Assays were performed in duplicate. Student t test or the Mann-Whitney rank-sum test was used to compare the control group and the IL-1╬▓ ┬▒ OSM-induced groups. Differences between groups were evaluated by one-way analysis of variance with post hoc tests. p values < 0.05 were considered to indicate statistical significance.
IL-1╬▓ ┬▒ SKI306X (up to 200 ┬Ąg/mL) or its components (up to 50 ┬Ąg/mL) did not affect chondrocyte viability. Only CM reduced viability to less than 80%, although this occurred in only one strain of chondrocytes (data not shown). Therefore, we used 200 ┬Ąg/mL of SKI306X and 50 ┬Ąg/mL of its herbal components in subsequent assays.
To investigate the effect of SKI306X and its herbal components on IL-1╬▓-induced degradation of cartilage, human OA cartilage explants were cultured with IL-1╬▓ in the presence or absence of IL-1Ra and SKI306X or its herbal components for 4 day. IL-1╬▓ induced GAG release (152.7% ┬▒ 25.2% vs. control, p = 0.026) (Fig. 1), which was blocked by IL-1Ra. SKI306X and PV, but not CM or TK, significantly inhibited IL-1╬▓-induced GAG release (SKI306X: 106.1 ┬▒ 4.3, p = 0.018 vs. IL-1╬▓; PV: 122.8 ┬▒ 14.0, p = 0.009 vs. IL-1╬▓).
We next investigated the effect of SKI306X and its herbal components on IL-1╬▓-induced expression of anabolic, catabolic, and TIMP genes. As shown in Fig. 2, all MMP genes examined were up-regulated by IL-1╬▓ (p < 0.001), and this up-regulation was inhibited by SKI306X and PV and CM, although not always statistically significantly so. In contrast, TK inhibited expression of only one of these genes, MMP-2.
IL-1╬▓ affected expression of the aggrecanase genes to varying degrees, including those implicated in the pathogenesis of OA (Fig. 3). Thus, SKI306X up-regulated ADAMTS-4 and down-regulated ADAMTS-5. TK strongly stimulated ADAMTS-4 expression, while SKI306X, PV, and CM did not. SKI306X and PV further reduced ADAMTS-5 expression.
IL-1╬▓ reduced AGC-1 expression (p = 0.017) (Fig. 4), and this effect was increased by SKI306X, PV, and CM, but not by TK (p < 0.001, p < 0.001, p = 0.035, and p = 0.980, respectively). COL2A1 expression was also slightly up-regulated, albeit not significantly so. However, PV and TK decreased COL2A1 gene expression (p < 0.05).
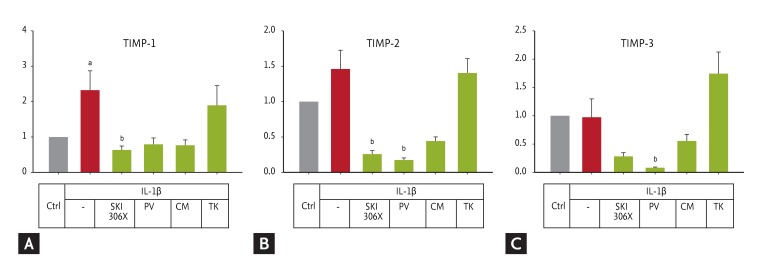
IL-1╬▓ up-regulated TIMP-1 expression (p = 0.032) (Fig. 5), but its effect on TIMP-2 and TIMP-3 was not significant. SKI306X, PV, and CM showed a tendency to reduce IL-1╬▓-induced expression of the TIMP genes, while TK had no effect.
Our results to this point did not show unambiguously that SKI306X was favorable in the treatment of OA, since some of its effects, such as the increased MMP and ADAMTS expression brought about by TK, and the reduced expression of anabolic genes and TIMPs, might not reduce OA. On the other hand, SKI306X clearly inhibited OA cartilage degradation. These findings prompted us to fractionate SKI306X and identify useful fractions. The results of fractionation of SKI306X are shown in Table 2.
SKI306X and its herbal components (25 to 200 ┬Ąg/mL), as well as 36 fractions of SKI306X (300 ┬Ąg/mL) and eight index materials (300 ┬Ąg/mL) were screened using fluorescently labeled substrate peptides; cleavage liberates FRET to activate fluorescence. Neither SKI306X nor its three herbal components significantly inhibited the activity of ADAMTS-4 or MMP-13 (data not shown). Furthermore, only one fraction of SKI306X (fraction 2) inhibited the activity of MMP-13 (31.6% compared to the positive control). On the other hand, five fractions (fractions 32 to 36) significantly inhibited ADAMTS-4 activity (activity; 18.5%, 8.3%, 14.3%, 16.8%, and 14.1%, respectively, compared to 100% in the control).
Although clinical trials have demonstrated that SKI306X has therapeutic efficacy comparable to that of diclofenac and celecoxib, no in vitro studies have yet examined its chondro-protective effects in human cartilage or chondrocytes [10,12].
In this study we identified a mechanism by which SKI306X and some of its herbal components may protect against cartilage damage in OA: inhibition of IL-1╬▓-induced GAG degradation. This effect was in part a result of reduction of IL-1╬▓-induced expression of catabolic genes, such as MMP-13 and ADAMTS-4.
Whereas previous in vitro experiments using rabbit and bovine chondrocytes employed concentrations of SKI306X of up to 300 ┬Ąg/mL, we used 200 ┬Ąg/mL of SKI306X and 50 ┬Ąg/mL of its herbal components based on their effects on human OA chondrocyte viability [6,7,8]. This difference in sensitivity may be due to differences between species or between chondrocyte sources, since the human chondrocytes used in our experiments originated from elderly patients with OA, while the animal chondrocytes used in previous studies were derived from the cartilage of healthy young animals.
We examined the effects of SKI306X and its herbal components on IL-1╬▓-induced expression of anabolic and catabolic genes in human OA cartilage, as has been performed previously for glucosamine [13]. Almost all MMPs and ADAMTS-4 were up-regulated by IL-1╬▓ and/or OSM, and SKI306X, PV and CM, but not TK inhibited this up-regulation, although the effects were sometimes not significant or variable among cell strains. MMPs were markedly up-regulated by IL-1╬▓ (10 ng/mL); because IL-1╬▓ had no effect on ADAMTS expression, we added OSM [14]. ADAMTS-1 was down-regulated by IL-1╬▓ and OSM, as reported previously [15].
Unlike SKI306X, PM, and CM, TK had little effect on or even increased cytokine-induced up-regulation of gene expression. Moreover, TK also did not inhibit GAG degradation in cartilage explant experiments. These results were contrary to the previous observation that TK inhibits cartilage degradation more effectively than CM and PV, probably by down-regulating MMPs [6,7]. However, cytokines do not always up-regulate enzyme expression, as shown recently in the case of ADAMTS-4 [16]. Therefore, how SKI306X and its three herbal components affect MMPs and aggrecanase (ADAMTS-4 and -5) activity in human cartilage and chondrocytes needs further study. In vivo experiments in animal OA models using varying proportions of the three components of SKI306X, or perhaps mixtures of only CM and PV, would also help elucidate which components affect degradative enzymes.
MMP-13 and ADAMTS-4 and -5 have been thoroughly investigated as promising therapeutic targets in the management of OA, including high-throughput screens for novel inhibitors [17,18,19,20,21]. We screened SKI306X fractions for inhibitors of MMP-13 and ADAMTS-4, using as controls well-known inhibitory compounds and some index materials present in SKI306X. Only one fraction weakly inhibited MMP-13 activity, but fractions 32 to 36 inhibited the activity of ADAMTS-4 (18.5%, 8.3%, 14.3%, 16.8%, and 14.1%, respectively), which represents considerably greater inhibition than that possible using TAPI-0, a specific inhibitor of ADAMTS-4, (34.0% activity at 1,000 nM). Interestingly, neither SKI306X nor its three herbal components inhibited ADAMTS-4. Identifying the various active agents in the SKI306X fractions is an important next step towards potential OA therapeutics.
In summary, SKI306X and its component herbs inhibit cartilage degradation in OA patient explants. This inhibition may result in part from down-regulation of catabolic genes such as MMP-13 and ADAMTS-4. We also found that fractions of SKI306X contain ADAMTS-4 inhibitor(s). Inhibition of MMPs and ADAMTS by various mixtures of the three herbs that comprise SKI306X, and fractions thereof, warrant further investigation, as such activity would validate their potential as OA therapeutics.
References
1. Issa SN, Sharma L. Epidemiology of osteoarthritis: an update. Curr Rheumatol Rep 2006;8:7ŌĆō15PMID : 16515759.


2. Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol 2008;22:351ŌĆō384PMID : 18455690.


3. Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res 2004;427(Suppl):S27ŌĆōS36PMID : 15480070.

4. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum 2000;43:1905ŌĆō1915PMID : 11014340.


5. Cameron M, Gagnier JJ, Little CV, Parsons TJ, Blumle A, Chrubasik S. Evidence of effectiveness of herbal medicinal products in the treatment of arthritis. Part I: osteoarthritis. Phytother Res 2009;23:1497ŌĆō1515PMID : 19856319.


6. Choi JH, Choi JH, Kim DY, et al. Effects of SKI 306X, a new herbal agent, on proteoglycan degradation in cartilage explant culture and collagenase-induced rabbit osteoarthritis model. Osteoarthritis Cartilage 2002;10:471ŌĆō478PMID : 12056850.


7. Kim JH, Ryu KH, Jung KW, Han CK, Kwak WJ, Cho YB. SKI306X suppresses cartilage destruction and inhibits the production of matrix metalloproteinase in rabbit joint cartilage explant culture. J Pharmacol Sci 2005;98:298ŌĆō306PMID : 16034188.


8. Hartog A, Hougee S, Faber J, et al. The multicomponent phytopharmaceutical SKI306X inhibits in vitro cartilage degradation and the production of inflammatory mediators. Phytomedicine 2008;15:313ŌĆō320PMID : 17949960.


9. Jung YB, Roh KJ, Jung JA, et al. Effect of SKI 306X, a new herbal anti-arthritic agent, in patients with osteoarthritis of the knee: a double-blind placebo controlled study. Am J Chin Med 2001;29:485ŌĆō491PMID : 11789591.


10. Lung YB, Seong SC, Lee MC, et al. A four-week, randomized, double-blind trial of the efficacy and safety of SKI306X: a herbal anti-arthritic agent versus diclofenac in osteoarthritis of the knee. Am J Chin Med 2004;32:291ŌĆō301PMID : 15315266.


11. Billinghurst RC, Dahlberg L, Ionescu M, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest 1997;99:1534ŌĆō1545PMID : 9119997.



12. Song YW, Lee EY, Koh EM, et al. Assessment of comparative pain relief and tolerability of SKI306X compared with celecoxib in patients with rheumatoid arthritis: a 6-week, multicenter, randomized, double-blind, double-dummy, phase III, noninferiority clinical trial. Clin Ther 2007;29:862ŌĆō873PMID : 17697905.


13. Uitterlinden EJ, Jahr H, Koevoet JL, et al. Glucosamine decreases expression of anabolic and catabolic genes in human osteoarthritic cartilage explants. Osteoarthritis Cartilage 2006;14:250ŌĆō257PMID : 16300972.


14. Koshy PJ, Lundy CJ, Rowan AD, et al. The modulation of matrix metalloproteinase and ADAM gene expression in human chondrocytes by interleukin-1 and oncostatin M: a time-course study using real-time quantitative reverse transcription-polymerase chain reaction. Arthritis Rheum 2002;46:961ŌĆō967PMID : 11953973.


15. Wachsmuth L, Bau B, Fan Z, Pecht A, Gerwin N, Aigner T. ADAMTS-1, a gene product of articular chondrocytes in vivo and in vitro, is downregulated by interleukin 1beta. J Rheumatol 2004;31:315ŌĆō320PMID : 14760803.

16. Rogerson FM, Chung YM, Deutscher ME, Last K, Fosang AJ. Cytokine-induced increases in ADAMTS-4 messenger RNA expression do not lead to increased aggrecanase activity in ADAMTS-5-deficient mice. Arthritis Rheum 2010;62:3365ŌĆō3373PMID : 20662062.


17. Lauer-Fields JL, Spicer TP, Chase PS, et al. Screening of potential a disintegrin and metalloproteinase with thrombospondin motifs-4 inhibitors using a collagen model fluorescence resonance energy transfer substrate. Anal Biochem 2008;373:43ŌĆō51PMID : 17949675.



18. Fosang AJ, Little CB. Drug insight: aggrecanases as therapeutic targets for osteoarthritis. Nat Clin Pract Rheumatol 2008;4:420ŌĆō427PMID : 18577998.


19. Lauer-Fields JL, Minond D, Chase PS, et al. High throughput screening of potentially selective MMP-13 exosite inhibitors utilizing a triple-helical FRET substrate. Bioorg Med Chem 2009;17:990ŌĆō1005PMID : 18358729.



Figure┬Ā1
Glycosaminoglycan (GAG) release from human osteoarthritis (OA) cartilage explants with interleukin (IL)-1╬▓ (10 ng/mL) in the absence or presence of an IL-1 receptor antagonist (IL-1Ra, 500 ┬Ąg/mL) and SKI306X (200 ┬Ąg/mL) or its herbal components, Clematis mandshurica (CM), Prunella vulgaris (PV), and Trichosanthes kirilowii (TK) (50 ┬Ąg/mL).
ap < 0.05 vs. control by t test, bp < 0.05 vs. IL-1╬▓ by one-way analysis of variance with a post hoc test.

Figure┬Ā2
(A-E) Changes in matrix metalloproteinase (MMP) gene expression in human osteoarthritic cartilage after culture with interleukin (IL)-1╬▓ (10 ng/mL) in the absence or presence of SKI306X (200 ┬Ąg/mL) or its herbal components, Clematis mandshurica (CM), Prunella vulgaris (PV), and Trichosanthes kirilowii (TK) (50 ┬Ąg/mL). Y axis: relative expression compared to control (Ctrl).
ap < 0.05 vs. control by Mann-Whitney rank-sum test, bp < 0.05 vs. IL-1╬▓ by one-way analysis of variance with a post hoc test.

Figure┬Ā3
(A-C) Change in aggrecanase (a disintegrin and metalloprotease with thrombospondin motifs, ADAMTS) gene expression in human osteoarthritic cartilage after culture with interleukin (IL)-1╬▓ (0.02 ng/mL) + oncostatin M (OSM; 10 ng/mL) in the absence or presence of SKI306X (200 ┬Ąg/mL) or its herbal components Clematis mandshurica (CM), Prunella vulgaris (PV), and Trichosanthes kirilowii (TK) (50 ┬Ąg/mL). Y axis: relative expression compared to control (Ctrl).
ap < 0.05 vs. control by Mann-Whitney rank-sum test, bp < 0.05 vs. IL-1╬▓ by one-way analysis of variance with a post hoc test.

Figure┬Ā4
(A) Changes in aggrecan (AGC1) and (B) collagen type II (COL2A1) gene expression in human osteoarthritic cartilage after culture with interleukin (IL)-1╬▓ (10 ng/mL) in the absence or presence of SKI306X (200 ┬Ąg/mL) or its herbal components, Clematis mandshurica (CM), Prunella vulgaris (PV), and Trichosanthes kirilowii (TK) (50 ┬Ąg/mL). Y-axis: relative expression compared to the control (Ctrl). Data are expressed as means ┬▒ SD of 10 chondrocyte strains.
ap < 0.05 vs. control by Mann-Whitney rank-sum test, bp < 0.05 vs. IL-1╬▓ by one-way analysis of variance with a post hoc test.

Figure┬Ā5
(A-C) Changes in tissue inhibitors of metalloproteinase (TIMP) gene expression in human osteoarthritic cartilage after culture with interleukin (IL)-1╬▓ (10 ng/mL) in the absence or presence of SKI306X (200 ┬Ąg/mL) or its herbal components, Clematis mandshurica (CM), Prunella vulgaris (PV), and Trichosanthes kirilowii (TK) (50 ┬Ąg/mL). Y-axis: relative expression compared to the control (Ctrl).
ap < 0.05 vs. control by t test or Mann-Whitney rank-sum test (TIMP-3), bp < 0.05 vs. IL-1╬▓ by one-way analysis of variance with a post hoc test.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



