 |
 |
| Korean J Intern Med > Volume 29(4); 2014 > Article |
|
Abstract
Background/Aims
Increased resting energy expenditure (REE) in rheumatoid arthritis (RA) patients is thought to be caused by hypermetabolism associated with production of proinflammatory cytokines. Our aim in the present study was to explore the possible association between REE and disease activity in females with RA.
Methods
A total of 499 female RA patients were recruited to this cross-sectional study assessing REE scores on disease activity indices (the routine assessment of patient index data 3 [RAPID3], the disease activity score 28, and the clinical/simplified disease activity index [CDAI/SDAI]) and the levels of RA-associated autoantibodies (rheumatoid factor and anticyclic citrullinated peptide [anti-CCP] antibodies). Age-matched healthy female controls (n = 131) were also enrolled.
Results
REE did not differ between RA patients (all patients, and those in remission or not) and controls, or between RA patients in remission or not (p > 0.05 for all comparisons). Increased REE in total RA patients was associated with younger age and a higher body mass index (BMI) (p < 0.001 and p < 0.001, respectively), but not with disease activity index scores on any of RAPID3, CDAI, or SDAI. BMI was the only clinical parameter exhibiting a significant relationship with REE quartiles (Q1 to Q4; p < 0.001); none of disease duration, functional status, or anti-CCP antibody titer in RA patients was significantly related to REE, based on analysis of covariance.
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by synovial hyperplasia and inflammation, neovascularization, and enhanced osteoclastogenesis in the affected joints. These processes eventually trigger joint deformities, functional impairment, and poor quality-of-life [1,2]. Other characteristics of RA-associated clinical phenotypes are alterations in energy metabolism and the compositions of body compartments. RA patients exhibit increased resting energy expenditure (REE), wasting of fat-free mass (FFM), and loss of body cell mass [3,4]. Changes in energy metabolism in RA patients have been attributed (in part) to chronic inflammation mediated by excessive production of proinflammatory cytokines including tumor necrosis factor-╬▒ (TNF-╬▒), interleukin-1╬▓ (IL-1╬▓), and IL-6 [3,5,6,7]. Such findings imply that changes in energy metabolism are associated with RA pathogenesis.
REE, the thermogenic effect of food (TEF), and physical activity (PA), are all measures of 24-hour energy expenditure, and account for 65% to 70%, 10% to 15%, and 20% to 30% of total energy expenditure (TEE), respectively [8,9]. Currently, REE is the best predictor of 24-hour energy expenditure because TEF and PA data tend to be inconsistent. The widely used anthropometric method for measurement of REE was developed by Mifflin et al. [10]. Earlier studies found that REE was higher in RA patients than healthy controls [3,7,11]. Aberrant energy expenditure, reflected in higher REE, is a common clinical problem in RA patients and has been attributed to excessive production of proinflammatory cytokines. However, two clinical trials of anti-TNF agents in RA patients did not show significant changes in body composition or REE, although improvements in disease activity and functional status were evident [11,12].
Cytokine production in RA patients is associated with protein breakdown [5] and increased REE [3,7]. Proinf lammatory cytokine production, contributing to the chronic inf lammatory response, is closely associated with the extent of disease activity in RA [1,2]. Therefore, a close relationship between REE and the extent of RA disease activity is possible. Recently, Arshad et al. [7] found an association between increased REE and elevated disease activity in RA patients. Furthermore, a clinical trial showed that basal metabolic rate served as a useful indicator of RA disease activity, and as a predictor of remission [13]. However, more data are required before it can be safely concluded that an association exists between REE, on the one hand, and the extent of disease activity, on the other. Our aim in the present study was to determine whether scores on activity indices, namely the disease activity score 28 (DAS28) [14], the clinical disease activity index (CDAI) [15], the simplified disease activity index (SDAI) [16], and the routine assessment of patient index data 3 (RAPID3) [17,18], were associated with REE in female patients with RA.
A total of 499 female patients was consecutively recruited from the outpatient clinic of the Daegu Catholic University Medical Center; all met the revised criteria for RA proposed by the American College of Rheumatology (ACR) in 1987 [19]. In addition, 131 age- and gender-matched healthy controls were enrolled from our Heath Promotion Center. All subjects were over 18 years of age at the time of enrolment. We excluded those with current infections, chronic obstructive pulmonary disease, congestive heart failure, thyroid disease, diabetes mellitus, renal failure, and chronic or current diarrhea; via review of medical records and individual interviews. At enrolment, all subjects gave written informed consent. The study protocol and proposal were reviewed and approved by the Institutional Review Board of the Daegu Catholic University Medical Center.
Baseline data including age, height, weight, and disease duration, were recorded. Seven ACR Core Data Set measures; thus the swollen joint count, the tender joint count, physician global assessment, erythrocyte sediment rate (ESR)/C-reactive protein (CRP) level, patient-reported functional disability, pain, and patient global assessment, were assessed; and these measures converted into multiple disease activity composites including those of the DAS28 [14], CDAI [15], SDAI [16], and RAPID3 [17,18]. Disease activity was classified as in remission (< 2.6), low (Ōēź 2.6 to < 3.2), moderate (Ōēź 3.2 to Ōēż 5.1), and high (> 5.1), based on DAS28-ESR criteria [14]. We arbitrarily classified the four disease activity categories into two: remission (< 2.6) and nonremission (Ōēź 2.6).
Rheumatoid factor (RF) and anticyclic citrullinated peptide (anti-CCP) antibody levels were measured in all subjects. RF levels were assessed via immunoturbidometry using the Cobas Integra RFII assay (Roche Diagnostics GmbH, Mannheim, Germany). The cutoff value for RF was < 10 IU/mL. Anti-CCP antibody levels were assessed using an enzyme-linked immunosorbent assay (Diastat, Axis-Shield Diagnostics, Dundee, UK). Negativity for anti-CCP antibody was defined as < 17 U/mL. The ESR and CRP levels were measured in blood samples taken at the time of study commencement. Joint assessment in terms of swelling and tenderness was performed by an experienced rheumatologist (H.L.).
Nonbiological disease-modifying antirheumatic drugs (DMARDs) taken at the time of enrolment included methotrexate (MTX), steroids, hydroxychloroquine (HCQ), sulfasalazine (SSZ), leflunomide, and tacrolimus. In addition, biological DMARDs treating RA were also identified; these included infliximab, etanercept, adalimumab, and abatacept. REE was calculated using the formula of Mifflin et al. [10]: REE for females = [10 ├Ś weight (kg) + 6.25 ├Ś height (cm) - 5 ├Ś age (yr)] - 161. Body mass index (BMI; kg/m2) was calculated as weight (kg) divided by the square of the height (m).
Data are expressed as mean ┬▒ standard deviation (SD) for continuous parameters and as frequencies with percentages for categorical parameters. Descriptive analysis was undertaken to identify the mean values and frequencies of each parameter. Differences in frequencies among groups (remission vs. nonremission and RA vs. controls) were evaluated, in terms of statistical significance, using the Mantel-Haenszel chi-square test for categorical variables and Student t test for continuous variables.
Correlations between REE and clinical/laboratory parameters were evaluated via Pearson correlation analysis. Multiple linear regression analysis of data from all patients, and RA patients in remission or not, were used to evaluate associations between REE and clinical/laboratory variables. Covariates of the multivariate-adjusted model were chosen via stepwise selection. Thus, we adjusted for age, disease duration, BMI, patient-reported functional status, ESR, DAS28-ESR, and anti-CCP antibody titer, for each model, if appropriate. We categorized REE into quartiles: Ōēż 1,029 kcal/day, Q1; 1,030 to 1,096 kcal/day, Q2; 1,097 to 1,168 kcal/day, Q3; and Ōēź 1,169 kcal/day, Q4. To allow comparisons among the four RA groups (Q1 to Q4), we first applied a one-way analysis of variance (ANOVA) for continuous variables. Analysis of covariance (ANCOVA) was used to determine REE differences between the groups (remission RA group vs. controls; nonremission RA group vs. controls; remission RA group vs. the nonremission RA group; and the total RA group vs. controls).
Statistical significance was considered present when the two-sided significance level was Ōēż 0.05. All statistical analyses were performed using the IBM SPSS version 19.0 (IBM Co., Armonk, NY, USA).
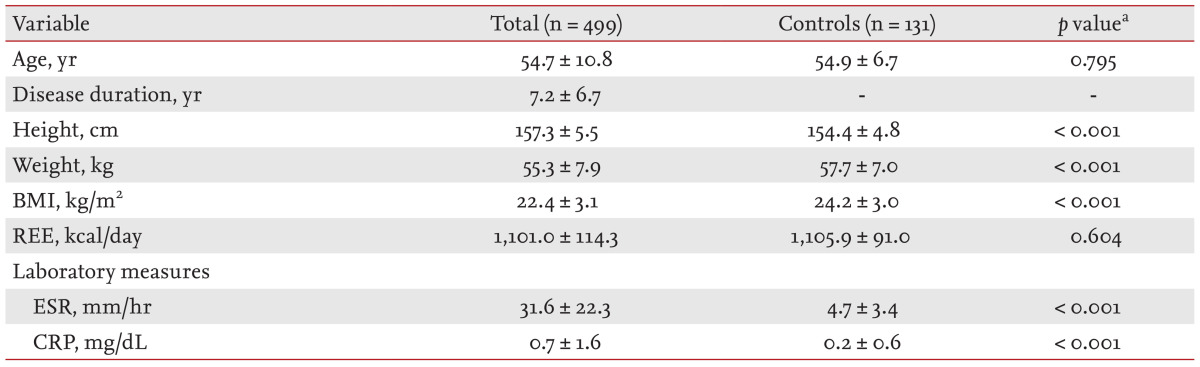
Baseline characteristics of the study population are shown in Table 1. A total of 499 female patients with RA was consecutively enrolled. Mean age at study commencement and disease duration in RA patients were 54.7 years (SD, 10.8) and 7.2 years (SD, 6.7), respectively. Age-matched controls (n = 131) were also enrolled. Differences in BMI, ESR, and CRP levels between RA patients and controls were all significant (p < 0.001 for all comparisons). REEs in RA and control subjects were estimated to be 1,101.0 kcal/day (SD, 114.3) and 1,105.9 kcal/day (SD, 91.0), respectively. These values did not significantly differ after adjustment for covariates including age, ESR, CRP level, and BMI (p = 0.659). In addition, the REE of controls was similar to that of RA patients in remission or not; the differences were not significant upon ANCOVA analysis (p = 0.906 and p = 0.758, respectively) (Fig. 1).
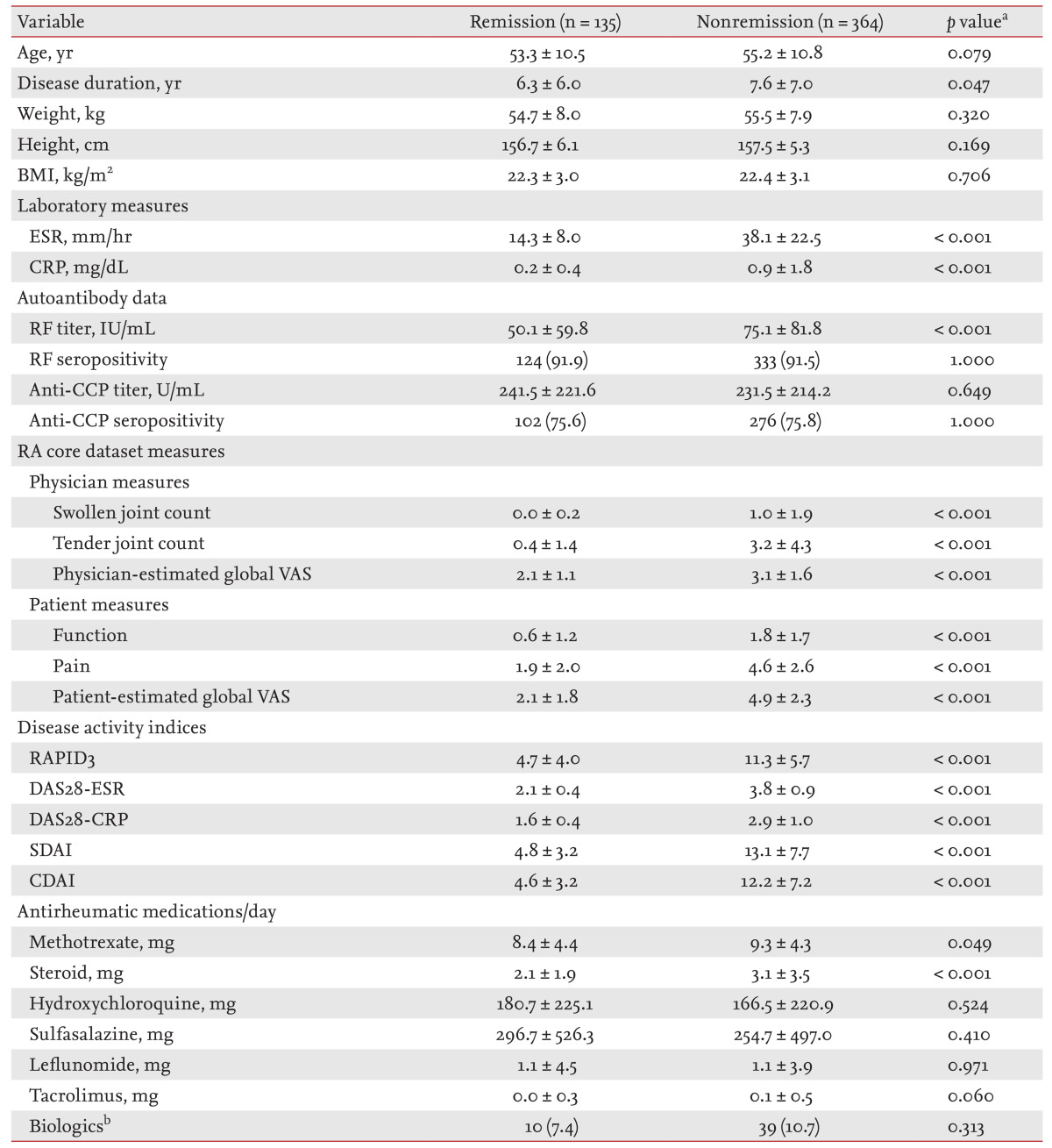
Differences in clinical variables and the use of DMARDs between the remission and nonremission RA groups are shown in Table 2. Whereas some variables including age, BMI, autoantibody positivity, and anti-CCP antibody titer did not differ significantly between the remission and nonremission RA groups; measures of laboratory markers, RA core dataset measures, and five disease activity index composite scores were higher in the nonremission group than the remission group (p < 0.001 for all comparisons). The levels of antirheumatic medications, including MTX and steroids, taken in the nonremission group were higher than in the remission group, but those of HCQ, SSZ, leflunomide, tacrolimus, and biologics were similar between the two groups.
To determine whether any clinical variable was associated with REE, we performed Pearson correlation analysis (Table 3). Age and BMI were associated with REE in all patients (r = -0.549, p < 0.001 for age; and r = 0.461, p < 0.001 for BMI). Age, disease duration, BMI, and ESR were associated with REE in the RA remission group, whereas REE in the RA nonremission group was associated with both age and BMI (Table 3). However, we found no correlation between REE and scores on disease activity indices, including RAPID3, SDAI, and CDAI (data not shown).
The results of multiple linear regression analysis are shown in Table 4. In all patients, those with higher REEs were younger and had higher BMIs than did those with lower REEs. In both the remission and nonremission RA groups, age and BMI were significantly associated with REE. Neither disease duration nor the ESR was significantly associated with REE in the remission group.
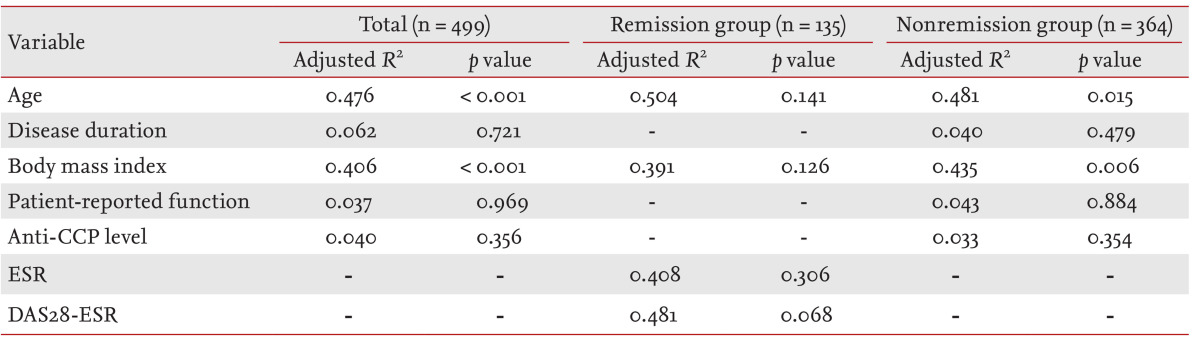
We next performed one-way ANOVA to identify significant differences in clinical variables among subjects in the four REE quartiles. BMI, patient-reported functional scores, disease duration, and anti-CCP antibody titer differed significantly in all RA patients in terms of their REE quartiles (p < 0.001, p < 0.001, p = 0.001, and p = 0.003, respectively). However, the apparently significant associations between patient-reported functional scores, disease duration, and anti-CCP antibody level disappeared after adjustment for age, patient-reported functional capacity, BMI, disease duration, and anti-CCP antibody level, using the ANCOVA method (Table 5). Age and BMI differed significantly among the REE quartiles when all patients were considered (R2 = 0.476, p < 0.001 for age; R2 = 0.406, p < 0.001 for BMI). A similar trend was also evident in the nonremission group. However, no clinical variable showed a significant association with REE quartile in RA patients in remission.
It has well known that RA patients have higher REEs than healthy controls, and also a lower body cell mass and less body fat. We hypothesized that REE might be affected by RA-related disease severity or activity, and might serve as a potentially useful marker of such activity or as a predictor of remission. We therefore performed the present study to determine whether individual components or composites of disease activity indices were associated with REE. We found that REE did not reflect RA disease activity status in our present cross-sectional study, although age and BMI were indeed associated with REE levels.
An earlier work found that an increase in REE was closely associated with TNF-╬▒ and IL-1╬▓ levels in RA patients, but not controls, affording some insight into the interaction between in vitro cytokine production and bodily metabolism [3]. Additional studies have since reported similar results, namely an inverse relationship between TNF-╬▒ production level and lean body mass or the extent of protein breakdown [5,6]. A recent cross-sectional study with 14 controls and 14 RA patients reported a positive correlation between IL-6 level and increased REE [7]. Aberrant inflammatory changes presenting in RA patients may enhance protein catabolism, triggering a decrease in FFM and an increase in REE. Additionally, smokers with RA have been shown to have higher basal metabolic rates than nonsmokers [20,21,22]. These results indicate that cigarette smoking causes persistent inflammation and functional disability, and also increases the basal metabolic rate of RA patients. However, we found no difference in REE between RA patients and controls, even after adjustment of covariates, in contrast to previous studies [3,5,6,7]. Consistent with our findings, a recent cross-sectional study showed that the REE of RA patients did not differ from that of age- and BMI-matched controls [23].
Daily TEE is composed of REE, TEF, and PA [8,9]. REE is a major component of TEE, accounting for more than 65% thereof, and is regarded as a predictor of TEE. Although REE can be accurately assessed via indirect calorimetry, the required equipment is expensive, and personnel must be suitably trained. Several predictive equations have been developed to calculate REE using readily available parameters including age, gender, height, and weight. The original REE equation was developed by Harris and Benedict [24] about 100 years ago. Since that time, Mifflin et al. [10] have introduced a new REE predictive equation based on body weight and height, without considering metabolic activity. Although REE may be increased in RA patients and may also be the principal component of TEE, a recent study showed that TEE was dependent on PA energy expenditure, rather than REE [23]. These findings suggest that REE data should be interpreted cautiously in the context of the energy metabolism of RA patients.
Several proinflammatory cytokines, including TNF-╬▒, IL-1╬▓, and IL-6, produced by inflammatory or immune cells in the synovium, play crucial roles in perpetuation of chronic inflammation in that tissue, associated with the aberrant neovascularization and enhanced osteoclastogenesis of RA [1,2]. Such pathogenic mechanisms trigger inflammatory responses in the joints involved in RA, ultimately causing joint deformities, functional disabilities, and poor quality-of-life. In addition, several earlier studies showed that increased REE in RA patients was linked to excessive production of proinflammatory cytokines, including TNF-╬▒, IL-1╬▓, and IL-6 [3,5,6,7]. REE has therefore been suggested to be associated with disease activity and to be a potential marker of clinical responses to antirheumatic therapies. Although data supporting a direct correlation between REE and disease activity are lacking, some studies have suggested that REE increases when disease activity rises, based on positive correlations between cytokine production and alterations in energy metabolism [3,5,6,7]. Recently, Jones et al. [13] reported that basal metabolic rate could be used as an indicator of RA disease activity. In the present study, however, REE was not associated with scores on any of the disease activity indices RAPID3, DAS28, SDAI, or CDAI.
The lack of association between REE and disease activity can be explained as follows. Enhanced REE in RA patients is thought to be caused by cytokine-induced hypermetabolism [3,5,6] and, therefore, TNF-blocking agents should decrease REE. Marcora et al. [12] enrolled 26 RA patients and randomized them into MTX or etanercept groups. The cited authors found no changes in body composition index scores after 24 weeks of treatment. Another work analyzed 20 RA patients treated with an anti-TNF blocker [11]. After 12 weeks of anti-TNF therapy, significant improvements were evident in terms of disease activity, physical function, and protein intake. However, neither REE nor FFM changed. These studies imply that alterations in energy metabolism, reflected by a change in REE, may not be associated with a reduction in the systemic inflammation characteristic of RA. Therapy with anti-TNF antagonists appears to reduce disease activity, and improve both functional status and protein intake in the short term. It is possible that changes in body composition or metabolism, such as REE or FFM, may become obvious only after several months of such treatment. Furthermore, we calculated REE using covariates including age, height, and weight, but found a close association between REE, age, and BMI, which may explain why REE was not associated with disease activity index scores. Metsios et al. [25] proposed two new predictive equations for REE in RA patients, based on CRP level and FFM, respectively, because the existing equations may underestimate REE in RA as they do not consider RA-associated cytokine-driven hypermetabolic status. Upon additional analysis using the CRP-based equation, we found that CRP level was closely associated with REE (data not shown). The CRP-based equation of the authors cited above may be a better means of measuring REE in RA patients. However, the equation is not yet not widely used in either the clinic or research. In addition, it is difficult to directly compare data derived from existing equations with those afforded by the new REE predictive method. Therefore, further studies are required to compare anthropometric variable- or CRP-based REE prediction equations measuring energy metabolism in RA patients.
The present study had some limitations. First, the work was cross-sectional in nature; we sought to determine if an association existed between REE and the level of disease activity. REE should be measured prospectively over a longer follow-up duration. However, neither of two clinical studies on anti-TNF blocker therapies with follow-up times of 12 and 24 weeks found any significant change in body composition index scores. Second, we calculated REE using the anthropometric variables of age, weight, and height. Therefore, REE was not directly measured, but rather predicted. Indirect calorimetry provides accurate REE measurements, but the use thereof is limited in clinical practice and studies of large populations. A CRP-based anthropometric equation has also been developed [25], but is not widely used. Further studies comparing REE prediction equations in RA patients are required.
In conclusion, REE was not associated with scores on RA disease activity indices and is therefore not a useful marker of disease activity in RA patients.
1. It is known that increased resting energy expenditure (REE) in rheumatoid arthritis (RA) patients can be caused by hypermetabolism associated with proinflammatory cytokine production.
2. We found no association between REE and RA disease activity in a Korean population.
3. Energy metabolism in RA may be independent of RA-associated systemic inflammation.
References
1. Firestein GS. Evolving concepts of rheumatoid arthritis. Nature 2003;423:356ŌĆō361PMID : 12748655.


2. Muller-Ladner U, Pap T, Gay RE, Neidhart M, Gay S. Mechanisms of disease: the molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat Clin Pract Rheumatol 2005;1:102ŌĆō110PMID : 16932639.


3. Roubenoff R, Roubenoff RA, Cannon JG, et al. Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest 1994;93:2379ŌĆō2386PMID : 8200971.



4. Rall LC, Roubenoff R. Rheumatoid cachexia: metabolic abnormalities, mechanisms and interventions. Rheumatology (Oxford) 2004;43:1219ŌĆō1223PMID : 15292530.


5. Rall LC, Rosen CJ, Dolnikowski G, et al. Protein metabolism in rheumatoid arthritis and aging: effects of muscle strength training and tumor necrosis factor alpha. Arthritis Rheum 1996;39:1115ŌĆō1124PMID : 8670319.


6. Roubenoff R, Roubenoff RA, Ward LM, Holland SM, Hellmann DB. Rheumatoid cachexia: depletion of lean body mass in rheumatoid arthritis: possible association with tumor necrosis factor. J Rheumatol 1992;19:1505ŌĆō1510PMID : 1464859.


7. Arshad A, Rashid R, Benjamin K. The effect of disease activity on fat-free mass and resting energy expenditure in patients with rheumatoid arthritis versus noninflammatory arthropathies/soft tissue rheumatism. Mod Rheumatol 2007;17:470ŌĆō475PMID : 18084698.


8. Ravussin E, Burnand B, Schutz Y, Jequier E. Twenty-four-hour energy expenditure and resting metabolic rate in obese, moderately obese, and control subjects. Am J Clin Nutr 1982;35:566ŌĆō573PMID : 6801963.


9. Jequier E, Schutz Y. Long-term measurements of energy expenditure in humans using a respiration chamber. Am J Clin Nutr 1983;38:989ŌĆō998PMID : 6650455.


10. Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241ŌĆō247PMID : 2305711.


11. Metsios GS, Stavropoulos-Kalinoglou A, Douglas KM, et al. Blockade of tumour necrosis factor-alpha in rheumatoid arthritis: effects on components of rheumatoid cachexia. Rheumatology (Oxford) 2007;46:1824ŌĆō1827PMID : 18032540.


12. Marcora SM, Chester KR, Mittal G, Lemmey AB, Maddison PJ. Randomized phase 2 trial of anti-tumor necrosis factor therapy for cachexia in patients with early rheumatoid arthritis. Am J Clin Nutr 2006;84:1463ŌĆō1472PMID : 17158431.


13. Jones H, Szumski A, Koenig AS. Basal metabolic rate as an indicator of rheumatoid arthritis disease activity and predictor of remission. Arthritis Rheum 2012;64(Suppl 10):S174.



14. Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44ŌĆō48PMID : 7818570.


15. Aletaha D, Nell VP, Stamm T, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 2005;7:R796ŌĆōR806PMID : 15987481.



16. Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003;42:244ŌĆō257PMID : 12595618.


17. Lee SS, Park MJ, Yoon HJ, Park YW, Park IH, Park KS. Evaluating the Korean version of the Multidimensional Health Assessment Questionnaire in patients with rheumatoid arthritis. Clin Rheumatol 2006;25:353ŌĆō357PMID : 16273310.


18. Pincus T, Swearingen CJ, Bergman M, Yazici Y. RAPID3 (Routine Assessment of Patient Index Data 3), a rheumatoid arthritis index without formal joint counts for routine care: proposed severity categories compared to disease activity score and clinical disease activity index categories. J Rheumatol 2008;35:2136ŌĆō2147PMID : 18793006.


19. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315ŌĆō324PMID : 3358796.


20. Metsios GS, Stavropoulos-Kalinoglou A, Nevill AM, Douglas KM, Koutedakis Y, Kitas GD. Cigarette smoking significantly increases basal metabolic rate in patients with rheumatoid arthritis. Ann Rheum Dis 2008;67:70ŌĆō73PMID : 17502358.


21. Collins LC, Walker J, Stamford BA. Smoking multiple high- versus low-nicotine cigarettes: impact on resting energy expenditure. Metabolism 1996;45:923ŌĆō926PMID : 8769345.


22. Metsios GS, Flouris AD, Jamurtas AZ, et al. A brief exposure to moderate passive smoke increases metabolism and thyroid hormone secretion. J Clin Endocrinol Metab 2007;92:208ŌĆō211PMID : 17077134.


23. Roubenoff R, Walsmith J, Lundgren N, Snydman L, Dolnikowski GJ, Roberts S. Low physical activity reduces total energy expenditure in women with rheumatoid arthritis: implications for dietary intake recommendations. Am J Clin Nutr 2002;76:774ŌĆō779PMID : 12324290.


Figure┬Ā1
Comparison of resting energy expenditures (REEs) among enrolled study subjects. REEs in the rheumatoid arthritis (RA) remission (RA_remi) and nonremission (RA_nonremi) groups were similar to that of healthy controls (p > 0.05 for both comparisons). p values were calculated by analysis of covariance after adjustment for age, body mass index, erythrocyte sediment rate, and C-reactive protein level.

Table┬Ā1
Baseline demographic and clinical characteristics of rheumatoid arthritis (RA) patients and controls
Table┬Ā2
Comparisons of clinical variables between rheumatoid arthritis (RA) patients in remission and not
Values are presented as mean ┬▒ SD or number (%).
BMI, body mass index; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; RF, rheumatoid factor; CCP, cyclic citrullinated peptide; VAS, visual analog scale; RAPID3, routine assessment of patient index data 3; DAS28, disease activity score 28; SDAI, simplified disease activity index; CDAI, clinical disease activity index.
ap values refer to comparisons between rheumatoid arthritis patients in remission or not using the Mantel-Haenszel chi-squared test for categorical variables and Student t test for continuous variables.
bBiologics included infliximab, etanercept, adalimumab, and abatacept.
Table┬Ā3
Correlations between resting energy expenditure and other clinical parameters in rheumatoid arthritis patientsa

Table┬Ā4
Determination of clinical/laboratory parameters relevant to resting energy expenditure in rheumatoid arthritis patientsa




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



